- *Corresponding Author:
- Saisruthi Kaveripakam
Division of Pharmaceutical Chemistry, Institute of Pharmaceutical Technology, Sri Padmavathi Mahila Visvavidyalayam (Women’s University), Tirupati-517 502, India
E-mail: sruthisai7@gmail.com
| Date of Submission | 28 July 2017 |
| Date of Revision | 12 January 2018 |
| Date of Acceptance | 01 August 2018 |
| Indian J Pharm Sci 2018;80(5):844-851 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study attempted to develop an experimental model that would exhibit pathological features seen in individuals with obesity and nephrotoxicity suitable for pharmacological screening of drugs. A combination of high-fat diet and cisplatin at a dose of 5 mg/kg was used to induce obesity along with nephrotoxicity in Wistar rats. Animals were divided into four groups of six each. Group-I served as control, group-II served as obesity control, group-III served as nephrotoxicity control in normal rats to which cisplatin was injected on day 35 and group-IV rats served as nephrotoxic obese rats, which were fed with high fat diet and on day 35 nephrotoxicity was induced by a single cisplatin injection (5 mg/kg, ip). Various parameters that represent obesity and nephrotoxicity such as the body weight, lipid profile, blood glucose, liver function markers, serum creatinine, blood urea nitrogen, urinary total protein and creatinine clearance were estimated. The assessed parameters of obesity and nephrotoxicity indicated that in the group-IV animals and exhibited prominent nephrotoxicity compared to the group-III rats. Histological assessment of kidney and liver substantiated the findings from the biochemical parameters. In summary this study revealed that it is possible to develop a unique rodent model of nephrotoxicity co-existing with obesity.
Keywords
Body weight, cisplatin, high-fat diet, serum creatinine
Obesity is a serious health problem and rapidly becoming a major challenge to health care systems worldwide [1]. Over the last three decades the prevalence of overweight and obesity has increased substantially [2]. By 2025, obesity will affect 18 % of men and over 21 % of women worldwide [3]. In some nations, obesity is already present in more than one-third of the adult population and contributes significantly to overall poor health and high annual medical costs [4]. This increasing prevalence of obesity has implications for coronary heart diseases, hypertension, diabetes mellitus, fatty liver, gall bladder disease, osteoarthritis, various cancers, mental disorders, and kidney diseases [5]. Numerous population-based studies have shown an interrelationship between obesity and of kidney disease. People who are overweight or obese have 2 to 7 times of developing renal diseases compared to those of normal weight [6]. A growing body of evidence indicates that obesity is also a potent risk factor for the development of chronic kidney disease and end-stage renal disease [7].
Renal diseases coexisting with obesity have become more predominant in the society due to changes in life style and dietary habits [8]. The number of people with kidney disease and obesity is substantial and the prevalence is increasing throughout the world [9]. Efforts should be directed to treating both the diseases together as a whole rather than independently by finding better novel therapeutic strategies for nephrotoxicity co-existing with obesity. In order to do so, appropriate and clinically relevant experimental models are necessary to test new drugs, understand the molecular basis and pathogenesis of the condition and mechanism of actions of these therapeutic agents. Thus to control these diseases, it is of paramount importance to establish such a unique animal model that closely mimic the changes subsequent to development of nephrotoxicity and obesity in humans. These viable animal models should address all the aspects of this human disease, developing all major signs of obesity as well as nephrotoxicity.
However there are no such animal models where nephrotoxicity and obesity co-exist, which could be used to screen therapeutic agents beneficial in such conditions. Hence the present study was designed to develop a unique animal model that would mimic pathological features of nephrotoxicity and obesity.
Materials and Methods
Male Wistar rats weighing 150-180 g were selected and a total of 33 animals were used for the study. The animals were supplied by Sri Venkateswara Enterprises, Bangalore. The experimental protocol was approved by the Institutional Animal Ethics Committee and carried out as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (Registration No.: 1677/PO/a/12/CPCSEA). The animals were housed in polypropylene cages with paddy husk bedding and maintained under standard laboratory conditions with controlled temperature 24±2° and relative humidity of 30 to 70 %, in an airconditioned room with 12:12 h light/dark cycle. The animals were allowed free access to standard rat chow or high-fat diet (HFD) as the case may be and water ad libitum.
