- *Corresponding Author:
- H. G. Shivakumar
Department of Pharmaceutics, J. S. S. College of Pharmacy, Sri Shivarathreeshwar Nagar, Mysore-570 015, India
E-mail: researchlab05@hotmail.com
| Date of Submission | 17 November 2005 |
| Date of Revision | 19 June 2006 |
| Date of Acceptance | 26 January 2007 |
| Indian J Pharm Sci, 2007, 69 (1): 58-63F |
Abstract
Semi-interpenetrating polymer networks of poly(vinyl alcohol) and poly(methacrylic acid) were prepared by free radical polymerization of methacrylic acid in presence of poly(vinyl alcohol) using N,N-methylenebisacrylamide as cross linking agent. The dynamic swelling behavior of the semi-interpenetrating polymer networks was studied to determine the water transport mechanism. The effect of cross linking agent and methacrylic acid concentration on the swelling and insulin release characteristics were evaluated. Swelling ratio and water diffusion coefficients were used to characterize the water uptake process. Swelling rates correlated well with the polymer composition, the slowest rate of water uptake was observed in hydrogel samples containing large quantities of N,N-methylenebisacrylamide and maximum swelling was observed with hydrogels containing higher methacrylic acid content. The mechanism of water transport in these semi-interpenetrating polymer network hydrogels was significantly affected by the pH of the swelling medium. At pH 2.0 mechanism was Fickian (diffusion controlled) and became case II (relaxation controlled) at pH 7.4. Insulin was incorporated by active loading technique and the insulin incorporated in the semi-interpenetrating polymer network hydrogels was in the range of 28-35%. The formulations showed decreased insulin release in pH 2.0, while complete release was observed in pH 7.4 pH buffer. The diffusion coefficient of insulin through the hydrogels was in the range of 3.84×10 -6 to 5.59×10 -6 cm 2 /min in pH 2.0 buffer and in pH 7.4 buffer it was in the range of 7.55×10 -6 to 21.636×10 -6 cm 2 /min.
In the recent years therapeutic peptides and proteins have risen to prominence as potential drugs of the future. These peptide and protein drugs though potent and specific in their action are generally given by parenteral route. The reason is that most peptide and protein drugs are very unstable in gastrointestinal tract owing to hydrolysis and digestion by the acids and enzymes. Parenteral route is marked by disadvantages like rapid fluctuations in the drug blood level, patient noncompliance and pain associated with repeated injections, etc. To overcome the disadvantages of parenteral route numerous routes including oral, transdermal, rectal, ocular, nasal have been tried [1-4]. Various delivery systems like microparticles, nanoparticles, mucoadhesive polymers, liposomes, pH sensitive hydrogels are some of the systems studied for oral protein delivery [5-9].
Hydrogels can be defined as polymeric networks capable of swelling and retaining large quantities of water in the swollen structures without dissolution or loss of their three dimensional structure [10]. Anionic hydrogels exhibiting pH dependent swelling contain carboxylic or sulfonic groups. These pH sensitive hydrogels exhibit swelling when the pH of the environment exceeds the pKa of the ionizable groups. The opposite behavior is observed for the cationic hydrogels [11]. The dynamic swelling change of the anionic hydrogels can be used in the design of intelligent controlled release devices for site-specific drug delivery of therapeutic proteins to upper small intestine where their biological activity is prolonged [12]. The change in the pH of the external environment will act as a stimulus and the response to the stimulus is the change in swelling properties of the hydrogels, causing the release of protein. An important route to modify the physico-chemical properties of a hydrogel is the preparation of interpenetrating polymer networks (IPNs) which are conventionally defined as intimate combination of two polymers, at least one of which is synthesized or crosslinked in the immediate presence of other [13].
The aim of the present study was to prepare semiinterpenetrating polymer network (semi-IPN) of poly(vinyl alcohol) and poly(methacrylic acid) and evaluate its potential for sorption and oral delivery of insulin. The effect of experimental parameters such as varying methacrylic acid content, crosslinking agent concentration and pH of the release medium on the release dynamics of the insulin was investigated. On the basis of Fick’s equation the diffusional exponent (n) was calculated for different hydrogel compositions. From the kinetic parameters data, an attempt was made to explore the nature of the mechanism of swelling of semi-IPN hydrogels in different pH and the release process of insulin.
