- Corresponding Author:
- J. R. Jain
Department of Quality Assurance, Maliba Pharmacy College, Bardoli-Mahuva Road, Tarsadi, Dist. Surat-394 350, India
E-mail: jyoteshjain@yahoo.com
| Date of Submission | 2 April 2010 |
| Date of Revision | 19 March 2011 |
| Date of Acceptance | 5 April 2011 |
| Indian J Pharm Sci, 2011, 73 (3): 296-300 |
Abstract
and aceclofenac from tablet dosage form. Method I is a simultaneous equation method (Vierodt’s method), wavelengths selected are 306.5 and 276 nm. Method II is the absorbance ratio method (Q-Analysis), which employs 298.5 nm as λ1 and 276 nm as λ2 (λmax of AF) for formation of equations. Both the methods were found to be linear between the range of 8-32 μg/ml for drotaverine and 10-40 μg/ml for aceclofenac. The accuracy and precision were determined and found to comply with ICH guidelines. Both the methods showed good reproducibility and recovery with % RSD in the desired range. The methods were found to be rapid, specific, precise and accurate and can be successfully applied for the routine analysis of drotaverine and aceclofenac in their combined tablet dosage form.
Keywords
Aceclofenac, absorbance ratio, drotaverine hydrochloride, simultaneous equation
Chemically, drotaverine (DV, fig. 1a) is (1-(3,4- diethoxybenzylidene)-6,7-diethoxy-1,2,3,4 tetrahydroisoquinoline) hydrochloride. It is a benzylisoquinoline derivative[1]. It is a highly potent spasmolytic agent and has excellent smooth muscle relaxant properties[2]. Aceclofenac (AF, fig. 1b) is 2-[(2,6-Dichlorophenyl)amino]benzeneacetic acid carboxymethyl ester[3]. It is used as an antiinflammatory drug. Literature survey revealed that assay of AF in bulk and dosage form is official in Indian Pharmacopoeia 2007[4] and British Pharmacopoeia 2008[3]. Several analytical methods have been reported for estimation of DV like spectrophotometry[5-7], HPLC[8], flow injection chemiluminescence analysis[9], thin layer chromatography[10,11] and voltammetry[12]. The analytical methods reported for estimation of AF are spectrophotometry[13-15], HPLC[16-18], LC-MS[19] and fluorimetry[20]. The present paper describes simple, accurate, specific and precise methods for simultaneous estimation of DV and AF in their combined tablet dosage form using two UV spectrophotometric methods (a) simultaneous equation method and (b) absorbance ratio method[21]. The proposed methods are optimized and validated as per the ICH guidelines[22,23].
A Shimadzu UV/Vis double beam spectrophotometer (model UV-1800) with a pair of 1 cm matched quartz cells was employed in this investigation. All weighing was done on a Shimadzu analytical balance (Model AU-220). Pure samples of DV and AF were obtained as gift samples from Astran Labs, Ahmedabad. Combined tablet formulation (Esnil) was procured from local pharmacy. Methanol AR was used as solvent.
Accurately weighed quantity of DV (80 mg) and AF (100 mg) was transferred to two separate 100 ml volumetric flasks, dissolved in little amount of methanol and diluted to the mark with methanol stock solutions: 800 μg/ml of DV and 1000 μg/ml of AF).
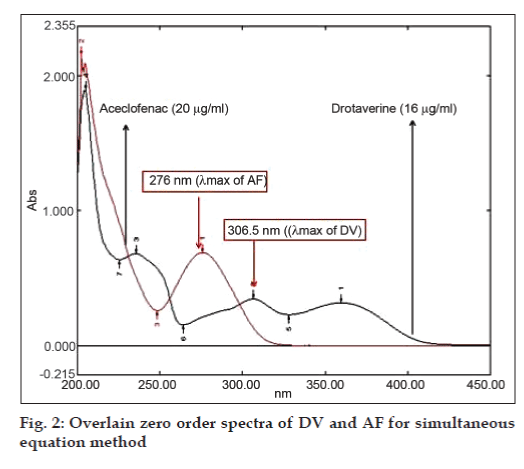
Simultaneous equation method uses the absorbances at two selected wavelengths, both being the λmax of the two drugs. Working standard solutions were scanned in the entire range of 200-400 nm to determine the λmax of both the drugs. The λmax of DV and AF were found to be 306.5 nm and 276 nm respectively (fig. 2). Seven standard solutions having concentrations 8, 12, 16, 20, 24, 28, 32 μg/ ml for DV and 10, 15, 20, 25, 30, 35, 40 μg/ml for AF were prepared in methanol. The absorbances of resulting solutions were measured at 306.5 and 276 nm and calibration curves were plotted at these wavelengths. The absorptivity coefficient of these two drugs was determined using the calibration curve equation. Two simultaneous equations were formed using these specific absorbance values. A1= 221.88Cx+63.43Cy, A2= 99.29Cx+332.86Cy, where, Cx and Cy are concentrations of DV and AF, respectively, in g/100 ml in sample solution. A1 and A2 are absorbances of the sample solution at 306.5 and 276 nm, respectively.
