- Corresponding Author:
- C. L. SINGH
R. V. Northland Institute, Chithera, Dadri, Gautam Budh Nagar-203 207,New Delhi-110 017, India
E-mail: chhotelal007@gmail.com
| Date of Submission | 11 May 2014 |
| Date of Revision | 21 January 2015 |
| Date of Acceptance | 26 July 2015 |
| Indian J Pharm Sci 2015;77(4):399-404 |
Abstract
In the present study a simple, accurate, precise, economical and specific UV-spectrophotometric method for estimation of besifloxacin in bulk and in different pharmaceutical formulation has been developed. The drug shows maximum λmax 289 nm in distilled water, simulated tears and phosphate buffer saline. The linearity range of developed methods were in the range of 3-30 µg/ml of drug with a correlation coefficient (r2) 0.9992, 0.9989 and 0.9984 with respect to distilled water, simulated tears and phosphate buffer saline, respectively. Reproducibility by repeating methods as %RSD were found to be less than 2%. The limit of detection in different media was found to be 0.62, 0.72 and 0.88 µg/ml, respectively. The limit of quantification was found to be 1.88, 2.10, 2.60 µg/ml, respectively. The proposed method was validated statically according to International Conference on Harmonization guidelines with respect to specificity, linearity, range, accuracy, precision and robustness. The proposed methods of validation were found to be accurate and highly specific for the estimation of besifloxacin in different pharmaceutical formulations.
Keywords
Besifloxacin, UV−Spectrophotometric method, Simulated tears, ICH guidelines, Validation
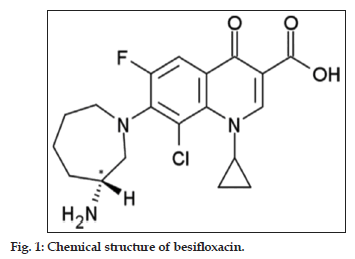
Besifloxacin (BSF) is a fourth−generation fluoroquinolone antibiotic with systemic n a m e ( I U PA C ) a s 7 − [ ( 3 R ) − 3 − a m i n o a z e p a m − 1 − y l ] − 8 − c h l o r o − 1 − c y c l o p r o p y l − 6 − f l u o r o − 4 − oxo−1,4−dihydroquinoline−3−carboxylic acid (fig. 1). It is fourth generation ophthalmic fluoroquinolone of synthetic origin that was approved by the US−FDA in May, 2009 for the treatment of bacterial conjunctivitis[1]. The first marketed formulation of BSF salt was ‘Besivance’ having 0.6% ophthalmic suspension and sold first in USA under the trade name of Besivance®[2−6]. BSF is a novel 8−chlorofluoroquinolone with an N−1 cyclopropyl substituent[7]. The amino azepinyl substituent at the C−7 position and the chlorine at the C−8 position give BSF a unique structure and activity profile. In vitro studies show BSF to be highly active against both gram−positive and gram−negative bacteria, including multidrug−resistant strains, and to be rapidly bactericidal. The literature reported the estimation of BSF in human tears by tandem mass HPLC[8] bioassay method[9] and by chiral HPLC[10], but no published research papers were found by UV−Spectrophotometer.
The purpose of this proposed work was to develop the methods for quantitative estimation of BSF in different simulated body fluid and in pharmaceutical formulations. The literature survey does not report any UV−Spectrophotometric method for estimation of BSF in different body simulated fluids. This paper reports three newly developed methods for quantitative estimation of BSF in simulated body fluids, in bulk and in pharmaceutical formulations, according to International Conference on Harmonization (ICH) guidelines[11,12]. The results were analyzed and validated statistically. Thus, reported methods were found to be simple, accurate, precise and economical.
Materials and Methods
An UV/Vis double beam spectrophotometer (Pharmaspec−1700, Shimadzu, Japan), having 1 cm matched quartz cells, loaded with UV Probe software, spectral bandwidth of 1 nm, having wavelength accuracy of ±0.3 nm. All weighing was performed over 1 mg sensitive electronic balance (Vibra DJ−150S−S, Shinko Denshi, Japan). For mixing and dissolving bath sonicator (Sarthak SUC− 322) was used.
The API reference standard of BSF hydrochloride was obtained as kind gift from Indoco Remedies Ltd, Mumbai, India arranged by KPS Clinical Services, Greater Noida, UP, India. Besifloxacin ophthalmic suspension 0.6% w/v (Besix® eye drops) containing 5 ml of product was procured from a local pharmacy. Double distilled water was used as solvent for experimental purposes. Disodium hydrogen phosphate, potassium dihydrogen phosphate, sodium chloride, sodium bicarbonate, calcium chloride, hydrochloric acid and sodium hydroxide were purchased from Qualigens (Fischer), Mumbai, India. All chemicals and reagents used for experimental purposes were of analytical grade.
