- Corresponding Author:
- A. V. Yadav
Department of Biopharmaceutics, Government College of Pharmacy, Karad–415 124, India
E–mail: avyadav03@rediffmail.com
| Date of Submission | 23 February 2007 |
| Date of Revision | 16 January 2008 |
| Date of Acceptance | 6 March 2008 |
| Indian J Pharm Sci., 2008, 70 (2): 170-174 |
Abstract
Domperidone microspheres for intranasal administration were prepared by emulsification crosslinking technique. Starch a biodegradable polymer was used in preparation of microspheres using epichlorhydrine as cross-linking agent. The formulation variables were drug concentration and polymer concentration and batch of drug free microsphere was prepared for comparisons. All the formulations were evaluated for particle size, morphological characteristics, percentage drug encapsulation, equilibrium swelling degree, percentage mucoadhesion, bioadhesive strength, and in vitro diffusion study using nasal cell. Spherical microspheres were obtained in all batches with mean diameter in the range of above 22.8 to 102.63 μm. They showed good mucoadhesive property and swelling behaviour. The in vitro release was found in the range of 73.11% to 86.21%. Concentration of both polymer and drug affect in vitro release of drug.
Keywords
Microspheres, domperidone, starch, nasal drug delivery, mucoadhesion, bioadhesive, crosslinking
For systemic therapy, the nasal route has attracted increasing attention as a suitable method for drug delivery. The nasal cavity as a site for systemic absorption of drug has some advantages which include large surface area, porous endothelium basement membrane, highly vascularised epithelial layer, high total blood ß ow per cm3, avoids first pass metabolism and easy access. The nasal route can also be used for patients under emesis and with respect to parental route the nasal route is not invasive [1-3].
The design of nasal dosage form has to consider the anatomical and physiological characteristics of nasal mucosa and more particularly the rapid mucociliary clearance that limits the time allowed for drug absorption to occur and effectively rule out sustained nasal administration. To overcome the rapid clearance and to facilitate the absorption through the barrier of nasal mucosa, mucoadhesive material and absorption promoters are used in nasal formulations [4].
Starch was used as polymeric material. Starch is biocompatable, biodegradable and bioadhesive in nature [5]. The concept of using bioadhesive delivery system in the form of degradable starch microsphere (DSM) for nasal delivery of drug was introduced by Illum et al [6]. DSM is not only biodegradable, but also shows a high degree of swelling when in contact with aqueous medium. Starch forms a gel like system, with prolonged residence time in the nose and significant contact with the nasal mucosa. In addition starch microspheres do not produce immune response. Starch microspheres are most promising for nasal administration [7].
Domperidone is a potent antiemetic [8], antimigraine [9] drug effective for preventing different kinds of emesis. Its conventional dosage form such as tablet and suspension give poor bioavailability (18%) due to extensive first pass effect, whereas on rapid i.v. injection it has been shown to cause cardiac arrhythmias [10]. This study investigates the design, development and evaluation of microspheres for nasal delivery.
Starch microspheres were prepared by emulsificationcrosslinking technique, using epichlorohydrin as crosslinking agent. The microspheres were characterized in terms of particle size morphology, percentage yield, percentage drug encapsulation, mucoadhesive property, swelling behaviour, bioadhesive strength and in vitro diffusion using nasal cell.
Materials and Methods
Domperidone was procured as a gift sample from Torrent Pharmaceutical, Ahmedabad .Soluble starch, epichlorohydrine, cyclohexane, chloroform, span-60, ethanol, sodium hydroxide were purchased from Loba Chemie, Mumbai. All reagents used were of analytical reagent grade.
Preparation of starch microspheres
Cross-linked starch microspheres were prepared by emulsion-crosslinking method11. For a typical batch, the aqueous phase was prepared by dissolving 8 g of soluble starch in 12 g of a 2 M sodium hydroxide solution under mechanical stirring. The aqueous phase was pre-emulsified in 1000 ml of cyclohexane:chloroform mixture (4:1, v/v) containing 0.5% (v/v) of span-60. The emulsion was homogenized by high-speed mechanical stirring for 3 min. Further, a suitable amount of epichlorohydrin (2.5%) was added under magnetic stirring at 1000 rpm. The stirring was maintained for 18 h at 400. Microspheres were isolated by centrifugation and washed twice with cyclohexane and distilled water. Finally microspheres were freeze dried and kept in a closed container. Formulation variables such as different amounts of drug concentration and concentration of polymer was evaluated to obtain the microspheres of optimum properties. Composition of variable is given in Table 1.
| Formulation code | Soluble starch (% w/v) | Domperidone (mg) |
|---|---|---|
| Ds1 | 8 | 100 |
| Ds2 | 8 | 125 |
| Ds3 | 8 | 150 |
| Ps1 | 6 | 100 |
| Ps2 | 10 | 100 |
| Ps3 | 12 | 100 |
Table 1: Formulation Variable for Different Batches of Starch Microspheres of Domperidone.