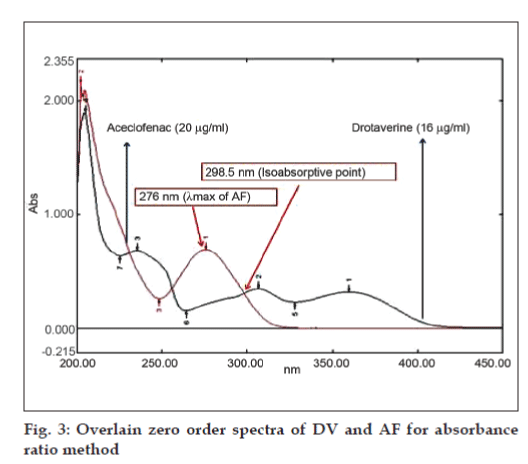
The concentration of Cx and Cy can be obtained as, Cx= (A2ay1-A1ay2)/(ax2ay1-ax1ay2) and Cy = (A1ax-A2ax1)/ (ax2ay1-ax1ay2), where, A1 and A2 are the absorbances of mixture at 306.5 and 276 nm respectively, ax1 and ax2 are absorptivities of DV at 306.5 and 276 nm respectively, ay1 and ay2 are absorptivities of AF at 306.5 and 276 nm respectively, Cx is concentration of DV, Cy is concentration of AF.Absorbance ratio method uses the ratio of absorbances at two selected wavelengths one at iso-absorptive point and other being the λmax of one of the two components. From the overlain spectra of two drugs, it is evident that DV and AF show an iso-absorptive point at 298.5 nm and the second wavelength used was 276 nm, which is the λmax of AF (fig. 3). Seven standard solutions having concentration 8, 12, 16, 20, 24, 28, 32 μg/ml for DV and 10, 15, 20, 25, 30, 35, 40 μg/ml for AF were prepared in methanol. The absorbances at 298.5 nm (isoabsorptive- point) and 276 nm (λmax of AF) were measured and absorptivity coefficients were calculated using calibration curve.
The concentrations Cx and CY of DV and AF, respectively in the sample mixture can be calculated using equations Cx= [(Qm–Qy)/(Qx–Qy)]×A1/ax1 and Cy= [(Qm–QX)/(Qy–Qx)]×A1/ay1. The Q-values and absorptivities for both drugs were calculated as follows, Qm=[Absorbance of sample solution at 276 nm/absorbance of sample solution at 298.5 nm (A1)], Qx=[Absorptivity of DV at 276 nm/ absorptivity of DV at 298.5 nm], Qy=[Absorptivity of AF at 276 nm/Absorptivity of AF at 298.5 nm], ax1=[Absorbance of DV at 298.5 nm/Concentration of DV in g/100 ml], ay1=[Absorbance of AF at 298.5 nm/Concentration of AF in g/100 ml], where, Qx and Qy are Q values of DV and AF, respectively, ax1 and ay1 are absorptivities at isoabsorptive point for DV and AF, respectively. These values were found to be Qx= 0.511, ax1=194.46, Qy= 2.301, ay1= 144.64.
Ten tablets were weighed and crushed to obtain a fine powder. An accurately weighed tablet powder equivalent to about 80 mg of DV and 100 mg of AF was transferred to 100 ml volumetric flask and dissolved in 50 ml of methanol. The volume was made up to the mark using methanol as solvent. The resulting solution was filtered through Whatmann filter paper and 10 ml of this filtrate was appropriately diluted to get concentration of 80 μg/ml of DV and 100 μg/ml of AF. This solution was further diluted to get concentration of 16 μg/ml of DV and 20 μg/ml of AF. Absorbance of sample solutions was measured at 306.5 and 276 nm and the concentration of two drugs in the sample were determined using Eqns (1) and (2) (method I). For method II, the absorbance of the sample solution A1 and A2 were measured at 298.5 nm (iso-absorptive point) and 276 nm (λmax of AF) respectively and ratio of absorbance were calculated which was known as Qm. Relative concentrations of two drugs were calculated using equations (3) and (4). The result of analysis of tablet formulation is shown in Table 1.