Preparation of HFD
The HFD rodent feed was prepared by mixing the following ingredients, casein-20 %, D,Lmethionine- 0.3 %, corn starch-15 %, sucrose-27.5 %, cellulose powder-5 %, mineral mixture-3.5 %, vitamin mixture-1 %, choline bitartrate-0.2 %, corn oil-9.9 % and lard oil-17.6 % [10].
Induction of nephrotoxicity
A pilot study was carried out with different doses of cisplatin (3, 4, and 5 mg/kg) in order to select an appropriate dose of cisplatin for inducing nephrotoxicity. Based on the pilot study results, it was found that 5 mg/kg cisplatin produced the desired nephrotoxicity in experimental rats. Therefore, a single cisplatin injection (5 mg/kg, intraperitoneal) was used in this study to induce nephrotoxicity.
Experimental design.
Twenty four animals were divided into 4 groups of 6 animals each. Group I (normal controls), rats were fed with standard rat chow diet for 40 d; group II (obese) rats were fed with HFD for 40 d; group III (nephrotoxic) rats were fed with standard rat chow diet and nephrotoxicity was induced by as single intraperitoneal injection of cisplatin at a dose of 5 mg/kg on day 35; group IV (obese nephrotoxic) rats were fed with HFD for 40 d to induce obesity and on day 35, nephrotoxicity was induced by single dose of cisplatin (5 mg/kg).
Assessment of various parameters of obesity and nephrotoxicity
Body weight was monitored once every 5 d. The Lee’s index was expressed as a cubic root of body weight in g divided by the naso-anal length in millimetre multiplied by 104 [11]. At the end of the 40 d experimental session, urine was collected into metabolic cages and the urinary functional parameters were estimated. After 12 h starvation, blood was collected from retroorbital plexus and animals were sacrificed by cervical decapitation. Serum was separated and used for estimation of serum markers. The liver, kidney, spleen and fat pads (mesenteric and perirenal fat pads) were dissected out, washed in ice cold saline, blotted dry and weighed. Kidney and liver tissues were used for antioxidant and histological studies.
Determination of lipid profile, blood glucose and liver function markers
Total serum cholesterol (TC), triglyceride (TG) and high-density lipoprotein (HDL) levels were estimated in serum using standard commercial kits. Low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) cholesterol were calculated using Friedewald Eqn., VLDL = TG/5; LDL = TC–(HDL+VLDL) [12]. The concentrations of blood glucose and levels of liver function markers such as serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT) and alkaline phosphatase (ALP) were measured following the manufacturer’s instructions using commercial kits [13].
Atherogenic index (AI) of experimental rats was calculated using the formula, LDL-cholesterol/HDLcholesterol ratio while coronary risk index (CRI) was the ratio of total cholesterol to HDL cholesterol [14]. Kidney function markers such as blood urea nitrogen (BUN), serum creatinine (SC), urinary total protein (UTP) and creatinine clearance (Clcr) were determined by standard methods using commercial kits [15]. Liver and kidney were homogenized in ice cold phosphate buffer to obtain a 10 % (w/v) homogenate. The homogenates were centrifuged at 10 000 rpm for 15 min and the clear supernatant was obtained. Oxidative stress markers were assessed by determining the levels of lipid peroxidation (LPO), reduced glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT), which were estimated in supernatant of both liver and kidney homogenate using standard procedures [16-19].
Histological studies
Kidney and liver was dissected, removed, washed with normal saline and fixed in 10 % neutral buffered formalin. The fixed specimens were then trimmed, washed and dehydrated in ascending grades of alcohol. The tissue specimens were cleared in xylene, embedded in paraffin, sectioned at 4-6 μ thickness and stained with haematoxylin and eosin [20].
Statistical analysis
The data was expressed as mean±standard error of the mean. Mean values between the groups was considered statistically significant (p<0.001) after analysing using one way ANOVA and was compared using Tukey- Kramer multiple comparison test.