Characterization of microspheres
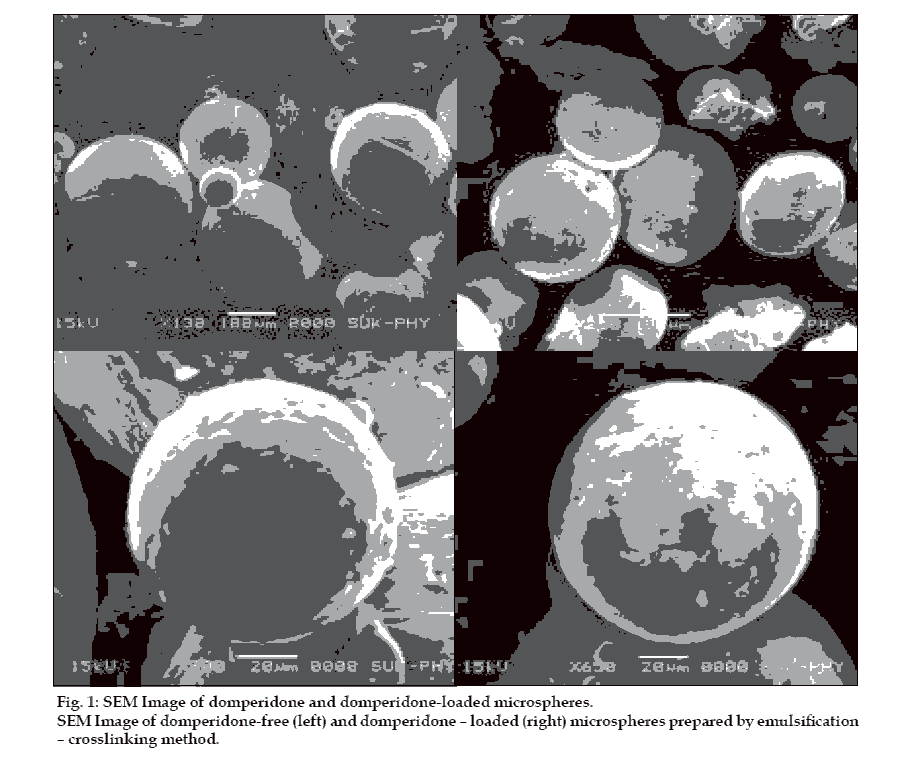
Total amounts of microspheres obtained were weighed and the percentage yield calculated taking into consideration the weight of drug and polymer. The particles were grossly separated into different fractions passing through a set of a sieves and then the particle size was determined using optical microscopic method with the help of calibrated eye piece micrometer. The size of around 300 particles were measured and the average particle size determined [12]. Surface morphology of microspheres was studied using scanning election microscopy (SEM) using Hitachi (Model S-2400) [13]. Microspheres were sprinkled on to double side tape, sputter-coated with platinum and examined in the microscope at 15 kV. Encapsulation was determined by taking weighed quantity of starch microspheres (approximately 25 mg) in a 25 ml volumetric ß ask, sufficient quantity of ethanol was added to make 25 ml. The suspension was shaken vigorously and then left for 24 h at room temperature with intermittent shaking. Supernatant was collected by centrifugation and drug content in supernatant was determined using UV spectrophotometer at a suitable wave length (286 nm) [14]. Efficiency of drug entrapment for each batch was calculated in terms of percentage drug entrapment (PDE) as per the formula, % drug entrapment= Actual drug content/ theoretical drug content ×100.
The equilibrium swelling degree (ESD) of starch microspheres was determined by swelling a suitable volume of dried microspheres in 5 ml ethanol, overnight in a measuring cylinder. The ESD (ml/g) was expressed as the ratio of the swollen volume to the mass of dried microspheres [15].
The bioadhesive strength of all batches was determined using measuring device [16]. Section of nasal mucosa was cut from the sheep nasal cavity and instantly secure with mucosal side out on glass vial. The vial using nasal mucosa was stored at 37° for 5 min. Next, one vial with a section of mucosa was connected to the balance and the other vial was placed on a height-adjusted pan. Microspheres were placed in between the adjusted vial. The weight was increased until two vials were detached. Bioadhesive force was determined for the minimum weight that detached two vials.