Materials and Methods
Methacrylic acid (Aldrich, Steinheim, Germany) was made inhibitor free by vacuum distillation. Poly(vinyl alcohol) (average molecular weight range 1 24 000-1 86 000 and degree of hydrolysis 99%) was purchased from Aldrich (Steinheim, Germany). MBA (Aldrich, Steinheim, Germany), potassium persulphate (Rankem, India) were used without purification. Insulin from porcine pancreas was purchased from Sigma (St. Louis, USA). Double istilled water was used throughout the experiment.
Hydrogel synthesis
The IPNs of varying composition were prepared by the free radical polymerization method. Poly(vinyl alcohol) (1% w/v) was dissolved in water heated to 90° and stirred for 12 h. When the solution attained room temperature, methacrylic acid (5 mM-15 mM), MBA (0.02 mM-0.08 mM) and potassium persulphate (0.2 mM) were added.This process was continued till constant weights were obtained.This clearly assured a complete removal of unreacted initiator, crosslinking agent and monomer from the hydrogels. Feed compositions of the monomers and other chemicals are listed in.Table 1
| Sample No. | PVA (1% w/v) | MAA (mM) | MBA (mM) | KPS (mM) | % Insulin loading ±SD |
|---|---|---|---|---|---|
| F1 | 1 | 5 | 0.02 | 0.2 | 29.8±1.22 |
| F2 | 1 | 10 | 0.02 | 0.2 | 33.4±0.81 |
| F3 | 1 | 15 | 0.02 | 0.2 | 34.4±1.45 |
| F1a | 1 | 5 | 0.04 | 0.2 | 28.8±1.06 |
| F1b | 1 | 5 | 0.08 | 0.2 | 27.6±0.97 |
The IPNs of varying composition were prepared by the free radical polymerization of MAA in presence of PVA using KPS as polymerization initiator and MBA as crosslinking agent. The insulin loading efficiency was calculated by subtracting the amount of insulin remaining in the phosphate buffer and combined washings from the concentration of insulin stock solution
Table 1: Feed Composition of Various Samples Of Pva-Maa Semi-Ipn Hydrogels
Scanning electron microscopy (SEM)
The surface morphology of dried hydrogels was determined using a scanning electron microscope (Stereoscan 120, Cambridge Instruments, USA). The hydrogel samples were mounted on the base plate and coated with gold using vapor deposition techniques. The surface was then scanned using magnification of 5000.
Dynamic swelling experiments
To determine the dynamic swelling behavior the hydrogels, disks of known weight was placed in pH 2.0 hydrochloric acid buffer and pH 7.4 phosphate buffer at 37°. The ionic strength of each buffer was adjusted to 0.1 M by the addition of potassium chloride. At regular intervals of time, the disks were taken out of the buffer, blotted for the removal of surface water and weighed again. The swelling process was characterised by the weight swelling ratio q,q=Ws/Wd where Ws is the weight of the swollen hydrogel and Wd is the weight of initially dried hydrogel. The swelling studies were carried out in triplicate.
Insulin incorporation
Insulin was first dissolved in 10 ml of 0.1 N HCl and this solution was neutralized with 0.1 N NaOH and then diluted with pH 7.4 phosphate buffer solution to make a 10 mg/ml insulin stock solution. To ensure that insulin did not adsorb on the glass surface, 0.02 mg of Tween-80 was added. The hydrogels were placed in 5 ml insulin stock solution and allowed to stand for 12 h. After 12 h the hydrogel disks were placed in 0.1 N HCl to cause collapse of the hydrogels. The hydrogels were then removed and washed first with 0.1 N HCl and then with distilled water. The hydrogels were then dried under vacuum and stored at 4° for further use. The loading efficiency was calculated by subtracting the amount of insulin remaining in the phosphate buffer and combined insulin remaining in the phosphate buffer and combined
In vitro release studies
Drug release from the dried insulin loaded hydrogels was carried both in pH 2.0 hydrochloric acid buffer and in pH 7.4 phosphate buffer at 37°. During the experiment the stirring speed was maintained at 100 rpm and the volume of dissolution media used was 50 ml. At different time points 1 ml of release medium was collected and replaced by the same volume of the buffer. Insulin released was estimated by reversed-phase high performance liquid chromatography method (RP-HPLC). The RP-HPLC consisted of C-18 column; the mobile phase was 0.2 M sodium sulphate (pH 2.3) and acetonitrile in the ratio of 74:26. The flow rate was 1ml/min and the insulin was detected using UV detector at 214 nm [14].