Results and Discussion
Animals of group II throughout the treatment schedule showed significant increase in body weight and Leeindex when compared to normal control. Animals of group III showed normal increase in body weight as those in the control group up to the administration of cisplatin, but from day 35 to 40, body weight slightly decreased in these animals when compared to group I. The group IV animals showed significant increase in body weight and Lee-index up to day 35 when compared to group I animals, but increase in body weight was not sustained from day 35 to 40 i.e. after administration of cisplatin in group IV (Table 1).
| Parameters | Group-I | Group-II | Group-III | Group-IV | |
|---|---|---|---|---|---|
| Body weight (g) | day 1 | 171.7 ± 3.07 | 174.2 ± 4.36 | 170.8 ± 3.27 | 170.0 ± 2.88 |
| day 35 | 210.8 ± 2.71 | 299.2 ± 3.75a# | 216.7 ± 2.47 | 290.0 ± 5.16a# | |
| day 40 | 215.0 ± 1.83 | 306.7 ± 3.58a# | 207.5 ± 1.71 | 275.8 ± 4.16a# | |
| Lee-index | day 1 | 232.4 ± 1.19 | 230.7 ± 1.66 | 231.8 ± 1.50 | 228.0 ± 2.38 |
| day 35 | 239.6 ± 3.85 | 305.5 ± 4.07a# | 236.9 ± 2.32 | 303.1 ± 1.23a# | |
| day 40 | 237.7 ± 2.48 | 317.0 ± 3.74a# | 233.2 ± 2.27 | 291.6 ± 1.76a# | |
Each value represents the mean ± SEM from 6 animals in each group. #p<0.001, ns: not significant, a: group-II, III and IV compared to group-I
Table 1: Change in body weight and Lee-index in experimental animals
TC, TG, LDL and VLDL significantly increased in group II and IV compared to group I. The levels of HDL decreased significantly in group II and IV compared to group I animals. Further there is no significant difference in raise in lipid profile between animals of group II and IV. In group III, there was no significant difference in TC, TG, HDL, LDL and VLDL levels when compared to group I controls (Table 2). Significant increase in blood glucose levels was observed in groups II and IV when compared to normal control. Group III animals did not show any significant increase in blood glucose levels when compared to group I levels (Table 2). The group II and IV rats showed a significant raise in levels of SGOT, SGPT and ALP in serum as compared to the control group (Table 2). AI and CRI of experimental rats were increased significantly in groups II and IV when compared to normal controls. There was no significant change in AI and CRI in group III compared to groups I, II and IV (Table 2).
| Parameters | Group-I | Group-II | Group-III | Group-IV |
|---|---|---|---|---|
| TC (mg/dl) | 59.50 ± 1.58 | 184.8 ± 4.74a# | 61.50 ± 1.48 | 189.5 ± 5.91a# |
| TG (mg/dl) | 43.67 ± 1.31 | 165.2 ± 3.29a# | 52.67 ± 1.36 | 176.8 ± 6.50a# |
| HDL (mg/dl) | 45.33 ± 1.12 | 26.67 ± 0.81a# | 42.00 ± 0.73 | 25.67 ± 0.80a# |
| LDL (mg/dl) | 5.43 ± 0.98 | 125.4 ± 5.59a# | 8.97 ± 2.14 | 128.5 ± 6.07a# |
| VLDL (mg/dl) | 8.73 ± 0.26 | 33.00 ± 0.68a# | 10.53 ± 0.27 | 35.37 ± 1.30a# |
| BG (mg/dl) | 70.83 ± 2.39 | 154.2 ± 3.00a# | 72.50 ± 3.35 | 160.0 ± 4.47a# |
| AI | 0.12 ± 0.02 | 4.74 ± 0.34a# | 0.22 ± 0.05 | 5.06 ± 0.36a# |
| CRI | 1.31 ± 0.02 | 6.98 ± 0.37a# | 1.47 ± 0.06 | 7.45 ± 0.43a# |
| SGOT (U/L) | 35.83 ± 1.49 | 126.1 ± 5.94a# | 79.00 ± 3.37 | 152.1 ± 7.07a# |
| SGPT (U/L) | 31.17 ± 1.70 | 114.9 ± 3.83a# | 69.47 ± 3.13 | 135.8 ± 7.41a# |
| ALP (U/L) | 46.33 ± 2.40 | 240.8 ± 17.27a# | 99.67 ± 2.33 | 291.5 ± 15.45a# |
Each value represents the mean ± SEM from 6 animals in each group. #p<0.001, ns: not significant, a: group II, III and IV compared to group I
Table 2: Lipid profile, blood glucose, atherogenic index, coronary risk index and liver functional markers in experimental animals
BUN, SC, UTP significantly increased and Clcr was significantly decreased in groups III and IV when compared with normal controls. In group II animals, mild increase in BUN, SC and UTP and a decrease in Clcr was observed when compared to group I. Moreover, animals of group IV showed significant increases in BUN, SC, UTP and decrease in Clcr levels when compared to those of groups III, II and I animals (Table 3). In group II and IV animals there was a significant increase in organ and fat pad weights. Further, kidney weight in group III animals increased significantly when compared to group I (Table 4).