Preparation of stock solutions and calibration curve
The standard stock solution (60 μg/ml) of drug was prepared by dissolving accurately 6 mg of API in 100 ml of volumetric flask with distilled water. Aliquots of the range 0.5 to 5.0 ml of standard stock solution of drug were taken in a series of 10 ml volumetric flask where volume was made up to the mark with distilled water, simulated tears (pH 7.4) and phosphate buffer (pH 7.4) separately to make the concentration range of 3−30 μg/ml. The absorbance of each standards were measured at 289 nm against the respective medium as a blank.
The stock dilution (60 μg/ml) of marketed product was prepared by pipetting out 1 ml of 0.6% w/v ophthalmic suspension (with continuously sonication during pipetting), which was equivalent to 6 mg of BSF that was dissolved with 100 ml distilled water. The calibration curves were prepared by plotting graph between absorbance and concentration.
Preparation of phosphate buffer saline
Phosphate buffer saline of pH 7.4 was made by dissolving 2.38 g of disodium hydrogen phosphate, 0.19 g of potassium dihydrogen phosphate and 8.0 g of sodium chloride in sufficient distilled water to produce 1000 ml. The pH was maintained by concentrated hydrochloric acid and sodium hydroxide solution.
Preparation of simulated tears
A simulated tear of pH 7.4 was prepared by dissolving 0.2 g of sodium bicarbonate, 0.008 g calcium chloride and 0.67 g of sodium chloride in volumetric flask to produce 100 ml. The pH was maintained by concentrated hydrochloric acid and sodium hydroxide solution.
Methods validation
The development and validation of analytical procedures with respect to specificity, linearity, range, accuracy, precision, detection limit (DL), quantitation limit (QL) and robustness were developed according to ICH guidelines.
Specificity
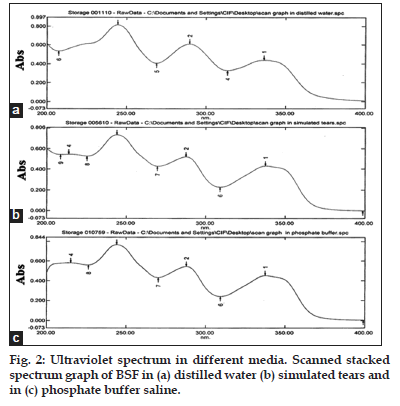
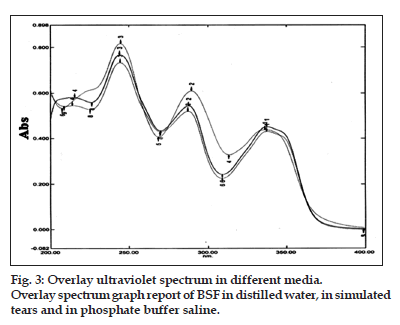
The specificity of the different method was analyzed by UV scan of the standard stock solution in different media like distilled water, simulated tears (pH 7.4) and phosphate buffer solution (pH 7.4), to determine the λmax (wavelength of maximum absorption) with different media. The scanning can be helpful to specificity of the method by evaluating interaction study obtained from scan of individual standard drug solution in different media and with others excipients of different formulations. In the present study, no interfering peak was observed (figs. 2 and 3) from the standard drug with different simulating media. Thus, scanning can be helpful to specificity.
Linearity
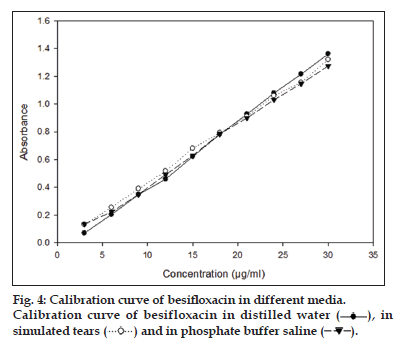
The linearity was established across the range and the absorbance of standard stock solution in different media in the range of 3−30 μg/ml was measured at 289 nm.The calibration curves were prepared by plotting graph between average absorbance (n=3) and concentration. Linearity was determined by least square regression method.
Range
The specified range was selected from linearity studies. The method for specified range was established by analyzing at 80, 100 and 120% of 3, 6 and 9 μg/ml samples in triplet at 289 nm. The range was expressed as mean recovery with SD and %RSD.