Mathematical analysis of water uptake and drug release
Analysis of the swelling behavior of semi-IPN hydrogels know was carried out using the equation Mt/M∞=4(Dt/πδ2)1/2, where D is the water diffusion coefficient in the polymer, δ the half thickness of the semi-IPN film, Mt the amount of water uptake at time t and M∞ is the water uptake at equilibrium stage. Diffusion coefficients of insulin through the semi-IPNs at pH 2.0 and 7.4 was calculated from the equation M∞=1-8/π2 exp(-π2Dt/4δ2), where D is the diffusion coefficient of insulin in the semi-IPN, δ is the half thickness of the semi-IPN, Mt the amount of insulin released at time t, M∞ is the maximum amount of insulin released [15].
Results and Discussion
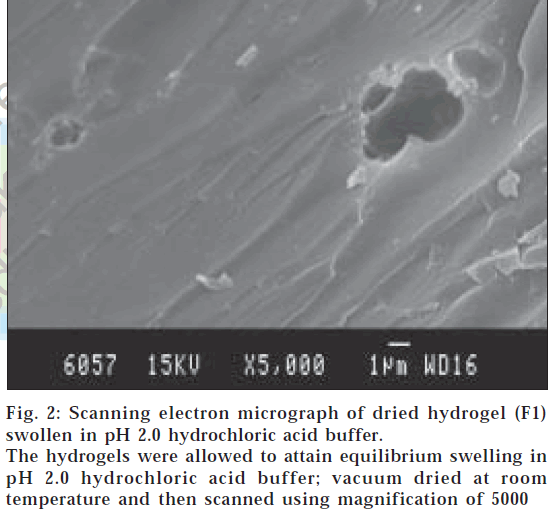
The surface morphology of dried hydrogels was carried out by SEM studies to observe the cross-sectional structures of dried hydrogels, and it was observed that surface of the hydrogels swollen in pH 7.4 showed porous structure (fig. 1) with pore size greater than 2 μm and for hydrogels swollen in pH 2.0 buffer showed nonporous nature (fig. 2).
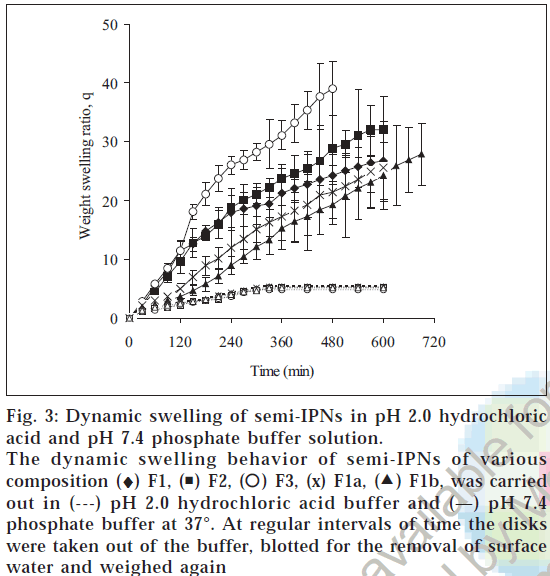
To study the time dependent swelling behaviors of the hydrogels, the disks were tested in pH 2.0 and pH 7.4 buffer solutions, which represent the gastric and intestinal pH. Fig. 3shows the weight swelling ratio (q) values of hydrogels as a function of time. At pH 7.4, the amount of water absorbed by the hydrogel was much larger than at pH 2.0 at the same time point. The portion of the water absorption curve with the fractional water uptake (Mt / M∞) less than 0.6 was fitted to the equation Mt/ M∞=ktn, where Mt is the mass of water absorbed at time t, M∞ is the mass of water absorbed at equilibrium, k is the characteristic constant of the hydrogel, and n is the characteristic exponent describing the penetrant mechanism [15]. For planar geometry, the value of n=0.5 0.5<n<1.0 indicates non-Fickian or anomalous transport, indicates a Fickian water diffusion mechanism, for and n=1 implies case II (relaxation controlled) transport. The constant n and k was calculated from the slope and intercepts of the plots of log (Mt/ M∞) versus logt. The values of n and k are reported in Table 2. The swelling of hydrogels was much higher in pH 7.4 than in pH 2.0 buffer. This is due to the fact that as the pH of the external environment was above the pKa of poly(methacrylic acid), the ionization of the carboxylic acid groups occurred which resulted in a more hydrophilic polymer network resulting in rapid absorption of water and higher swelling ratio in pH 7.4 buffer. The n values at pH 7.4 was in the range of 0.83-1.07 indicating that the transport mechanism was case II (relaxation controlled) whereas at pH 2.0, n values was 0.49-0.54 indicating Fickian diffusion. In ionic hydrogels, the swelling is significantly affected by the ionization of the functional groups of the polymer. Increased in ionization
| Sample No. | n | k ×102 (min-1) | ||
|---|---|---|---|---|
| pH 2.0 pH 7.4 | pH 2.0 pH 7.4 | |||
| F1 | 0.53 (±0.037) | 1.02 (±0.031) | 0.26 (±0.051) | 3.8(±0.447) |
| F2 | 0.52 (±0.016) | 1(±0.009) | 0.26(±0.016) | 4.12(±0.138) |
