- *Corresponding Author:
- Preeti Sable
Y. B. Chavan College of Pharmacy, Aurangabad, Maharashtra 431001, India
E-mail: bcoppreeti26sable@gmail.com
| Date of Received | 09 October 2022 |
| Date of Revision | 18 July 2023 |
| Date of Acceptance | 07 September 2023 |
| Indian J Pharm Sci 2023;85(5):1268-1280 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Deferasirox is an iron chelator used in the treatment of iron overload. It shows good oral bioavailability of 70 % but is plagued with various side effects that could be addressed if the dose of the drug was reduced. It is a drug that has to be taken chronically and hence, would benefit from a convenient dosage form. With these aims in focus, an orally disintegrating tablet containing a self-microemulsifying drug delivery system of deferasirox was attempted. Various oils, surfactants and co-surfactants were screened and sunflower oil, Cremophor RH 40 and Capmul MCM C8 were respectively selected, and the microemulsion region was identified by constructing pseudo-ternary phase diagrams. The self-microemulsifying drug delivery system could be successfully loaded onto Aerosil 200, and these solid self-microemulsifying drug delivery system could be converted into orally disintegrating tablets with all the desired attributes. The tablets showed a 32 % increase in drug release within 1 h over the conventional formulation available locally. From the various studies performed, it could be concluded that the prepared orally disintegrating tablet of drug-loaded solid self-microemulsifying drug delivery system has the potential to improve the bioavailability of deferasirox, which could lead to titration and further reduction of dose, which would reduce the incidence of side effects.
Keywords
Deferasirox, iron chelator, self-emulsifying, orally disintegrating tablet, sunflower oil, Cremophor, Capmul
Iron is an essential nutrient with limited bioavailability required for a multitude of metabolic processes such as oxygen transport, deoxyribonucleic acid synthesis and electron transport[1]. A physiological mechanism to excrete iron from the human body doesn’t exist. What occurs is a fine balance between its availability and utilization. Iron is made available to the body via two sources, namely intestinal absorption and release from hepatocytes and macrophages. This iron homeostasis makes use of complex feedback mechanisms regulated by the iron regulatory hormone-hepcidin[2]. An excessive amount of iron could manifest a variety of toxicity consequences largely due to its ability to generate free radicals which could attack cellular macromolecules, damage them leading to tissue injury and ultimately cell death[3]. These free radicals could lead to serious consequences such as mental retardation, early onset of neurological diseases such as Alzheimer’s, Huntington, multiple sclerosis, delays in sexual maturity, impotence and infertility, cardiac dysfunctions like arrhythmia, cardiomyopathy and hemosiderosis, hepatic manifestations like hepatitis, cirrhosis and cancer, and metabolic dysfunctions such as diabetes, hypogonadism, thyroid and parathyroid disorders as well as decreased levels of adrenal gland secretions. Other outcomes may include arthritis, chronic fatigue, depression, hair loss and change in skin colour, abdominal pains, splenomegaly, venous thrombosis, and osteoporosis[4,5]. Iron overload can manifest in a variety of situations such as abuse of iron supplements, acquired iron overload due to chronic hepatitis, genetic causes such as all kinds of hereditary hemochromatosis, African iron overload, sickle cell disease, major β-thalassemia, sideroblastic anaemia, deficiency of enzymes such as pyruvate kinase and glucose-6-phosphate dehydrogenase, disorders of transporting proteins namely atransferrinemia and aceruloplasminemia, and lastly, frequent blood or red blood cells transfusions[4,6]. Patients receiving chronic transfusions should be closely monitored and frequently screened for iron overload to reduce the morbidity and mortality risks associated. These patients generally develop portal fibrosis within the first 2 y, liver cirrhosis within the first decade of treatment and cardiac damage in the second or third decade of treatment. This cardiac damage is the main cause of death in thalassemia patients. An increased predisposition to cardiac diseases is seen in patients with Liver Iron Concentration (LIC) values above 15 mg of iron per gram of dry weight of liver (15 mg/g Fe), and in patients with serum ferritin values above 2500 μg/l[7]. This could be effectively dealt with chelation therapy[8]. At physiological pH, the soluble ferrous ion (Fe+2) is rapidly oxidized to the insoluble ferric form (Fe+3)[9]. These chelators would bind with unbound iron, form a complex and excrete it via urine or faeces. Deferasirox (4-[3,5-bis-(2- hydroxyphenyl)-[1,2,4]-triazol-1-yl]benzoic acid, (DEF)) is an N-substituted bis-hydroxyphenyltriazole with a low molecular weight (373.4 Da). It is given in the dose of 20/30 mg/kg/d for the treatment of iron overload due to blood transfusion (transfusional hemosiderosis) in patients of 2 y of age and older[10]. It is marketed as Exjade® (tablets for oral suspension, 125, 250 and 500 mg)[11], Jadenu® Sprinkle (granules 90, 180 and 360 mg)[12], and Jadenu® (film-coated tablets, 90, 180 and 360 mg)[13]. Its bioavailability, when compared to intravenous administration, is 70 %. It is very highly bound to plasma proteins (approx. 99 %) and has an elimination half-life of 11-19 h which accounts for its once-a-day administration. The drug though effective has a large number of side effects associated with it which could benefit by dose reduction. DEF is a Class II molecule as per the Biopharmaceutical Classification System developed by Amidon et al.[14] suggesting that improving its solubility (practically insoluble at low pH and solubility of 0.4 mg/ml at pH 7.4) could lead to an improvement in its oral bioavailability. Although its bioavailability is acceptable for oral administration but the aim of this work is to improve its solubility and thereby bioavailability thereby leading to dose reduction which would directly reduce the incidence and intensity of side effects. As this is a drug meant for very long-term administration it could benefit from dose reduction. There are various pharmaceutical approaches to improve the solubility of a drug ranging from milling, complexation, nanotechnology to self-emulsifying systems. We are focussing on developing a Self-Microemulsifying Drug Delivery System (SMEDDS) of DEF which would not only improve its solubility due to reduction in particle size, the contribution of excipients like surfactants and co-surfactants, and activation of endogenous solubilizers like bile salts due to the high lipid content in the formulation but it could also help in bypassing the metabolism by lymphatic uptake of the drug. Both these actions together we think could help in improving the bioavailability of the drug. As this is a drug that the patients have to take daily for very long periods and in a lot of cases almost throughout their lives, adherence to therapy and patient compliance come at the forefront. One formulation that enjoys excellent patient compliance is the Orally Disintegrating Tablet (ODT) which forms a palatable dispersion on the patient’s tongue within a few seconds and can be swallowed without the aid of water. The present study aims at marrying both these pharmaceutical technologies into one dosage form to make a system for DEF which would improve its bioavailability as well as patient acceptance as it could have a potential of dose reduction which could be the overall therapy much more efficacious and safer.