Mucoadhesive properties of microspheres were studied by rat gut loop method described by Dhawan et al [17]. The required amount of starch microspheres were suspended in physiological saline solution and sonicated (100 mg in 5 ml). The suspension of microspheres was filled in to a loop of small intestine (~15 cm in length) of sheep and sealed. This tube was incubated in saline solution at 37° for 60 min, microspheres suspension was then removed. The adhered microspheres amount was estimated from the difference between the applied microspheres amount and the flowed microspheres amount. The ratio of the adhered microspheres to the applied microspheres was computed as percent mucoadhesion. In vitro nasal diffusion study was done by using nasal diffusion cell [18], having three openings each for sampling, thermometer and donor tube chamber. The nasal mucosa of sheep was separated and it was attached to donor chamber tube. Diffusion of domperidone from microspheres was studied at 37±2°. An amount of microspheres equivalent to about 5 mg of domperidone were added on the mucosal surface at zero time. Samples were withdrawn at different time intervals upto 8 h and it was further diluted with ethanol upto 5 ml and absorbance was measured spectrophotometrically at 286 nm to evaluate the amount of drug release.
Results and Discussion
Physical characteristics of microsphere are shown in Table 2. DSM prepared in all the batches were spherical in shape. In previous studies, Illum et al [6], found that particle size was related to intranasal drug absorption. A size range of 40-60 µm was suitable for nasal administration. The size of starch microspheres prepared in this study was in the range of 22.8 to 102.63 µm, which is favourable for intranasal absorption. It was observed that as the amount of polymer and drug concentration increased in the microspheres the particle size also increased proportionally. The increase in the particle size observed with increase in polymer and drug concentration was due to increase in viscosity of the droplet [19].
| Formulation code |
Particle size (µm)a |
Product yield (%) |
Encapsulation efficiency (%) |
Equilibrium swelling degree (ml/g)a |
Mucoadhesion (%)a |
Bioadhesive strength (g) |
|---|---|---|---|---|---|---|
| Ds1 | 22.43±1.30 | 56.20 | 26.30 | 3.61±0.091 | 82.93±1.85 | 7.86±0.04 |
| Ds2 | 37.50±0.88 | 68.30 | 29.82 | 3.27±0.14 | 78.27±2.13 | 7.45±0.070 |
| Ds3 | 42.70±1.34 | 66.25 | 34.46 | 3.18±0.17 | 83.81±1.7 | 7.31±0.050 |
| Ps1 | 47.50±1.25 | 73.56 | 43.13 | 3.52±0.090 | 79.80±1.23 | 8.32±0.065 |
| Ps2 | 62.64±2.08 | 79.26 | 45.23 | 4.05±0.095 | 74.77±1.44 | 8.72±0.070 |
| Ps3 | 102.63±2.61 | 78.21 | 56.20 | 4.54±0.10 | 72.51±1.90 | 9.46±0.51 |
| Plain | 23.26±1.36 | 67.48 | - | 3.27±0.16 | 75.15±0.88 | 8.89±0.32 |
aMean±SD
Table 2: Physical Characteristics of Prepared Microspheres of Domperidone
The yield of starch microspheres was obtained in range of (84-89%) SEM analysis of microspheres revealed that all microspheres prepared were spherical and smooth in shape. Fig. 1 shows morphological image of domperidone free and domperidone loaded starch microspheres. The percentage drug encapsulation was found to be 26.8% to 56.2% as shown in Table 2. It was observed that polymer and drug concentration affect PDE. Highest PDE was found (56.2%) for 10% w/v of starch. Increase in polymer concentration attributed to increase in viscosity, which resulted in formation of large droplets, thus increasing the PDE.
Equilibrium swelling degree increases as the concentration of polymer increases while it decreases as concentration of drug increases as compared to plain starch microspheres. It can be concluded that incorporation of drug in microspheres decrease ESD. ESD was found to be 3.12 to 4.63 ml/g.
Percentage mucoadhesion was found in the range from 72.4% to 83.5%. Bioadhesive strength was in range from 8.51 g to 9.67 g. Thus starch microspheres prepared were found to be having good mucoadhesive property.