Accuracy
Accuracy can be analyzed by percentage recovery of added (2, 3 and 4.5 μg/ml) drug solution to fixed concentration (4.5 μg/ml) of standard drug solution. For standard stock solution, the accurately weighed amount of BSF 6 mg was dissolved in volumetric flask with 100 ml of distilled water. While for sample stock 1 ml of ophthalmic suspension was pipetted out and dissolved in volumetric flask with 100 ml of distilled water.
In a separate dilution three volumetric flask of 10 ml was taken and each having 4.5 μg/ml of standard stock solution were added with 2, 3 and 4.5 μg/ml of sample stock solution respectively to get final concentration of 6.5, 7.5 and 9 μg/ml after diluting with distilled water. The same procedure was repeated with the simulated tears and phosphate buffer solution. The accuracy was reported as percentage recovery by the assay of known amount of sample in the standard solution with SD and %RSD.
Precision
Repeatability precision was calculated by analyzing 6 determinations of strength 15 μg/ml as 100% of BSF standard solution. The repeatability precision was expressed as %RSD. The intermediate precision was evaluated on the standard solution of strength 9 μg/ml on same day and also on two consecutive weeks in each media.
Detection limit and quantitation limit
The detection limit and the quantitation limit were based on the slope of the calibration curve and standard deviation of Y− intercept of regression line.
Robustness
The robustness of the method was evaluated by stability study of BSF on the same day and on two consecutive days. The robustness was expressed as amount recovered in %RSD.
Results and Discussion
The proposed method was validated according to the guidelines of ICH. The method discussed in this analysis provides a simple, accurate, economical and convenient for the analysis of BSF by UV−spectroscopy. The absorbance spectra of BSF in different media like distilled water, simulated tears and phosphate buffer solution were shown in (fig. 2). The found reported average λmax was 289 nm that was the same in different scanning media (fig. 3). Thus proposed method was found to be specific and selective.
In the developed method, linearity was observed in the concentration range of 3−30 μg/ml. Linear absorbance versus concentration gives regression equation; Y=0.0438X−0.0905, Y=0.0437X+0.0001 and Y=0.0434X–0.0207 with a correlation coefficient (r2) of more than 0.99 in distilled water, simulated tears and in phosphate buffer saline solutions (fig. 4). The above linear regression equation with a high correlation coefficients indicates good linearity between absorbance and concentration in the range of 3−30 μg/ml Table 1.
The accuracy of the proposed method by standard addition method were found within the specified range, thus indicates the accuracy of the method Table 2. The specified range test of the proposed method of each 3, 6 and 9 μg/ml studied at 80, 100 and 120% were analyzed and calculated for their percentage recovery with SD and % RSD. The SD and % RSD within the range (<2%) proves the specific range test within limit Table 3.
| Parameters | Media 1 | Media 2 | Media 3 |
|---|---|---|---|
| Linearity range (µg/ml) | 3–30 | 3–30 | 3–30 |
| Regression equation | Y=0.0484X-0.0905 | Y=0.0437X+0.0001 | Y=0.0434X-0.0207 |
| Correlation coefficient (r2) | 0.9992 | 0.9989 | 0.9984 |
| Molar absorptivity (ε) (L/mol/cm) | 0.0382±103 | 0.0430±103 | 0.0410±103 |
| Sandell’s sensitivity (µg.cm2/0.001 abs. unit) | 10296±10-3 | 9142±10-3 | 9605±10-3 |
| 95% CI for slope | <0.0001 | <0.0001 | <0.0001 |
| 95% CI for intercept | <0.0001 | 0.9893 | 0.1111 |
| SE of slope | 0.0005 | 0.0005 | 0.0006 |
| SE of intercept | 0.0091 | 0.0096 | 0.0116 |
| SE of estimate | 0.0134 | 0.0141 | 0.0169 |
| Repeatability (%RSD) | 0.72 | 1.16 | 0.31 |
| DL (µg/ml) | 0.62 | 0.72 | 0.88 |
| QL (µg/ml) | 1.88 | 2.1 | 2.67 |
| Media 1: Distilled water, Media 2: simulated tear (pH=7.4), Media 3: phosphate buffer saline (pH=7.4), %RSD: percent residual standard deviation of six determinates. | |||
| CI: confidence interval, SE: standard error, DL: detection limit, QL: quantitation limit, BSF: besifloxacin, UV: ultraviolet | |||
Table 1: Regression, Analysis And System Suitablity Parameters Of Bsf From Uv−Spectrophotometric Method.