| F3 | 0.54 (±0.021) | 1.07 (±0.049) | 0.28(±0.032) | 5.66(±0.646) |
| F1a | 0.54 (±0.038) | 0.86(±0.012) | 0.27(±0.044) | 4.72(±0.293) |
| F1b | 0.49(±0.013) | 0.83(±0.027) | 0.22(±0.016) | 5.84(±0.412) |
Parameters n and k were obtained as slope and intercept from the plot of logMt/M∞ versus logt from the swelling studies of semi-IPNs in pH 2.0 and 7.4 buffer solutions at 37°
Table 2: Parameters N And K For Water Transport In Semi-Ipns
Figure 3: Dynamic swelling of semi-IPNs in pH 2.0 hydrochloric acid and pH 7.4 phosphate buffer solution.
The dynamic swelling behavior of semi-IPNs of various composition (♦) F1, ( ) F2, (
) F2, ( ) F3, (x) F1a, (
) F3, (x) F1a, ( ) F1b, was carried out in (---) pH 2.0 hydrochloric acid buffer and (—) pH 7.4 phosphate buffer at 37°. At regular intervals of time the disks were taken out of the buffer, blotted for the removal of surface water and weighed again
) F1b, was carried out in (---) pH 2.0 hydrochloric acid buffer and (—) pH 7.4 phosphate buffer at 37°. At regular intervals of time the disks were taken out of the buffer, blotted for the removal of surface water and weighed again
of functional groups at pH greater than pKa causes electrostatic repulsion between the ionized groups,leading to chain expansion,which in turn affects chain relaxation. As a result the hydrogels at pH 7.4 swelled by relaxation controlled mechanism. But at pH 2.0 there was no interaction between ionized functional groups and the transport mechanism was Fickian. The hydrophilicity of a hydrogel increases when the composition of methacrylic acid is increased. When the functional groups are ionized, their fixed ions repel one another, and this repulsion leads to further swelling of the network. The extent to which the ionized hydrogel swells at equilibrium increases with an increase in the concentration of functional ionizable groups on the network. The effect of increase in the concentration of methacrylic acid on swelling ratio of the hydrogel has been investigated by varying the concentration of ionizable monomer in the feed mixture in the range 5-15 mM. On varying the crosslinker concentration (MBA) in the range 0.02 mM-0.08 mM in the feed mixture of the hydrogels, there was a significant fall in the swelling of the IPNs. This may be explained by the fact that on increasing the crosslinker content there is a prominent decrease in the free volumes available between the chains of the macromolecular network and thus the swelling of hydrogel decreases. The water diffusion coefficient through the semi-IPNs was obtained by plotting Mt/M∞ versus t1/2 and from the slope the diffusion coefficient was calculated. Water diffusion coefficients in pH 7.4 was 5.44×10-5, 5.43×10-5, 8.16×10-5 cm2/min for the semi-IPNs containing 5, 10, 15 mM of MAA. This indicated that the semi-IPNs with higher MAA content showed rapid absorption of water because of increased electrostatic repulsion between the ionized groups, leading to chain expansion Also when the crosslinker concentration was increased from 0.02 mM to 0.06 the diffusion coefficient decreased from 5.44×10-5 to 4.14×10-5 cm2/min and when crosslinker concentration was increased to 0.08 mM the diffusion coefficient further decreased to 3.91×10-5 cm2/min.