| Parameters | Group-I | Group-II | Group-III | Group-IV |
|---|---|---|---|---|
| SC (mg/dl) | 0.68 ± 0.04 | 1.38 ± 0.08 | 2.22 ± 0.07 | 4.17 ± 0.31a#;b# |
| BUN (mg/dl) | 12.42 ± 0.42 | 19.28 ± 1.45 | 32.72 ± 0.82 | 50.83 ± 2.47a#;b# |
| UTP (mg/24 h) | 2.11 ± 0.04 | 3.60 ± 0.24 | 6.57 ± 0.26 | 9.52 ± 0.29a#;b# |
| Clcr ((ml/h/100 g) | 16.75 ± 0.86 | 13.57 ± 0.66 | 5.88 ± 0.41 | 2.55 ± 0.33a#;b# |
Each value represents the mean ± SEM from 6 animals in each group. #p<0.001, ns: not significant, a: group II, III and IV compared to group-I, b: group IV compared to group-III
Table 3: Kidney functional markers in experimental animals
| Organ and fat pads | Group-I | Group-II | Group-III | Group-IV |
|---|---|---|---|---|
| Kidney (g) | 0.78 ± 0.02 | 1.15 ± 0.03a# | 1.05 ± 0.04a# | 1.45 ± 0.06a# |
| Liver (g) | 5.91 ± 0.18 | 9.30 ± 0.46a# | 5.57 ± 0.22 | 9.07 ± 0.39a# |
| Heart (g) | 0.80 ± 0.02 | 1.23 ± 0.05a# | 0.78 ± 0.01 | 1.23 ± 0.04a# |
| Spleen (g) | 0.81 ± 0.01 | 1.22 ± 0.05a# | 0.79 ± 0.02 | 1.20 ± 0.03a# |
| Perirenal fat (g) | 0.91 ± 0.06 | 4.08 ± 0.12a# | 0.90 ± 0.04 | 4.17 ± 0.19a# |
| Mesenteric fat (g) | 3.25 ± 0.07 | 9.31 ± 0.23a# | 3.17 ± 0.10 | 8.96 ± 0.38a# |