Materials and Methods
The drug, DEF was kindly gifted by Alkem Labs, India. The remaining excipients and solvents were purchased and were of the highest grade of purity.
Determination of solubility of the drug in various formulation components:
The three main ingredients required to formulate a SMEEDS are the oil, surfactant, and co-surfactant. Their choice is dependent on the solubility of the drug. To select these, 2 ml of different excipients were taken in a vial and an excess amount of drug was added to each of them. The vials were tightly closed and subjected to continuous agitation on an orbital shaker for a period of 72 h at 25° to achieve equilibrium solubility. After the said period, the samples were centrifuged at 3000 rpm for 15 min and the supernatant was filtered by membrane filtration and collected. They were suitable diluted and their Ultraviolet (UV) absorbance was taken at 246 nm (UV-1800, Shimadzu).
Screening of surfactant and co-surfactant:
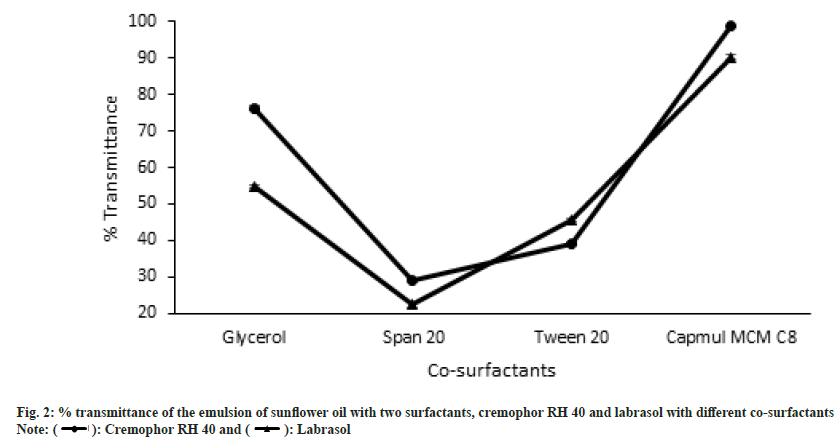
The surfactant was selected based on its emulsification ability. The emulsification ability was judged based on ease of emulsion formation and the clarity of the emulsion formed. To assess that, surfactant and the oil were mixed in equimolar proportions and heated to 40-45° for 30 sec to attain homogenization. 50 mg of this mixture was diluted with 50 ml of double-distilled water to obtain a fine emulsion in a volumetric flask. The volumetric flask was inverted a few times until a uniform and transparent emulsion was formed. The number of turns required to form the said emulsion was noted. The prepared emulsion was allowed to stand for 2 h, post which its % transmittance was assessed at 650 nm[15]. The surfactants screened were Cremophor RH 40 (HLB 14-16), Labrasol (HLB 14), and Span 80 (HLB 4.3). The selection of co-surfactants was also based on its ability to aid the surfactant selected in the previous study to form a stable and rapid emulsion in the shortest amount of time possible. The co-surfactants that were assessed for their suitability were glycerol, Span 20, Tween 20, and Capmul MCM C8.
Constructions of a pseudo-ternary phase diagram:
The water titration method at ambient temperature was used to construct a pseudo-ternary phase diagram using the excipients selected from the previous study. The procedure consisted of preparing solutions of different ratios of surfactant to co-surfactant (Smix) by weight (1:1, 1:2, and 2:1). They were mixed with oil in proportions ranging from 1:9 to 9:1 to make a total mass of 1 g. the prepared mixtures were subjected to vortexing for 5 min and titrated by dropwise addition of water. After each addition, each sample was visually observed for its clarity. The percentage of oil, Smix, water at which a clear mixture was formed was selected and the values were used to construct the phase diagram.
Preparation of SMEDDS:
From the ternary phase diagram ratio of surfactant to co-surfactant was optimized. A mixture of different proportions of surfactant and co-surfactant was prepared and the drug was added to it. The resultant mixture was heated at 35° to facilitate solubilization. Oil in the predetermined amount was added and was allowed to rest for an hour to form the isotropic mixture.
Evaluation of SMEDDS, dispersibility:
Evaluation of Solid-SMEDDS (S-SMEDDS): Water (500 ml) is taken in United States pharmacopeia apparatus II (Paddle apparatus) and maintained at 37±0.5°. the paddle is rotated at a speed of 50 rpm to mimic body conditions. The prepared SMEDDS are added and visually observed for one of the following outcomes as listed in Table 1.
| S No. | Dispersibility and appearance | Grade | Time to self-emulsify |
|---|---|---|---|
| 1 | Rapidly forming nano or microemulsion having a clear or bluish appearance | A | Within 1 min |
| 2 | Rapidly forming, slightly clear emulsion having a bluish-white appearance | B | Within 1 min |
| 3 | Fine milky emulsion that forms moderately fast | C | Within 2 mins |
| 4 | Dull, greyish-white emulsion having a slightly oily appearance that is slow to emulsify | D | Within 3 mins |
| 5 | Exhibit poor or minimal emulsification with large oil droplets present on the surface | E | Within 3 mins |
Table 1: Visual Observation and Classification of Self-Emulsifying Formulations Based on their Ability and Speed of Forming a Dispersion
Grade A and B formulation will remain as nano- or microemulsion in the gastrointestinal tract whereas those falling under Grade C would form an emulsion with a larger droplet size[16].
Time for self-emulsification:
The pre-concentrate and 0.1 N HCl were blended at a constant speed of 50 rpm at 37±5° temperature. Self-emulsification time was measured by visually observing the mixture and percent transmittance was measured at 650 nm in a UV-Visible spectrophotometer (UV-1800 Shimadzu) at the end of 24 h.
Cloud point determination:
The formulation was diluted 100 times with distilled water and was maintained in a water bath set to 25°. the temperature is gradually increased at the rate of 5°/min. The temperature at which the first sign of haziness appears is recorded as the cloud point[17].