Insulin was loaded by active loading technique. All the formulations showed insulin loading in the range of 2835% of incubated insulin. When the insulin was loaded at pH 7.4 buffer, due to electrostatic repulsion between the ionized carboxylic groups of poly(methacrylic acid) the pore size of the networks increased allowing the insulin to diffuse readily into the networks due to concentration gradient. When the pH was decreased (when placed in 0.1N HCl) the network collapses and the effective pore size of the network decreased. The insulin was entrapped inside the hydrogels due to the small pore size of the collapsed networks. On varying the crosslinker concentration (N,N-Methylenebisacrylamide) in the range 0.02 mM-0.08 mM in the feed mixture of the hydrogels, there was a significant fall in the swelling of the IPNs and, therefore, the percent loading of insulin decreased. This may be explained by the fact that on increasing the crosslinker content there is a prominent decrease in the free volumes available between the chains of the macromolecular network and thus the loading of insulin decreases. When the methacrylic acid composition in the hydrogels was increased, the swelling was greatly increased but the percent insulin loading increased slightly (Table 1).
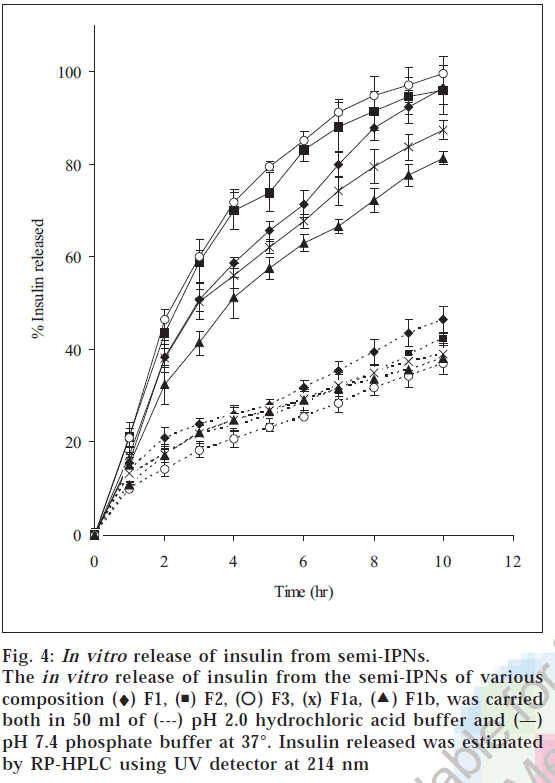
The release of insulin from the hydrogels was studied in both pH 2.0 and pH 7.4 buffer solutions. The release characteristic of insulin from hydrogels is shown in fig 4. The release of insulin from the hydrogels was analyzed using an exponential relation, which relates the amount of drug released from hydrogels as a function of time Mt/M∞=ktn for 0<Mt/M∞<0.6, where n is diffusional exponent. The in vitro release studies showed that the fraction insulin released in the pH 2.0 buffer was less than 0.5 in 10 h, whereas complete release was observed in pH 7.4 buffer in 10 h. When MBA concentration was increased fractional release was found to decrease. This is due to the fact that with increase in the crosslink density, the network becomes more compact resulting in the decrease in the fractional release. The increase in the compactness restricts the mobility of the network chains (chain relaxation) and also the diffusion of the insulin from the hydrogels into the release medium. When the value of n <0.5, the polymer relaxation does not affect the molecular transport and hence, diffusion is Fickian. For n>0.5 the solute transport will be non-Fickian, and when n=1 the system will be relaxation controlled. The n value for the hydrogels was in the range of 0.48-0.52 in pH 2.0 buffer and 0.83-0.92 in pH 7.4 buffer. The diffusion coefficient of insulin through the semi-IPNs was obtained by plotting log [π2/8(1- Mt/M∞)] versus t. The diffusion coefficients ranged from 3.84×10-6 to 5.59×10-6 cm2 min-1 in pH 2.0 buffer and from 7.55×10-6 to 21.636×10-6 cm2/ min in pH 7.4 buffer (Table 3). Diffusion coefficients increased by almost a factor of five when the pH of release medium was changed from 2.0 to 7.4 buffer (F3). Also the diffusion coefficient increased with increase in MAA content of semi-IPNs, with values increasing from 15.27×10-6 to 21.63×10-6 cm2/min as the MAA composition increased from 5 to 15 mM. And when the crosslinking concentration was increased from 0.02 to 0.08 mM the diffusion coefficient value of insulin decreased from 15.27×10-6 to 7.55×10-6 cm2/min. Reversed phase HPLC chromatograms demonstrated that insulin was not degraded nor aggregated after prolonged contact with the polymer. The retention times and the shapes of the peaks of freshly made insulin solution, loaded insulin and released insulin in pH 2.0 and pH 7.4 buffer were identical. This indicated the activity of insulin was retained after the loading procedure despite prolonged contact of the insulin with the polymer. From the above study it can be concluded that semi-IPNs of PVA/PMAA can be explored as a possible delivery systems for protein drugs that protects the proteins against the harsh environment of acidic pH but releases the drug in the distal part of intestine where the enzymatic activity is comparatively low.