Each value represents the mean ± SEM from 6 animals in each group. #p<0.001, ns: not significant, a: group II, III and IV compared to group I
Table 4: Organ and fat pad weights in experimental animals
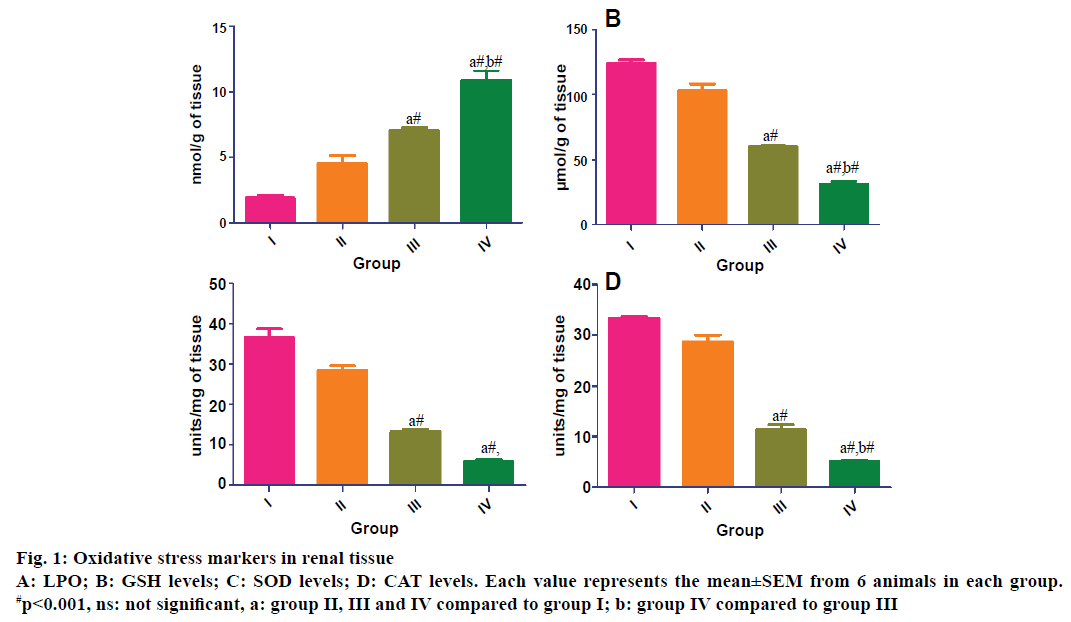
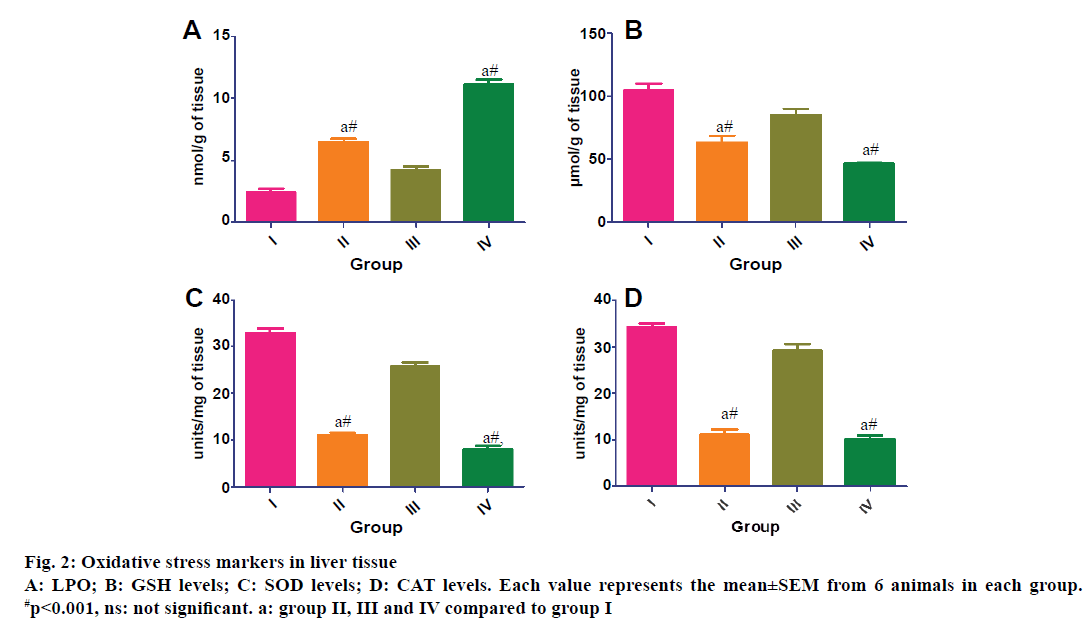
In the renal tissue of group III and IV animals, there was a significant raise in LPO levels and declines in GSH, SOD and CAT levels when compared to group I. While in group II there was significant raise in LPO levels and significant decrease in GSH, SOD and CAT levels compared to group I. Further in group IV animals when compared to group III, LPO levels were significantly increased and GSH and CAT levels were significantly decreased. SOD levels in group IV also decreased significantly in renal tissue when compared to group III (Figure 1). In group II and IV animals there was a marked significant increase in LPO levels and decrease in GSH, SOD and CAT levels when compared to normal animals in the hepatic tissue. In group III nephrotoxic animals, there was significant raise in LPO levels and significant decrease in GSH, SOD and CAT compared to group I in hepatic tissue (Figure 2).