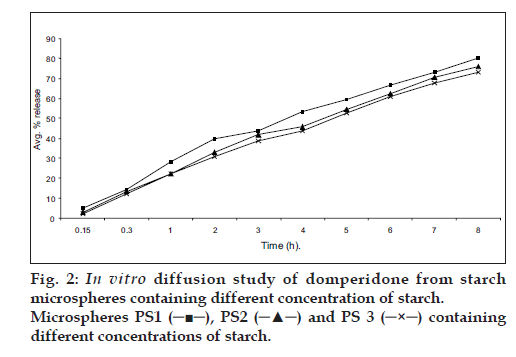
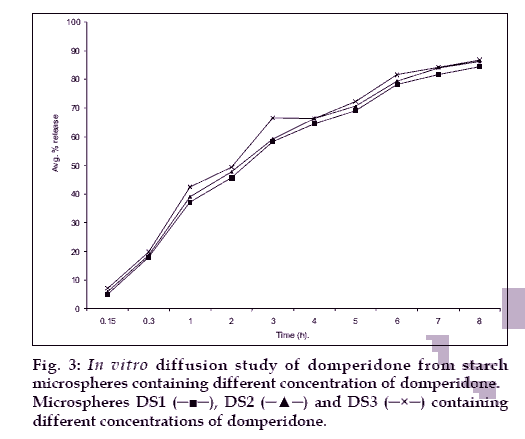
The in vitro diffusion profile of domperidone from microspheres is shown in figs. 2 and 3. The release profile of formulation code Ds3 and Ps3 were highest and lowest (86.21% and 73.11%) respectively. It was observed that domperidone is released from the microspheres in sustained release manner. Formulation variables such as concentration of polymer and drug affect rate and extent of drug release, which is due to increase in density of polymer and also increase in diffusion path length that the drug molecule has to travel [20]. It is also revealed that release of drug from microspheres is directly proportional to concentration of drug. Thus sustained release of domperidone from microspheres may be achieved as a desirable property for formulation, treating conditions involving vomitting and migraine.
Acknowledgements
The authors are grateful to Torrent Pharmaceuticals for providing gift sample of drug. The authors are thankful to Shivaji University, Kolhapur for providing, SEM data of microspheres.
References
- lllum LJ. Nasal drug delivery-possibilities, problems and solution. J Control Release 2003;87:187-98.
- Aurora J. Development of nasal delivery system: A review. Drug Deliv Technol 2002;2(7):70-3.
- Turker S, Anur E, Ozer Y. Nasal route and drug delivery system. Pharm World Sci 2004;26:137-42.
- Dondeti P, Zia H, Needham TE. Bioadhesive and formulation parameters affecting nasal absorption. Int J Pharm 1996;127:115-33.
- Wade A, Weller PJ. A Handbook of pharmaceutical excipients, A joint publication of the American Pharma. Association and the Pharma. Great Britain: Society of Great Britain; 1994. p. 289-95.
- Lllum L, Jorgensen J, Bisgaord H, Krogsgaard O, Rossing N. Bioadhesive microspheres as a potential nasal drug delivery system. Int J Pharm 1987;39:189-99.
- Mao S, Chen J, Wei Z, Liv H, Bi D. Intranasal administration of melatonin starch microspheres. Int J Pharm 2004;272:37-43.
- Reynolds JEF. Martindale; The Extra pharmacopoeia. 30th ed. Royal Society Of Great Britain. London: The Pharmaceutical Press; 1999.
- Krymchantowski YA. Acute treatment of migraine: Breaking the paradigm of monotherapy. BMC Neurol 2004;44:4-5.
- Tripathi KD. Essentials of medical pharmacology, 4th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2001. p. 649-50.
- Hamdi G, Pnchel G, Dunchene D. An original method for studying in vitro the enzymatic degradation of cross-linked starch microsphere. JControl Release 1998;55:193-201.
- Capan Y, Jiang G, Giovagnali S, Na KH, Deluca PP. Preparation and characterization of poly (D,L?lactide-co-glycolide) microspheres for controlled release of human growth harmon. AAPS Pharm Sci Tech 2003;4:1-10.
- Parikh RH, Parikh JR, Dubey RR, Soni HN, Kapadia KN. Poly(D,L-Lactide-Co-Glycolide) microspheres containing 5-ßuorouracil: Optimization of process parameters. AAPS Pharm Sci Tech 2003;4:1-8.
- Dubey RR, Parikh RH. Two stage optimization process for formulation of chitosan microsphere. AAPS Pharm Sci Tech 2004; 5:1-9.
- Hamdi G, Ponchel G, Enzymatic degradation of epichlorohydrine cross-linked starch microspheres by a-amylase. Pharm Res 1999;16:867-75.
- Matazovi SA, Smart JD, An in vitro method for assessing the duration of mucoadhesion. J Control Release 1994;31:207-12.
- Dhawan S, Singla AK, Sinha VR, Evaluation of mucoadhesive properties of chitosan microspheres prepared by different method. AAPS Pharm Sci Tech 2004;5:1-7.
- Pisal S, Shelke V, Mahadik K, Kadam S. Effect of organogels component on in vitro nasal delivery of propanolol hydrochloride. AAPS Pharm Sci Tech 2004;4:1-9.
- Sankar C, Mishra B. Development and in vitro evaluation of ketorolac tromethomine for intranasal administration. Acta Pharm 2003;53:101-10.
- Hascicek C, Gonul N, Ekr N. Mucoadhesive microspheres containing gentamycin sulphate for nasal administration. Farmaco 2003;58:11-6.