| Formulations | pH 2.0 | pH 7.4 | ||
|---|---|---|---|---|
| D ×10-6 cm2 min-1 | R2 | D ×10-6 cm2 min-1 | R2 | |
| F1 | 5.598 | 0.989 | 15.274 | 0.973 |
| F2 | 4.539 | 0.989 | 15.952 | 0.996 |
| F3 | 3.894 | 0.992 | 21.636 | 0.977 |
| F1a | 4.058 | 0.996 | 9.288 | 0.995 |
| F1b | 3.843 | 0.995 | 7.551 | 0.996 |
The diffusion coefficient of insulin through the semi-IPNs was obtained by plotting log [π2/8(1-Mt/M∞)]versus t The slope of the plot was π2D/2.303×4δ2 from which the diffusion coefficient D was calculated
Table 3: Diffusion Coefficient Of Insulin Through The Semi-Ipns Of Various Compositions
Figure 4: In vitro release of insulin from semi-IPNs. The in vitro release of insulin from the semi-IPNs of various composition (♦) F1, ( ) F2, (
) F2, ( ) F3, (x) F1a, (
) F3, (x) F1a, ( F1b, was carried both in 50 ml of (---) pH 2.0 hydrochloric acid buffer and (—) pH 7.4 phosphate buffer at 37°. Insulin released was estimated by RP-HPLC using UV detector at 214 nm
F1b, was carried both in 50 ml of (---) pH 2.0 hydrochloric acid buffer and (—) pH 7.4 phosphate buffer at 37°. Insulin released was estimated by RP-HPLC using UV detector at 214 nm
Acknowledgements
The authors wish to thank Principal of J. S. S. College of Pharmacy, Mysore and J. S. S. Mahavidyapeetha, Mysore for their encouragement and University Grants Commission (UGC) for funding the project.
References
- Kim, B. and Peppas, N.A., Int. J. Pharm., 2003, 266, 29.
- Mitragotri, S., Blankschtein, D. and Langer, R., Science , 1995, 269, 850.
- Ichikawa, K., Iohata, I. and Mitomi, M., J. Pharm. Pharmacol., 1980, 32 , 314.
- Nagai, T., Nishimoto, Y. and Suzuki, Y., J. Control. Release, 1984, 1 , 15.
- Agarwal, V., Reddy, I.K. and Khan, M.A., Int. J. Pharm., 2001, 225, 31.
- Ganorkar, C.R., Liu, F., Baudyš, M. and Kim, S.W., J. Control. Release, 1999, 59, 287.
- Pan, Y., Li, Y., Zhao, H., Zheng, J., Xu, H., Wei, G., Hao, J. and Cui, F., Int. J. Pharm., 2002, 249, 139.
- Luessen, H.L., Verhoe, J.C., Borcahrd, G., Lehr, C.M., de Boer, A.G. and Junginger, H.E., Pharm. Res., 1995, 12, 1293.
- Iwanaga, K., Ono, S., Narioka, K., Morimoto, K., Kakemi, M., Yamashita, S., Nango, M. and Oku, N., Int. J. Pharm., 1997, 157, 73.
- Kamath, K. and Park, K., Adv. Drug Deliv. Rev., 1993, 11, 59.
- Kim, B., Flamme, K.L. and Peppas, N.A., J. Appl. Poly. Science, 2003, 89, 1606.
- Lugo, M.T. and Peppas, N.A., Macromolecules , 1999, 32, 6646.
- Lopes, M.A. and Felisberti, M.I., Biomaterials, 2003, 24, 1279.
- Oliva, A., Fariρa, J.B. and Llabrιs, M., Int. J. Pharm., 1996, 143, 163.
- Brazel, C.S., and Peppas, N.A., Polymer, 1999, 40, 3383