| Media | Standard | Added sample | Percentage | %RSD |
|---|---|---|---|---|
| concentration (µg/ml) | concentration (µg/ml) | recovery (mean±SD) | ||
| Distilled water | 4.50 | 2.00 | 105.23±0.01 | 0.17 |
| 4.50 | 3.00 | 108.37±0.02 | 0.26 | |
| 4.50 | 4.50 | 108.66±0.04 | 0.35 | |
| Simulated tear (pH=7.4) | 4.50 | 2.00 | 99.84±0.03 | 0.38 |
| 4.50 | 3.00 | 101.06±0.03 | 0.40 | |
| 4.50 | 4.50 | 99.77±0.03 | 0.34 | |
| Phosphate buffer saline (pH=7.4) | 4.50 | 2.00 | 101.35±0.02 | 1.94 |
| 4.50 | 3.00 | 104.00±0.12 | 1.47 | |
| 4.50 | 4.50 | 99.00±0.03 | 0.28 |
Table 2: Accuracy
| Media | Concentration | Tested concentration | Tested concentration | Tested concentration | |||
|---|---|---|---|---|---|---|---|
| (µg/ml) | (80% concentration) | (100% concentration) | (120% concentration) | ||||
| Percentage | % RSD | Percentage | % RSD | Percentage | %RSD | ||
| recovery±SD | recovery±SD | recovery±SD | |||||
| Distilled water | 6 | 103.56±0.12 | 1.98 | 102.14±0.06 | 0.87 | 102.08±0.05 | 0.65 |
| 9 | 103.88±0.04 | 0.55 | 104.22±0.07 | 0.69 | 104.34±0.04 | 0.41 | |
| 12 | 97.40±0.05 | 0.51 | 98.00±0.12 | 1.06 | 101.08±0.07 | 0.52 | |
| Simulated tear (pH=7.4) | 6 | 97.93±0.04 | 0.80 | 98.00±0.03 | 0.45 | 99.38±0.02 | 0.30 |
| 9 | 99.64±0.08 | 1.08 | 99.06±0.03 | 0.28 | 102.60±0.09 | 0.91 | |
| 12 | 97.86±0.04 | 0.43 | 97.25±0.04 | 0.38 | 101.67±0.29 | 1.22 | |
| Phosphate buffer saline (pH=7.4) | 6 | 97.77±0.05 | 1.00 | 102.50±0.04 | 0.57 | 103.21±0.04 | 1.91 |
| 9 | 99.60±0.05 | 0.66 | 99.96±0.02 | 0.20 | 98.81±0.18 | 1.80 | |
| 12 | 98.84±0.04 | 0.42 | 101.66±0.04 | 1.12 | 98.50±0.03 | 0.19 | |
Table 3: Range
The precision of the method was found for 15 μg/ml samples within the limit (<2 %RSD) prove the precision of the methods Table 4. The intermediate precision (% RSD) for selected sample 9 μg/ml was found to be 1.26, 1.09, 0.99 in distilled water, in simulated tears 0.25, 0.12, 1.03 and in phosphate buffer found to be 0.13, 0.17 and 0.86 in the analysis on same day, also on the two consecutive weeks (Table 5). The % recovery and % RSD proves high precision of the method.
The robustness of the proposed method was established by % recovery and % RSD of the sample on same day and also on two consecutive days was found to be 1.23, 0.56, and 1.26 in distilled water, 0.77, 1.35 and 1.10 in simulated tears 2.09, 0.55 and 1.74 in phosphate buffer, respectively. The result proves the robustness of the method Table 6. The DL and QL was found to 0.62 and 1.88 μg/ml in distilled water, 0.72 and 2.10 μg/ml in simulated tears, and 0.88 μg/ml and 2.67 μg/ml in phosphate buffer, respectively.
Conclusion
The developed method was found to be very simple, sensitive, accurate and economical. The reported UV−methods can be used for the analysis of BSF in simulated body fluids, in bulk and in marketed formulations. Thus proposed method will be suitable for the analysis of besifloxacin hydrochloride.