Effect of pH on stability of the emulsion:
The selected formulation was subjected to 50-, 100-, 500-, and 1000-fold dilution with simulated gastric fluid (pH 1.2-without enzymes) and simulated intestinal fluid (pH 6.4-without enzymes). The diluted emulsions were very keenly observed for 24 h for any physical changes suggesting coalescence, precipitation, or phase separation[18].
Preparation of S-SMEDDS:
The drug is dissolved in a mixture of surfactant and co-surfactant with the aid of heat to aid the solubilization of the drug. Pre-weighed oil is added to this mixture with stirring using a magnetic stirrer to ensure complete mixing. This was used to conduct all further processing and evaluations. To determine the suitability of the carrier to prepare solid SMEDDS, they were going to be judged on two criteria, viz, maximum adsorption ability and time for 90 % drug release. The liquid mixture prepared in the aforementioned step was taken in a mortar to which adsorbent was added in increments with continuous trituration till a powder was formed. Based on this, the weight of the carrier needed to fully adsorb 1 g of the SMEDDS was determined. The amount of SMEDDS that could be adsorbed by 100 g on the carrier was then calculated as the adsorption capacity. Various carriers tried included talc, lactose, microcrystalline cellulose, mannitol and colloidal silicon dioxide.
Evaluation of S-SMEDDS, drug content:
Solid SMEDDS equivalent to 10 mg of DEF was diluted with a suitable amount of methanol. This was sonicated for 15 min to ensure complete extraction of the drug from the Solid SMEDDS. The resultant mixture was filtered using a 0.45 μm filter. The drug content was determined by measuring absorbance at 246 nm using the UV-1800 spectrophotometer (Shimadzu, Japan).
Determination of flow characteristics:
Parameters such as Carr’s index, angle of repose and flow rate were determined. 50 g of the sample was allowed to flow through a dry glass funnel with a stem. The time required for the entire sample to flow through the funnel was noted. The height and radius of the resulting cone were determined which helped in the calculation of the angle of repose. To determine the Carr’s index, 40 g of sample was taken in a 100 ml measuring cylinder and the cylinder was tapped 1250 times till constant volume. The volume before and after tapping was noted.
Particle size and zeta potential measurement:
The mean particle size (z-average) and zeta potential of the DEF solid SMEDDS were determined by dynamic light scattering technique using a zeta size analyser (Zetasizer 3000 Hs, Malvern Instruments Ltd., UK). The dried powder was redispersed with water to obtain the proper scattering intensity before measurement.
Morphological evaluation:
The morphological study was carried out using a scanning electron microscope (JSM-6390, Jeol USA Inc.). The sample was lightly sprinkled on double adhesive tape stuck on an aluminium stub. The stubs were then coated to a thickness of above 10 nm under an argon atmosphere using a gold sputter module using an electrical potential of 2.0 kV at 25 mA for 10 min under a high vacuum evaporator and the sample coated stub was placed in the scanning electron microscope chamber. An excitation voltage of 20 kV was used in the experiment.
In vitro drug release:
The in vitro drug release of prepared solid SMEDDS was assessed in triplicate using the Indian Pharmacopoeia (IP) Dissolution Type I apparatus (Paddle type) at 37±0.5°C. Solid SMEDDS containing 10 mg equivalent of the drug and 10 mg of pure drug powder was placed in 900 ml of dissolution medium (phosphate buffer pH 6.8 and 0.1 N HCl). The revolution speed of the paddle was maintained at 50 rpm. At predetermined time intervals, 5 ml of dissolution medium was collected, filtered and the same volume of fresh dissolution medium was replenished to maintain the sink conditions. The samples were analysed for the drug concentration using a UV-Visible spectrophotometer at 246 nm.
Preparation of ODT of S-SMEDDS:
The solid SMEDDS powder equivalent to 90 mg drug was mixed with microcrystalline cellulose as the filler, and a suitable superdisintegrants and compressed under optimum pressure to yield tablets that showed rapid disintegration within 30 secs when placed upon the tongue[19]. Various super-disintegrants such as croscarmellose sodium, Sodium Starch Glycolate (SSG), and Kollidon® CL were evaluated at a different concentration level to get the best possible combination to yield orally disintegrating tablets of the desired quality. Aspartame was added as the sweetener and magnesium stearate as the lubricant.
Evaluation of ODT of S-SMEDDS, tableting attributes:
Measurement of dimensions, weight variation, hardness, friability, wetting time, and in vitro disintegration time was carried out. The dimensions (diameter and thickness) were measured using a vernier calliper. Weight variation was done as per the procedure laid down by the IP 2019. As per IP, 20 tablets were taken and weighed individually. And their deviation from the average weight was assessed. Crushing strength or hardness was measured using a Monsanto tester. Friability was performed by selecting 20 pre-weighed tablets and introducing them into the drum of a Roche friabilator. The machine was programmed to run at 25 rpm for 4 min. The percentage weight loss was calculated. To determine the wetting time, a filter paper disc of 5 cm diameter is placed in a Petri plate and it was wet with a 2 ml solution of eosin blue (0.2 %). The tablet was placed on this filter paper gently using forceps and the time taken for the dye to reach the top of the surface was noted. The in vitro disintegration time of the tablet was also performed in a Petri plate of diameter 5 cm. 5 ml of phosphate buffer of pH 6.8 was used as the medium and the time taken for complete disintegration of the tablet was noted. Measurement of dimensions, weight variation, hardness, friability, wetting time, and in vitro disintegration time was carried out. The dimensions (diameter and thickness) were measured using a vernier calliper. Weight variation was done as per the procedure laid down by the IP 2019. As per IP, 20 tablets were taken and weighed individually. And their deviation from the average weight was assessed. Crushing strength or hardness was measured using a Monsanto tester. Friability was performed by selecting 20 preweighed tablets and introducing them into the drum of a Roche friabilator. The machine was programmed to run at 25 rpm for 4 min. The % weight loss was calculated. To determine the wetting time, a filter paper disc of 5 cm diameter is placed in a Petri plate and it was wet with a 2 ml solution of eosin blue (0.2 %). The tablet was placed on this filter paper gently using forceps and the time taken for the dye to reach the top of the surface was noted. The in vitro disintegration time of the tablet was also performed in a Petri plate of diameter 5 cm. 5 ml of phosphate buffer of pH 6.8 was used as the medium and the time taken for complete disintegration of the tablet was noted.