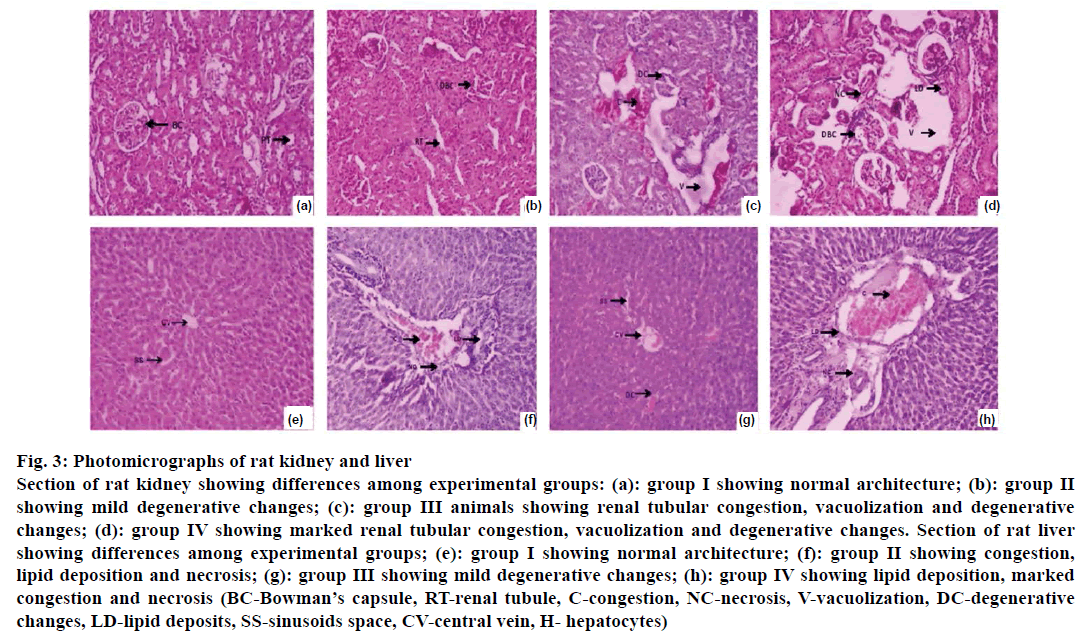
Histology of normal control group kidney showed absence of congestion of glomerular blood vessels and tubular necrosis resembling normal architecture. In kidneys of group II animals mild degenerative changes were observed. In contrast histological assessment of kidney tissue of group III animals showed congestion, necrosis and degenerative changes and group IV animals demonstrated severe congestion of glomerular blood vessels, tubular necrosis, vacuolization, and degeneration as compared to both group I and III (Figure 3).
Figure 3: Photomicrographs of rat kidney and liver
Section of rat kidney showing differences among experimental groups: (a): group I showing normal architecture; (b): group II
showing mild degenerative changes; (c): group III animals showing renal tubular congestion, vacuolization and degenerative
changes; (d): group IV showing marked renal tubular congestion, vacuolization and degenerative changes. Section of rat liver
showing differences among experimental groups; (e): group I showing normal architecture; (f): group II showing congestion,
lipid deposition and necrosis; (g): group III showing mild degenerative changes; (h): group IV showing lipid deposition, marked
congestion and necrosis (BC-Bowman’s capsule, RT-renal tubule, C-congestion, NC-necrosis, V-vacuolization, DC-degenerative
changes, LD-lipid deposits, SS-sinusoids space, CV-central vein, H- hepatocytes)
Histological assessment of the liver of the normal control rats showed normal architecture of central vein, peripheral vein, and hepatocytes. In contrast, the liver cells of the group II and IV showed degeneration, congestion, and fat deposition as compared to normal control (Figure 3).
Obesity is defined as abnormal or excessive fat accumulation that may impair health [21]. It increased the probability of being affected with various diseases particularly heart diseases, type 2 diabetes, certain types of cancer and osteoarthritis [22]. Recent reports pointed out at obesity as an important risk factor for chronic kidney diseases [23]. Potentially linking these two epidemiological observations it becomes evident that many obesity-induced derangements themselves were nephrotoxic. With increase in the proportion of such individuals with both the disease conditions co-existing, obesity and nephrotoxicity need to be addressed not as independent diseases but as a unique disease combination that required urgent attention.
There were several animal models of nephrotoxicity as well as obesity. However there is no experimental model where both nephrotoxicity and obesity co-existed. Increase in body weight, lipid profile, BG, adiposity levels, SGOT, SGPT, ALP, organ index, SC, BUN, UTP and decreased Clcr confer combined architecture of obesity and nephrotoxicity. In the search to combat these risk factors together efforts were directed to develop a suitable animal model that would mimic all the symptoms of obesity as well as nephrotoxicity to screen potential candidate drugs.
Such a unique experimental model would closely reflect the natural history and characteristics of obesity along with nephrotoxicity. Further it was kept in mind while developing the model it should be less expensive, easily available, quick to develop, reproducible and display various components of obesity and nephrotoxicity. In the absence of such a unique experimental model where both the diseases co-exist, development of an animal model is of paramount importance and utility.
Obesity included increased TC, TG, LDL, VLDL and decreased HDL levels. Obesity might also lead to increase in BG, increase in SGOT, SGPT and ALP levels [14,24]. Patients of obesity might not have overt nephrotoxicity. However, the objective of present study was to develop an animal model, which has essentially nephrotoxic and in addition it should possess obesity.