| Method | mg/Tablet | % of label claim | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DV | AF | DV | AF | DV | AF | ||||
| Label | Label | OBT | OBT | ||||||
| Method-1 | (SE) | 80 | 100 | 80.29 | 99.05 | 100.37 | 99.05 | ||
| Method-2 | (AR) | 80 | 100 | 80.40 | 99.65 | 100.50 | 99.65 | ||
*Average of five determinations; SE - Simultaneous equation; AR - Absorbance ratio; DV - Drotaverine; AF - Aceclofenac; OBT - Obtained.
Table 1: Results Of Simultaneous Estimation Of Dv And Af In Marketed Formulation By Method I And Ii
Aliquots of standard stock solutions of DV and AF were taken in volumetric flasks and diluted with methanol to get final concentrations in range of 8-32 μg/ml for DV and 10-40 μg/ml for AF. This calibration range was prepared five times and absorbances were measured at respective wavelengths for each drug separately.
Precision of the methods was determined by performing interday variation, intraday variation and repeatability studies. In interday variation, the absorbance of standard solutions of DV (8-32 μg/ml) and AF (10-40 μg/ml) were measured on five consecutive days. In intraday variation, the absorbances were measured five times in a day. In repeatability study, three concentrations of both the drugs were analysed in triplicate.To study the accuracy of the proposed methods, recovery studies were carried out by standard addition method at three different levels. A known amount of drug was added to pre-analyzed tablet powder and percentage recoveries were calculated.
The proposed methods were validated as per ICH guideline. The plot of absorbances versus respective concentrations of DV was found to be linear in the concentration range of 8-32 μg/ml with correlation coefficient 0.9996 at 306.5 nm and for AF it was found to be linear in the concentration range of 10- 40 μg/ml with 0.9990 correlation coefficient at 276 nm for simultaneous equation method (method I). For absorbance ratio method (method II) linearity range was same as for method I with correlation coefficient 0.9995 at 298.5 nm and 0.9990 at 276 nm. Precision was calculated as interday and intraday variations and % RSD was found to be less than 1 for both methods and for both drugs (Table 2). The accuracy of method was determined at 75, 100 and 125% level. The % recovery ranges from 98.23% to 100.49% for both the methods (Table 3). The two methods can be successfully used for simultaneous estimation of DV and AF in their combined tablet dosage form. Marketed tablets were analyzed and results obtained were in the range of 98-102% (Table 1). The proposed methods were found to be simple, accurate and rapid for the routine determination of DV and AF in tablet formulation.
| Parameters | Simultaneous equation method | Absorbance ratio method | |||
|---|---|---|---|---|---|
| DV | AF | DV | AF | ||
| Linearity range | 8-32 | 10-40 | 8-32 | 10-40 | |
| Correlation coefficient | 0.9996 | 0.9990 | 0.9995 | 0.9990 | |
| Precision (% RSD) | |||||
| Repeatability | 0.30-0.91 | 0.07-0.85 | 0.07-0.89 | 0.07-0.85 | |
| Intraday (n=5) | 0.32-0.90 | 0.14-0.74 | 0.43-0.78 | 0.07-0.85 | |
| Interday (n=5) | 0.52-0.88 | 0.34-0.52 | 0.47-0.54 | 0.34-0.52 | |
| % Recovery | 98.56-100.11% | 98.23-100.49% | 98.13-99.03% | 98.13-100.74% | |
| *For repeatability n=3. | |||||
Table 2: Validation Parameters
| Name of drug | Amount of drug added | Method I | Method II | ||||
|---|---|---|---|---|---|---|---|
| (µg/ml) | % Recovery* | SD | % Recovery* | SD | |||
| DV | 12 | 100.11 | 0.005 | 98.96 | 0.004 | ||
| 16 | 98.56 | 0.004 | 98.13 | 0.003 | |||
| 20 | 99.56 | 0.003 | 99.03 | 0.006 | |||
| AF | 15 | 100.49 | 0.004 | 100.74 | 0.004 | ||
| 20 | 98.23 | 0.003 | 98.40 | 0.003 | |||
| 25 | 100.16 | 0.005 | 100.27 | 0.006 | |||
| *Mean of three estimations | |||||||
Table 3: Recovery Studies
Acknowledgements
The authors are thankful to Astran Lab., Ahmedabad for providing pure gift samples of drotaverine hydrochloride and aceclofenac. The authors are also thankful to the Principal, Maliba Pharmacy College for providing necessary facilities.