In vitro drug release:
The in vitro drug release of prepared solid SMEDDS was assessed in triplicate using the IP Dissolution Type I apparatus (Paddle type) at 37±0.5°. The prepared orally disintegrating tablets of DEF solid SMEDDS selected after assessing for previously mentioned quality evaluations and marketed tablets were placed in 900 ml of dissolution medium (0.1 N HCl). The revolution speed of the paddle was maintained at 50 rpm. At predetermined time intervals, 5 ml of dissolution medium was collected, filtered and the same volume of fresh dissolution medium was replenished to maintain the sink conditions. The samples were analysed for the drug concentration using a UV-VIS spectrophotometer at 246 nm.
Results and Discussion
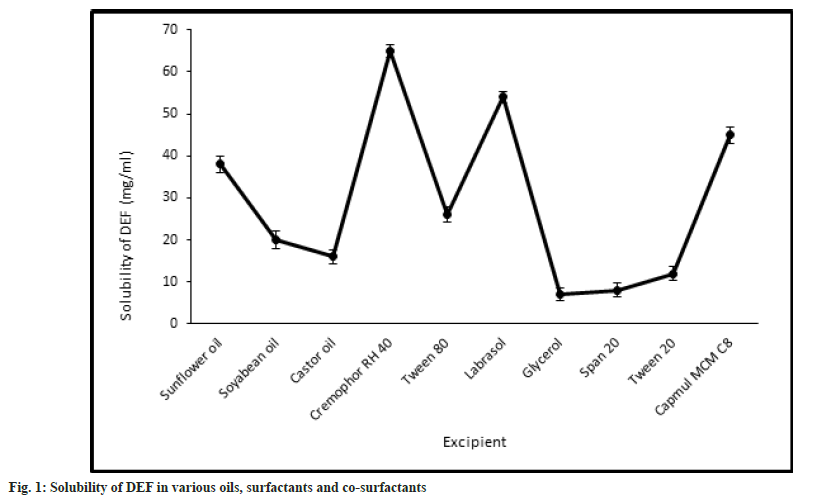
The selection of the components is of the utmost importance as that would dictate the emulsification and stability characteristics of the formulation. All the components have one main role to play, i.e., to improve the solubility of DEF in the formulation, so that on mixing with gastric contents the system emulsifies almost instantaneously without any precipitation of the drug. With this aim in mind, the solubility studies were carried out to assess the solubility of the drug in various oils, surfactants, and co-surfactants, that could be selected in preparing the formulation. There are various classes of lipids that could be used as oils to solubilize the drug. We decided to go for vegetable oils as published reports suggest that they are fully digestible and absorbable and hence have little to no issues in vivo[20]. They offer added advantages like their availability is not an issue and they are relatively inexpensive. The oils -explored in the present study are castor oil, soya ben oil, and sunflower oil. They are made up of longchain triglycerides like ricinoleic acid, linoleic acid, and linolenic acid respectively. These oils have a very large proportion of hydrophobic parts which helps in the solubilization of a large amount of the hydrophobic drug as compared to their mediumchain and synthetic counterparts[21]. The drug showed maximum solubility in sunflower oil (38±1.98 mg/ ml) as opposed to soyabean oil (20±1.14 mg/ ml) and castor oil (16.42±1.56 mg/ml). Of the surfactants and co-surfactants that were selected for screening, Cremophor RH 40 and Capmul MCM C8 showed the maximum solubilizing capacity for DEF. The solubility of DEF in various oils, surfactants, and co-surfactants is as shown in fig. 1.
Sunflower oil was selected as the lipid to formulate the SMEDDS from the observations made in the solubility testing of the drug in different vegetable oils. Of the three surfactants that were screened for their capacity to emulsify sunflower oil, Cremophor RH 40 and Labrasol showed good results as well as had an acceptable solubilizing capacity for DEF as shown in fig. 1 above. On the other hand, Span 80 did not give acceptable results. This could be attributed to its Hydrophilic-Lipophilic Balance (HLB) of 4.3 which is on the lower end of the spectrum and unsuitable to emulsify sunflower oil to form an o/w emulsion. Co-surfactants are a very important component of forming a SMEDDS as they in tandem with the surfactants aid not only to enhance the solubility of the drug in the system but they also help in achieving a homogenous distribution of the hydrophilic surfactant into the oil phase. They have another role wherein they get adsorbed at the oil- surfactant interface to reduce the interfacial tension, even more, to get instantaneous self-emulsification along with physically preventing the oil droplets from coalescing. Thereby they play a dual role in improving formulation stability[22]. Thus, their judicious selection can be a major stepping stone in the formulation of a successful SMEDDS. As two surfactants were found to be suitable for formulating SMEDDS of DEF in sunflower oil, the selection of cosurfactants was carried out in the mixture of oil with respective surfactants. The selection of co-surfactants was primarily based on its ability to not only form an emulsion rapidly but also that it remains stable without causing any coalescence or precipitation. This was assessed by making note of percent transmittance value. The results for both as depicted in fig. 2. The number of inversions for the different combinations ranged from 6 (Capmul MCM C8 with Cremophor RH 40) to 17 (Span 20 with Labrasol).
The composition of the SMEDDS depends on the “microemulsion region” which was determined by plotting the ternary phase diagram. This was done by the water titration method. Three different ratios of surfactant:co-surfactant were selected, viz, 1:1, 2:1, and 3:1. In each of the ratio-based formulation, nine batches were made depending on the proportion of oil and the mixture of surfactant (Smix). The entire range from 1:9 to 9:1 was scanned. The pseudo ternary phase diagrams of all 3 combinations are depicted in (fig. 3).