Anthropometric parameters like body weight and Leeindex were evaluated in experimental groups. Group II and group IV animals showed significant increase in body weight and Lee-index when compared to normal animals. These results agreed with earlier reports, which demonstrated that HFD feeding increased energy intake that led to increased fat deposition in tissues and organs there by leading to weight gain [25]. Compared to group II animals, increase in body weight and Lee-index in group IV animals did not sustain from day 35 to 40 when compared to obese animals (group-II). Further, in the group III animals injection of cisplatin decreased both body weight and Leeindex. This change in group-III and group-IV animals may be attributed to the state of nephrotoxicity that is known to cause weight loss as reported by earlier investigators [25]. Challenging with cisplatin caused decrease in body weight which is typically seen in nephrotoxicity but not obesity. Hyperlipidaemia was the main characteristic of obesity. The TC, TG, LDL and VLDL levels were significantly higher and HDL levels were significantly lower in animals of group II and group IV when compared to normal control. These results concurred with those reported by Duiyan et al. and Sung et al. [26,27]. However these investigators did not include cisplatin-induced nephrotoxicity in their study.
As reported in previous studies, in the present study also AI and CRI also increased substantially on administration of HFD, which suggested that group II and group IV rats were more prone to coronary artery diseases [24]. In the group II and group IV rats BG levels and liver function markers such as SGOT, SGPT and ALP were significantly increased when compared to normal rats. These results were in accordance with earlier reports which demonstrated that HFD consumption led to increase in BG, SGOT, SGPT and ALP levels [13,27]. By observing all the above parameters it can be concluded that administration of cisplatin in obese rats did not cause any changes in obesity markers.
In nephrotoxic animals of group-III, SC, BUN, UTP increased and Clcr decreased in cisplatin-treated rats. These results were in good agreement with earlier reports [28,25]. In the current study rats fed with HFD and administered cisplatin showed significant raise in SC, BUN and UTP and decline in Clcr even when compared to nephrotoxic rats of group III. This indicated development of remarkable nephrotoxicity in obese rats and thus the present model appeared to demonstrate unique pathogenesis, which was not showed by the models used by other investigators.
Organ and fat pad weights were significantly increased in group II and IV rats when compared to control rats. These results agreed with previous reports, which stated that increased organ weight might be due to increased energy intake leading to raised fat depositions in tissues and organs [24,29]. In addition, significant increase in kidney weight in group IV was also consistent with earlier findings that nephrotoxic effect of cisplatin produced marked increase in the kidney weight, which might be due to renal inflammation [30].
Generally obesity and nephrotoxicity were associated with oxidative stress, which resulted from an imbalance between the production of free radicals and an effective antioxidant activity leading to oxidative damage to cells or tissue [13,27]. Darouich et al. stated that oxidative stress identified in obesity-related renal diseases might be the mechanism underlying the initiation or progression of renal injury in obesity [31]. In the present study, GSH, SOD and CAT levels were remarkably decreased and LPO levels were increased profoundly in group IV. These results agreed with earlier reports, which suggested that feeding HFD to experimental animals depressed their antioxidant system due to increased LPO and formation of free radicals [32]. These results were also in good agreement with previous reports, which demonstrated that administration of cisplatin caused renal oxidative stress [33]. Histological studies of liver and kidney also substantiated the biochemical findings.
Based on all the above observations of the current study, HFD fed rats developed nephrotoxicity with the administration of cisplatin without affecting the obesity markers. Hence this approach could serve to induce nephrotoxicity in obese rats. The present study demonstrated the development of a unique rodent model of obesity co-existing with nephrotoxicity. The developed model could aid in screening different pharmacological agents in pathological conditions where obesity and nephrotoxicity co-existed.
Conflicts of interest
No conflict of interest was reported by the authors.
References
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioral, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:2287-323.
- Fujioka K. Management of obesity as a chronic disease: non-pharmacologic, pharmacologic, and surgical options. Obes Res 2002;10:116-23.
- Kovesdy CP, Furth S, Zoccali C. Obesity and kidney disease: Hidden consequences of the epidemic. Indian J Nephrol 2017;27:85-92.
- Subramanian SV, Perkins JM, Ozaltin E, Davey SG. Weight of nations: a socioeconomic analysis of women in low- to middle-income countries. Am J Clin Nutr 2010;93(2):413-21.
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Marks J. Prevalence of obesity, diabetes, and obesity related health risk factors. JAMA 2003;289:76-79.
- Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, et al. Is obesity a major cause of chronic renal disease? Adv Ren Replace Ther 2004;11:41-54.
- Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol 2006;17:1695-702.
- Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci 2002;324:127-37.
- Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001;59:1498-509.
- Vasselli JR, Weindruch R, Heymsfield SB, Pi-Sunyer FX, Boozer CN, Yi N, et al. Intentional weight loss reduced mortality rate in a rodent model of dietary obesity. Obes Res 2005;13:693-702.
- Lee MO. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am J Physiol 1929;89:24-31.
- Yugarani T, Tan BK, Teh M, Das NP. Effect of polyphenolic natural products on the lipid profiles of rats fed high-fat diets. Lipids 1992;27:181-86.
- Athesh K, Divakar M, Brindha P. Antiobesity potential of Cyperus rotundus aqueous tuber extract in rats fed on high fat cafeteria diet. Asian J Pharm Clin Res 2014;7:88-92.
- Abbott RD, Wilson PW, Kannel WB, Castelli WP. High density lipoprotein-cholesterol, total cholesterol screening and myocardial infarction. The Framingham Study. Arterosclerosis 1988;8:207-11.
- Godkar PB. Text book of Medicinal laboratory. Bombay: Bhalani Publishing House; 1994. p.1022-28.
- Wright JR, Colby HD, Miles PR. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys 1981;206:296-304.
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene in liver necrosis, Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974;11:151-69.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170-75.
- Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. CRC Press: Boca Raton, Florida, United States; 1985: p. 283-84.
- Jose S, Adikay S. Effect of the ethanolic extract of Scoparia dulcisin Cisplatin induced Nephrotoxicity in Wistar rats. IJPER 2015;49:s68-s74.
- Obesity: preventing and managing the global epidemic, Report of a WHO Consultation (WHO Technical Report Series 894). Available from: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/.
- Obesity and overweight. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- Weisinger JR, Kempson RL, Eldridge FL, Swenson RS. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med 1974;81:440-47.
- Adeneye AA, Adeyemi OO, Agbaje EO. Antiobesity and antihyperlipidaemic effect of Hunteria umbellata seed extract in experimental hyperlipidaemia. J Ethnopharmacol 2010;130:307-14.
- Moon J, Do HJ, Kim OY, Shin MJ. Antiobesity effects of quercetin rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat fed rats. Food Chem Toxicol 2013;58:347-54.
- Duiyan J, Yi X, Xin M, Qing M, Ying G, Bo L, et al. Antiobesity and lipid lowering effects of theaflavins on high-fat diet induced obese rats. J Funct Foods 2013;1142-50.
- Sung YY, Kim DS, Choi G, Kim SH, Kim HK. Dohaekseunggi-tang extract inhibits obesity, hyperlipidemia, and hypertension in high fat diet induced obese mice. BMC Complement Altern Med 2014;14:372.
- Mondi S, Kvsrg P, Jhansi D, Vijay R, Rao UMV. Prophylactic and curative effect of ethanolic extract of Bassia malabarica bark against cisplatin induced nephrotoxicity. Asian J Pharm Clin Res 2014;7:143-46.
- Huang YW, Liu Y, Dushenkov S, Ho CT, Huang MT. Antiobesity effects of epigallocatechin-3-gallate, orange peel extract, black tea extract, caffeine and their combinations in a mouse model. J Funct Foods 2009;1:304-10.
- Zhu X, Jiang X, Li A, Zhao Z, Li S. S-Allylmercaptocysteine Attenuates Cisplatin-induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation. Nutrients 2017;166:2-16.
- Darouich S, Goucha R, Jaafoura MH, Zekri S, Ben Maiz H, Kheder A. Clinicopathological characteristics of obesity associated focal segmental glomerulosclerosis. Ultrastruct Pathol 2011;35:176-82.
- Kang M, Oh JW, Lee HK, Chung HS, Lee SM, Kim C, et al. Antiobesity Effect of PM-F2-OB, an Antiobesity Herbal Formulation, on Rats Fed a High-Fat Diet. Biol Pharm Bull 2004;27(8):1251-56.
- Ajith TA, Jose N, Janardhanan KK. Amelioration of cisplatin induced nephrotoxicity in mice by ethyl acetate extract of a polypore fungus, Phellinus rimosus. J Exp Clin Cancer Res 2002;21:487-91.