Acknowledgements
The authors would like to grateful of the authorities of R. V. Northland Institute, Dadri, G. B. Nagar, India for funds and providing required facilities to carry out the proposed work and also thankful to the KPS Clinical Services, Greater Noida, UP, India for providing the standard drug besifloxacin hydrochloride.
| Media | Concentration | Percentage | %RSD |
|---|---|---|---|
| (µg/ml) | recovery±SD | ||
| Distilled water | 15 | 101.06±0.11 | 0.72 |
| Simulated tear (pH=7.4) | 15 | 102.13±0.18 | 1.16 |
| Phosphate buffer saline (pH=7.4) | 15 | 99.00±0.05 | 0.31 |
Table 4: Precision Repeatability
| Media | Time | Concentration | Percentage | %RSD |
|---|---|---|---|---|
| (days) | (µg/ml) | recovery±SD | ||
| Distilled water | 1 | 9.00 | 100.62±0.12 | 1.26 |
| 7 | 9.00 | 106.95±0.12 | 1.09 | |
| 14 | 9.00 | 108.34±0.12 | 0.99 | |
| Simulated tear | 1 | 9.00 | 99.22±0.03 | 0.25 |
| (pH=7.4) | 7 | 9.00 | 102.88±0.01 | 0.10 |
| 14 | 9.00 | 105.11±0.10 | 1.03 | |
| Phosphate buffer | 1 | 9.00 | 101.3±0.01 | 0.13 |
| saline (pH=7.4) | 7 | 9.00 | 109.11±0.18 | 0.17 |
| 14 | 9.00 | 104.21±0.10 | 0.86 |
Table 5: Intermediate Precision.
| Media | Concentration | Percentage | %RSD |
|---|---|---|---|
| (µg/ml) | recovery±SD | ||
| Distilled water | 3 | 106.22±0.08 | 2.09 |
| 6 | 100.50±0.04 | 0.55 | |
| 9 | 102.55±0.16 | 1.74 | |
| Simulated tear (pH=7.4) | 3 | 105.84±0.03 | 0.77 |
| 6 | 102.33±0.09 | 1.35 | |
| 9 | 102.24±0.10 | 1.10 | |
| Phosphate buffer saline (pH=7.4) | 3 | 103.26±0.04 | 1.23 |
| 6 | 101.83±0.04 | 0.56 | |
| 9 | 101.22±0.12 | 1.26 |
Table 6: Robustness
Financial support and sponsorship
R. V. Northland Institute, Dadri, G. B. Nagar, India provided the funds and facilities for the work.
Conflicts of interest
There are no conflicts of interest.
References
- Bausch, Lomb. New topical ophthalmic antibacterial for the treatment of bacterial conjunctivitis. Receives FDA Approval of Besivance; 29 May, 2009.
- Silverstein BE, Allaire C, Bateman KM, Gearinger LS, Morris TW, Comstock TL. Efficacy and tolerability of besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis: A multicenter, randomized, double-masked, vehicle-controlled, parallel-group study in adults and children. ClinTher 2011;33:13-26.
- McDonald MB, Protzko EE, Brunner LS, Morris TW, Haas W, Paterno MR, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology 2009;116:1615-1623.e1.
- Tepedino ME, Heller WH, Usner DW, Brunner LS, Morris TW, Haas W, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin 2009;25:1159-69.
- Zhang JZ, Ward KW. Besifloxacin, a novel fluoroquinolone antimicrobial agent, exhibits potent inhibition of pro-inflammatory cytokines in human THP-1 monocytes. J AntimicrobChemother 2008;61:111-6.
- Ward KW, Lepage JF, Driot JY. Nonclinical pharmacodynamics, pharmacokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. J OculPharmacolTher 2007;23:243-56.
- Haas W, Sanfilippo CM, Hesje CK, Morris TW. Contribution of the R8 substituent to the in vitro antibacterial potency of besifloxacin and comparator ophthalmic fluoroquinolones. ClinOphthalmol 2013;7:821-30.
- Arnold DR, Granvil CP, Ward KW, Proksch JW. Quantitative determination of besifloxacin, a novel fluoroquinolone antimicrobial agent, in human tears by liquid chromatography-tandem mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 2008;867:105-10.
- Costa MC, Barden AT, Andrade JM, Oppe TP, Schapoval EE. Quantitative evaluation of besifloxacin ophthalmic suspension by HPLC, application to bioassay method and cytotoxicity studies. Talanta 2014;119:367-74.
- Wang Z, Wang S, Zhu F, Chen Z, Yu L, Zeng S. Determination of enantiomeric impurity in besifloxacin hydrochloride by chiral high-performance liquid chromatography with precolumnderivatization. Chirality 2012;24:526-31.
- ICH, Q2A, Hamonised Tripartite Guideline. Test on Validation of Analytical Procedures, IFPMA. In: Proceedings of the International Conference on Harmonization. Geneva; 1994.
- ICH, Q2B, Hamonised Tripartite Guideline. Validation of Analytical Procedure: Methodology, IFPMA. In: Proceedings of the International Conference on Harmonization. Geneva; 1996.
 ) and in phosphate buffer saline (
) and in phosphate buffer saline ( ).
).