From the diagrams, it is evident that the area of the microemulsion region is the largest for the ratio of 1:1. Hence that was selected as the combination of choice for formulation development and further evaluation. It is an accepted fact that the solubility of the drug in the system should be less dependent on the Smix, as on eventual dilution in vivo such systems show greater drug precipitation[23]. For the finalization of the concentration of the main components, three compositions lying in the microemulsion region of the pseudo ternary phase diagram were selected. These contained oil 10, 20, and 30 % and were called F1, F2, and F3 respectively. The remaining amount had been made up with a 1:1 proportion of Smix. The formulations were evaluated for their dispersibility, time for self-emulsification, % transmittance, and their cloud point. The results of these have been given in Table 2. The results of this indicate that formulation F1 and F2 have the potential of forming stable SMEDDS within a very short period which was confirmed by the determination of the exact time required for self-emulsification. This indicated that in vivo the system would get emulsified almost instantaneously without significant precipitation. These results could be corroborated by the % transmittance values obtained of the formulations diluted with distilled water and kept undisturbed for 24 h. As both formulations F1 and F2 had a value of over 90 %, hence it could be concluded that those formulations would pose lesser in vivo stability and precipitation issues. Cloud point is the temperature at which the emulsion has the potential of turning cloudy from its current clear state. As the stability of these emulsions in vivo is of the essence for the success of the system hence a cloud point above 37° is required. At the cloud point, there is a precipitation of the drug and phase separation. This cloud point can be affected by the lipophilicity of the drug and other formulation components[24]. All three formulations had a cloud point significantly above the threshold and hence that couldn’t be useful in selecting the final composition. But amongst the three batches, F1 had the highest cloud point value indicating maximum in vivo stability. Based on all the above tests formulation F3 was found to be unsatisfactory and hence further evaluations on F3 were discontinued. Furthermore, the stability of the two formulations when diluted with simulated gastric fluid (pH 1.2-without enzymes) and simulated intestinal fluid (pH 6.4-without enzymes) was assessed at different dilution levels. 50-, 100-, 500-, and 1000-fold dilutions were carried out and kept for 2 h. Post the stipulated time, they were observed for any physical changes suggesting coalescence, precipitation, or phase separation. The results are as shown in Table 3. From all the tests performed to select the best formulation from the compositions derived from the microemulsion region, we concluded that formulation F1 was found to be suitable in all respects and hence would be continued for the preparation of S-SMEDDS.
| Test | F1 | F2 | F3 |
|---|---|---|---|
| Dispersibility test | A | A | B |
| Time for self-emulsification (secs) | 45±2 | 57±3 | 100±3 |
| % transmittance | 98.74±0.63 | 92.16±0.57 | 78.38±1.21 |
| Cloud point (°) | 68-72 | 66-69 | 50-53 |
Table 2: Observations of Various Evaluation Tests Performed on SMEDDS Shortlisted from the Microemulsion Region
| Batch | Simulated gastric fluid (pH 1.2-without enzymes) | Simulated intestinal fluid (pH 6.4-without enzymes) | ||||||
|---|---|---|---|---|---|---|---|---|
| 50 X | 100 X | 500 X | 1000 X | 50 X | 100 X | 500 X | 1000 X | |
| F1 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| F2 | +++ | +++ | ++ | ++ | +++ | +++ | ++ | + |
Note: (+++): No change observed; (++): Minor clouding observed and (+): Precipitation and clouding occurred
Table 3: Physical Observations Made to Assess the Effect of Dilution with Mediums of Different Ph to Varying Proportions
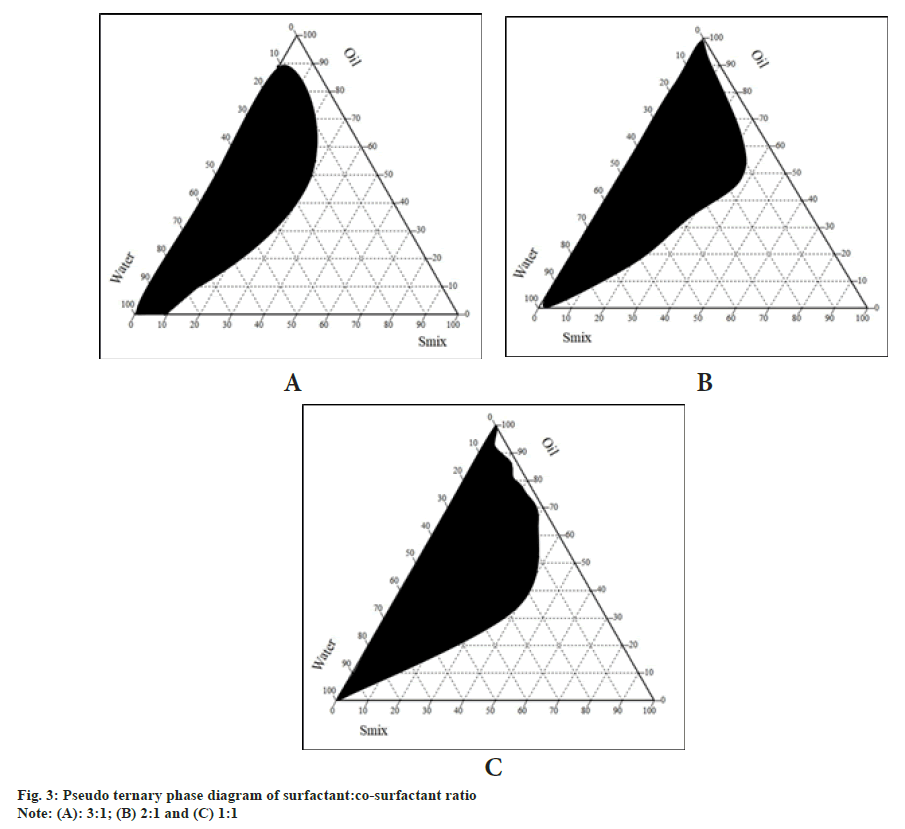
To prepare ODT of SMEDDS of DEF it was paramount to adsorb it onto a suitable carrier. The criteria for suitability were adjudged as maximum adsorption ability and time for 90 % drug release. The SMEDDS composition finalized which contained 10 % sunflower oil was taken in a mortar to which adsorbent was added in increments with continuous trituration till a powder was formed. Various carriers tried included talc, lactose, microcrystalline cellulose, mannitol and colloidal silicon dioxide. The adsorption capacity and time for 90 % drug release as represented in fig. 4. From the results, it could be emphatically concluded that both Aerosil 200 (Colloidal silicon dioxide) and Avicel PH 101 (microcrystalline cellulose) are good alternatives for the adsorption of the prepared SMEDDS. Similar results have been observed by other researchers[25]. As Aerosil 200 showed slightly better results as compared to Avicel PH 101, hence S-SMEDDS were prepared as using Avicel 200 as the carrier. This could be attributed to the amorphous nature of Aerosil 200 along with its particle size and void space[26]. S-SMEDDS to Aerosil 200 ratio was fixed at 2:1, the batch was scaled up, and further evaluations were carried out to confirm the suitability of Aerosil 200 and in the said proportion so that it could be used in the preparation of the final dosage form.