References
- Oneil MJ, Smith A, Heckelman PE. The Merck Index. 13th ed. Whitehouse Station NJ: Merck; 2001. p. 3489.
- Chitlange SS, Ranjana S, Wankhede SB, Kulkarni AA. Spectrophotometric methods for simultaneous estimation of nimesulide and drotaverine. Int J ChemTech Res 2009;1:135-8.
- British Pharmacopoeia. Vol. 1. London: HMSO Publication; 2008. p. 44-5.
- Indian Pharmacopoeia. Vol. 2. The Indian Pharmacopoeia Commission, Ghaziabad: Govt. of India Ministry of Health and Family Welfare; 2007. p. 681-2.
- Hisham EA, Magda MA, Suzan MS, Nadia FY. Spectrophotometric and spectrodensitometric determination of paracetamol and drotaverineHCl in combination. SpectrochimActa Part A 2007;66:1147-51.
- Borgmann SH, Parcianello LM, Arend MZ, Cardoso SG. Direct spectrophotometric determination of drotaverine in capsules. Pharmazie 2007;62:483-5.
- Borgmann HM, Parcianello L, Arend MZ, Bajerski L, Cardoso SG. Development and validation of a dissolution method with spectrophotometric analysis for drotaverine capsules. Sci Pharm 2008;76:541-54.
- Giannellini V, Salvatore F, Bartolucci G, Coran SA, Alberti MB. A validated HPLC stability- indicating method for the determination of Drotaverine in bulk drug substance. J Pharm Biomed Anal 2005;39:776-80.
- Yao HC, Yang XF, Li H. Sensitive determination of nanogram levels of diacerein in a pharmaceutical formulation by flow injection chemiluminescence analysis. J Chinese ChemSoc 2007;54:949-56.
- Ayad MM, Youssef NF, Abdellatif HE, Soliman SM. A comparative study on various spectrometries with thin layer chromatography for simultaneous analysis of drotaverine and nifuroxazide in capsules. Chem Pharm Bull 2006;54:807-13.
- Metwally FH, Abdelkawy M, Naguib IA. Determination of nifuroxazide and drotaverine hydrochloride in pharmaceutical preparations by three independent analytical methods. J AOAC Int 2006;89:78-87.
- Ziyatdinova GK, Samigullin AI, Budnikov GK. Voltammetric determination of papaverine and drotaverine. J Anal Chem. 2007;62:773-6.
- Kirti ST, Purushotam KS, Rajesh MJ, Mrinalini CD. Spectrophotometric methods for simultaneous estimation of diacerhein and aceclofenac.Int J Chem Tech Res 2009;1:991-5.
- El-Saharty YS, Refaat M, El-Khateeb SZ. Stability indicating spectrophotometric and densitometric methods for determination of aceclofenac. Drug Develop Ind Pharm 2002;28:571-82.
- Singhvi I, Goyal A. Visible spectrophotometric estimation of aceclofenac and indapamide from tablets using folin-ciocalteu reagent. Indian J Pharm Sci 2007;69:164-5.
- Bhinge JR, Kumar RV, Sinha VR. A simple and sensitive stability indicating RP-HPLC assay method for the determination of Aceclofenac. J Chromatogr Sci 2008;46:440-4.
- Shaikh KA, Devkhile AB. Simultaneous determination of aceclofenac, paracetamol, and chlorzoxazone by RP-HPLC in pharmaceutical dosage form. J Chromatogr Sci 2008;46:649-52.
- Hinz B, Auge D, Rau T, Rietbrock S, Brune K, Werner U. Simultaneous determination of aceclofenac and three of its metabolites in human plasma by high-performance liquid chromatography. Biomed Chromatogr 2003;17:268-76.
- Kang W, Kim EY. Simultaneous determination of aceclofenac and its three metabolites in plasma using liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal 2008;46:587-91.
- ElKousy NM. Spectrophotometric and spectrofluorimetric determination of etodolac and aceclofenac. J Pharm Biomed Anal 1999;20:185-94.
- Beckett AH, Stenlake JB. Practical Pharmaceutical Chemistry.4th ed. Part 2. London: Continuum International Publishing Group; 2002. p. 293-6.
- ICH, Q2 (R1): Validation of Analytical Procedures: Text and Methodology, Geneva, 2005.
- Robert AN, Alfred HW. Pharmaceutical Process Validation.An international.3rd ed. Vol. 129. New York: Marcel Dekker; 2005. p. 515-22.