Fig. 4: The adsorption capacity and time for 90 % drug release of the drug from SMEDDS adsorbed on various carriers
Note: ( ): Adsorption capacity (g of SMEDDS/100 g of carrier) and (
): Adsorption capacity (g of SMEDDS/100 g of carrier) and ( ): Drug release at the end of 1 h (%); : Talc; 2: Lactose; 3: Microcrystalline cellulose; 4: Mannitol and 5: Colloidal silicon dioxide
): Drug release at the end of 1 h (%); : Talc; 2: Lactose; 3: Microcrystalline cellulose; 4: Mannitol and 5: Colloidal silicon dioxide
The drug content was measured by diluting S-SMEDDS with methanol, which is a solvent in which the drug shows maximum solubility. Methanol was chosen as the solvent to ensure complete extraction of the drug from the formulation. The dilution was analysed by determining the absorbance of the sample at 246 nm using a UV-Vis spectrophotometer. Drug content was found to be 99.65±0.2 %. This confirmed the good loading and eventual release of the drug from the carrier.
Flow properties of the S-SMEDDS are of prime importance to formulate its orally disintegrating tablet preferably by direct compression. The tapped and untapped bulk densities of the S-SMEDDS were found to be 0.14 and 0.12 g/ml. Its Carr’s ratio of 14.29±0.52 % and Hausner’s ratio of 1.17±0.01 indicated a “good” flow of the blend. These results were validated by its angle of repose which was found to be 22.46±1.08°. The flow rate was found to be 2.5 g/s. All these results point at direct compression to be a favourable method of formulating the orally disintegrating tablets of S-SMEDDS of DEF.
Both particle size and zeta potential are the measures of the success of the formulation. Finer particle sizes indicate better solubility of the drug and stability of the formulation as seen by the absence of coalescence. Zeta potential is an indication that the particles would remain separated in the system and ensure its stability. The Z-average particle size of S-SMEDDS was found to be 338.4 nm with a polydispersity index of 0.291 indicating narrow particle size distribution. Due to the closeness in particle sizes, there isn’t much variation in achieving saturation solubility and concentration gradients.
This prevents the Ostwald ripening which could be responsible for the growth and eventually reconversion of the nano-sized particles to micro-sized ones[27]. Zeta potential is the measure of the chance of aggregation bought about by the coalescence of particles. Zeta potential measures the charge on the particle. The charge of the oil droplets in conventional SMEDDS is negative due to the presence of free fatty acids[28]. For the droplets in SMEDDS, a high numeric value of zeta potential will confer stability and long shelf life. When the potential is low, attractive forces may exceed this repulsion and the emulsion may break and aggregate. The zeta potential for our system was found to be -29.5 mV. This suggests the good stability of the SMEDDS formulation.
Morphological characterization was carried out using scanning electron microscopy using gold sputtering. Fig. 5 is the scans of solid drug and drugloaded S-SMEDDS. From the scans, it is evident that the crystalline drug was amorphized by solubilizing completely in liquid SMEDDS, indicating the ability of SMEDDS to improve the solubility and thereby bioavailability of DEF.
The S-SMEDDS was prepared with the aim of formulating an orally disintegrating tablet from which the drug would be released quickly along with a significant increase in the amount of drug releasing and dissolving. These studies were carried out using the IP Dissolution Type I apparatus (Paddle type) operating at 37±0.5°. The dissolution mediums selected were phosphate buffer pH 6.8 and 0.1 N HCl. The paddle speed was fixed at 50 rpm and samples were withdrawn over a period of 1 hr. The result obtained is as shown in fig. 6. From the above figure, it is clear that the drug-loaded S-SMEDDS showed a significant improvement in the amount of drug released within 1 h. This could also lead to an improvement in the rate and extent of drug absorption in vivo. This can indicate an improvement in the overall bioavailability of DEF.
The biggest challenge as a formulation developer is to make a dosage form that is acceptable and convenient to the patients. Orally Disintegrating Tablets (ODT) have found much popularity in this respect. As DEF is to be taken daily for several years, the convenience of the dosage form becomes of utmost importance. Thus, the S-SMEDDS were thought to be converted into an ODT. Three superdisintegrants, viz croscarmellose sodium, SSG, and Kollidon® CL, were screened at three different concentrations of 1, 2.5, and 5 % respectively. The details of the batches taken are as given in Table 4.
| Weight on ingredient (mg) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|---|
| S-SMEDDS | 270 | 270 | 270 | 270 | 270 | 270 | 270 | 270 | 270 |
| Microcrystalline cellulose | 25.9 | 21.4 | 13.9 | 25.9 | 21.4 | 13.9 | 25.9 | 21.4 | 13.9 |
| Croscarmellose sodium | 3 | -- | -- | 3 | -- | -- | 3 | -- | -- |
| SSG | -- | 7.5 | -- | -- | 7.5 | -- | -- | 7.5 | -- |
| Kollidon® CL | -- | -- | 15 | -- | -- | 15 | -- | -- | 15 |
| Aspartame | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Magnesium stearate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total tablet weight (mg) | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
Table 4: Trial Batches of ODT of S-SMEDDS
All the batches prepared were evaluated for measurement of dimensions, weight variation, hardness, friability, wetting time, and in vitro disintegration time. The results of the tests are as given in Table 5. From these results, we could conclude that tablets prepared with SSG and crosslinked polyvinylpyrrolidone (Kollidon CL) required shorter wetting and disintegration time as compared to those prepared by croscarmellose sodium as the superdisintegrants.
| Evaluation parameter | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|---|
| Diameter (mm) | 10±0.2 | 10±0.2 | 10±0.2 | 10±0.2 | 10±0.2 | 10±0.2 | 10±0.2 | 10±0.2 | 10±0.2 |
| Thickness (mm) | 2-3 | 2-3 | 2-3 | 2-3 | 2-3 | 2-3 | 2-3 | 2-3 | 2-3 |
| Weight variation | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| Hardness (kg/cm) | 2±0.5 | 2±0.5 | 2.5±0.5 | 2.5±0.5 | 3±0.5 | 3±0.5 | 1±0.5 | 1±0.5 | 1±0.5 |
| Friability (%) | Tablets break | Tablets break | 0.8±0.1 | 0.8±0.1 | 0.7±0.1 | 0.7±0.1 | Tablets break | Tablets break | Tablets break |
| Wetting time (s) | 67±2 | 59±2 | 65±2 | 25±2 | 12±2 | 9±2 | 35±2 | 24±2 | 13±2 |
| Disintegration time (s) | 85±3 | 79±3 | 83±3 | 35±3 | 15±2 | 15±3 | 47±3 | 36±3 | 24±3 |
Table 5: Evaluation of All 9 Batches of ODT of S-SMEDDS
This could be attributed to their high water absorption capacity due to which they demonstrate higher swelling which leads to a build-up of hydrodynamic pressure which forces these tablets to disintegrate quickly[29]. Of the two, we found SSG to be the most suitable. The effect of concentration was observed when SSG was increased from 1 to 2.5 % there was a noticeable drop in the disintegration and wetting time of the tablets. But the increase of the SSG concentration from 2.5 to 5 % did not make any significant difference in the disintegration time. This could be attributed to the gelling behaviour of SSG which could be occluding the pores in the tablet thereby preventing the intake of the medium by the tablets[30]. Hence 2.5 % of SSG was selected as the super-disintegrant of choice.
A comparison was made between the ODT prepared by drug-loaded S-SMEDDS and the marketed formulation available locally based on in vitro drug release. From the results, as depicted in fig. 7, it is evident that the prepared ODTs show quicker drug release as compared to the conventional dosage form available in the market. From the in vitro drug release studies of the S-SMEDDS in comparison with the drug powder, it could be concluded that the effect of the dissolution medium wasn’t significant on the drug’s release. Hence these studies were performed using only 0.1 N HCl as the dissolution medium. It was conspicuous that the drug release from the ODT of drug-loaded S-SMEDDS (98.69±1.13 %) was much higher than that observed for the marketed formulation (66.75±1.38 %) within 1 h. The small increase in the in vitro drug release of the ODT of S-SMEDDS over S-SMEDDS as assessed before could be attributed to the increased wettability offered by the superdisintegrants.
In conclusion, DEF, a Biopharmaceutics Classification System class II molecule was thought to be loaded into a SMEDDS to help in improving its solubility and overall oral bioavailability. The formulations were made with vegetable oils in combination with Cremophor RH 40 as the surfactant and Capmul MCM C8 as the co-surfactant. The SMEDDS could be successfully loaded on to half part of Aerosil 200. These S-SMEDDS could be converted into orally disintegrating tablets with all the desired attributes. The tablets showed a 32 % increase in drug release within 1 h over the conventional formulation available locally. From the various studies performed it could be concluded that the prepared ODT of drug-loaded S-SMEDDS has the potential to improve the bioavailability of DEF, which could lead to titration and further reduction of dose which would reduce the incidences of side effects. These studies could be continued further wherein pharmacokinetic assessment can be made to prove the hypothesis in its entirety and fulfil the aim envisaged at the outset.
Conflict of Interest:
Authors declare that there is no conflict of interest.
References
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci 2014;19(2):164.
[Google Scholar] [PubMed]
- Ganz T. Systemic iron homeostasis. Physiol Rev 2013;93(4):1721-41.
[Crossref] [Google Scholar] [PubMed]
- Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol 2005;202(2):199-211.
[Crossref] [Google Scholar] [PubMed]
- Mobarra N, Shanaki M, Ehteram H, Nasiri H, Sahmani M, Saeidi M, et al. A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Oncol Stem Cell Res 2016;10(4):239-45.
[Google Scholar] [PubMed]
- Sebastiani G, Pantopoulos K. Disorders associated with systemic or local iron overload: From pathophysiology to clinical practice. Metallomics 2011;3(10):971-86.
[Crossref] [Google Scholar] [PubMed]
- Tanaka C. Clinical pharmacology of deferasirox. Clin Pharmacokinet 2014;53(8):679-94.
[Crossref] [Google Scholar] [PubMed]
- Agarwal MB. Deferasirox: Oral, once daily iron chelator-an expert opinion. Indian J Pediatr 2010;77:185-91.
[Crossref] [Google Scholar] [PubMed]
- Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox Sang 2009;97(3):185-97.
[Crossref] [Google Scholar] [PubMed]
- Porter JB. Deferasirox: An update. Hemoglobin 2009;33(sup1):S70-5.
[Crossref] [Google Scholar] [PubMed]
- Deferasirox. Chemsrc. 2020
- Drug label information. DailyMed. 2020
- Sprinkle (deferasirox) granules. US FDA. 2020.
- In grief: Jadenu- A new formulation of deferasirox for iron overload. The Medical Letter. 2020
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413-20.
[Crossref] [Google Scholar] [PubMed]
- Pattewar S, Kasture SB, Pande VV, Patil DN, Sharma SK. Development and optimization of piroxicam-loaded solid self-microemulsifying drug delivery system. Indian J Pharm Sci 2018;80(2):350-8.
- Rao MR, Munjapara GS, Khole IA. Preparation and evaluation of self-microemulsifying drug delivery system of carvedilol. Latin Am J Pharm 2011;30:837-43.
- Patel AR, Vavia PR. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS 2007;9:E344-52.
[Crossref] [Google Scholar] [PubMed]
- Uppugalla SR, Rathnanand M, Srinivas P, Deepak K, Amit K, Priya S. Self-emulsifying systems of aceclofenac by extrusion/spheronization: Formulation and evaluation. J Chem Pharm Res 2011;7(6):1-8.
- Guidance for Industry Orally Disintegrating Tablets. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2008.
- Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems: An overview. Acta Pharm Sinica B 2013;3(6):361-72.
- Gurram AK, Deshpande PB, Kar SS, Nayak UY, Udupa N, Reddy MS. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J Pharm Sci 2015;77(3):249.
[Crossref] [Google Scholar] [PubMed]
- Li L, Zhou CH, Xu ZP. Self-nanoemulsifying drug-delivery system and solidified self-nanoemulsifying drug-delivery system. Nanocarriers Drug Deliv 2019;421-49.
- Qureshi MJ, Mallikarjun C, Kian WG. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: A study in diet induced hyperlipidemic rabbits. Asian J Pharm Sci 2015;10(1):40-56.
- Akula S, Gurram AK, Devireddy SR. Self-microemulsifying drug delivery systems: An attractive strategy for enhanced therapeutic profile. Int Sch Res Notices 2014;2014:964251.
[Crossref] [Google Scholar] [PubMedi]
- Tung NT, Tran CS, Nguyen HA, Nguyen TL, Chi SC, Nguyen DD. Development of solidified self-microemulsifying drug delivery systems containing l-tetrahydropalmatine: Design of experiment approach and bioavailability comparison. Int J Pharm 2018;537(1-2):9-21.
[Crossref] [Google Scholar] [PubMed]
- Krsti? M, Medarevi? ?, ?uriš J, Ibri? S. Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In: Lipid nanocarriers for drug targeting. 2018:473-508.
- Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: A promising drug delivery strategy. J Pharm Pharmacol 2004;56(7):827-40.
[Crossref] [Google Scholar] [PubMed]
- Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm 2000;50(1):179-88.
[Crossref] [Google Scholar] [PubMed]
- Khinchi MP, Gupta MK, Bhandari A, Sharma N, Agarwal D. Design and development of orally disintegrating tablets of famotidine prepared by direct compression method using different superdisintegrants. J Appl Pharm Sci 2011;1(1):50-8.
- Zhao N, Augsburger LL. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets. AAPS Pharmscitech 2005;6:E120-6.
[Crossref] [Google Scholar] [PubMed]
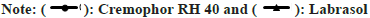
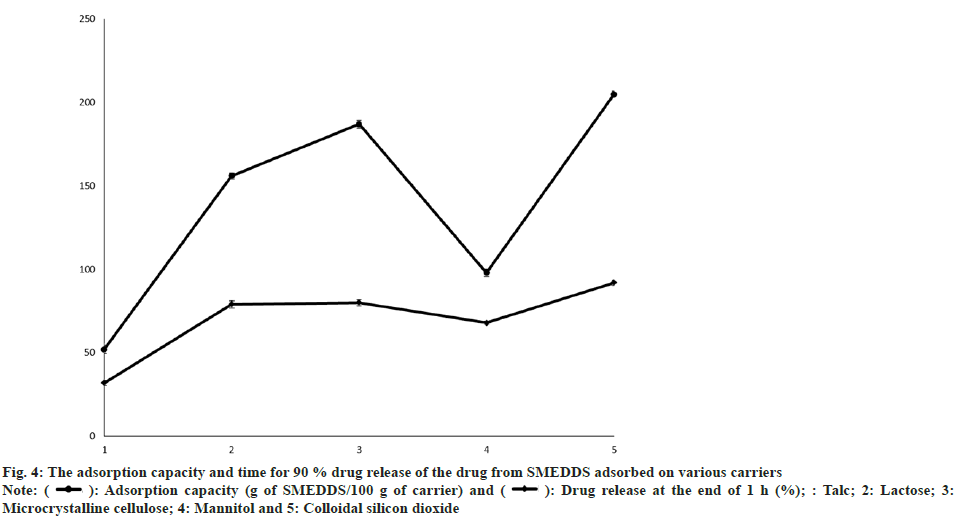

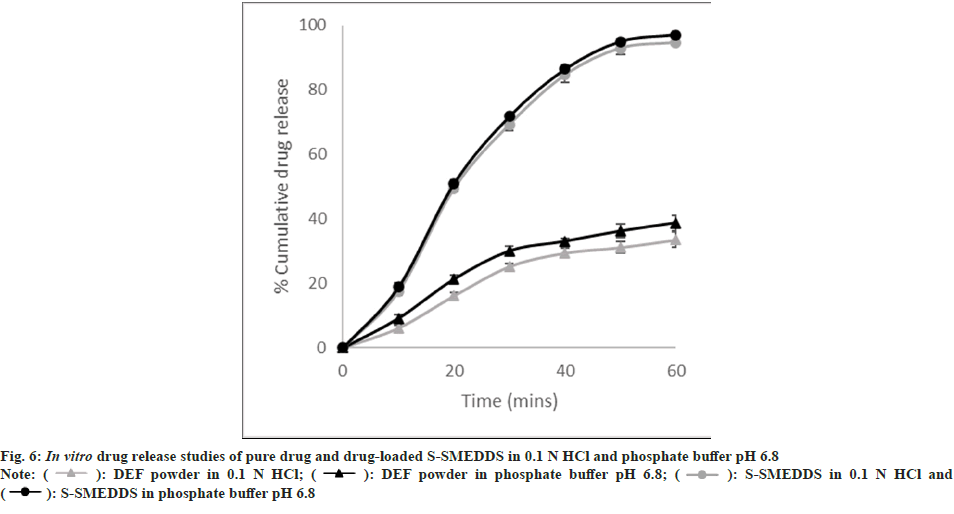
 ): DEF powder in 0.1 N HCl; (
): DEF powder in 0.1 N HCl; ( ): DEF powder in phosphate buffer pH 6.8; (
): DEF powder in phosphate buffer pH 6.8; ( ): S-SMEDDS in 0.1 N HCl and (
): S-SMEDDS in 0.1 N HCl and ( ): S-SMEDDS in phosphate buffer pH 6.8
): S-SMEDDS in phosphate buffer pH 6.8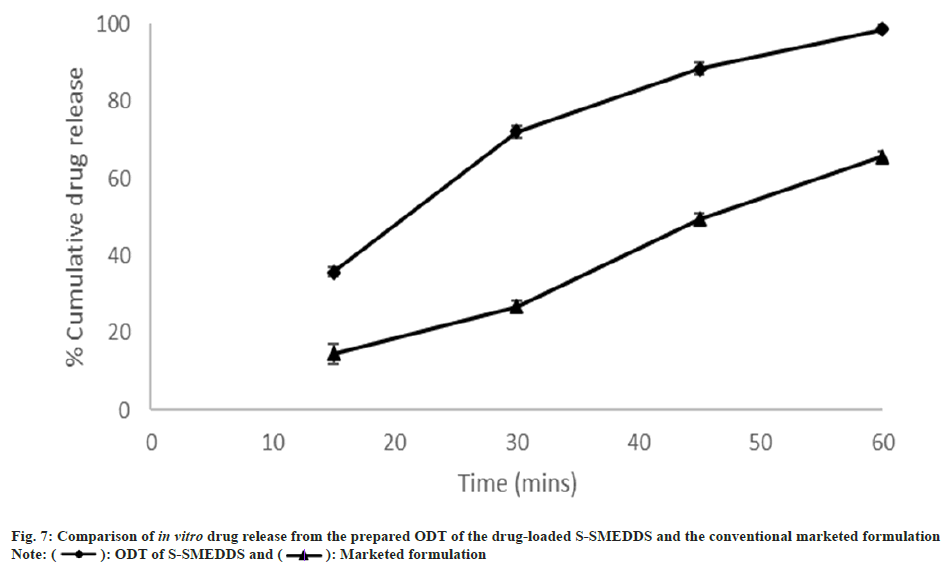
 ): ODT of S-SMEDDS and (
): ODT of S-SMEDDS and (  ): Marketed formulation
): Marketed formulation



