- *Corresponding Author:
- Qian Zhang
Department of Department of Digestive Diseases, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130000, China
E-mail: zqian@jlu.edu.cn
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “108-118” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, we analyzed the efficacy and influencing factors of programmed cell death protein 1 inhibitors for hepatitis B virus-associated liver cancer undergoing hepatic arterial chemotherapy. Study the therapeutic effect and influencing factors of programmed cell death protein 1 inhibitor on hepatitis B virus-related liver cancer undergoing hepatic arterial chemotherapy. This study was a retrospective study. Hepatitis B virus -infected patients with liver cancer who received hepatic arterial chemotherapy in hospital from January 2018 to January 2022 were selected. Among 158 patients, there was no statistically significant difference in hepatitis B virus reactivation and ALT elevation between hepatic arterial chemotherapy combined with programmed cell death protein 1 inhibitor and hepatic arterial chemotherapy alone. The overall tumor progression (odds ratio, 2.400 [95 % confidence interval, 1.183-4.871], p=0.015), tumor growth (odds ratio, 2.296 [95 % confidence interval, 1.098-4.803], p=0.027), lesion size increased (odds ratio, 2.401 [95 % confidence interval, 1.017-5.670], p=0.046), lesion number increased (odds ratio, 3.614 [95 % confidence interval, 1.443-9.053], p=0.006), new tumor metastasis (odds ratio, 2.742 [95 % confidence interval, 1.127-6.672], p=0.026) and new distant metastasis (odds ratio, 3.281 [95 % confidence interval, 1.226-8.779], p=0.018) were statistically significant. The number of tumor progression was lower in patients treated with antiviral therapy at baseline compared with those treated without antiviral therapy (odds ratio, 0.459 [95 % confidence interval, 0.214-0.984], p=0.045), with a statistically significant difference between the two groups. Programmed cell death protein 1 receptor inhibitors (odds ratio, 2.400 [95 % confidence interval, 1.183-4.871], p=0.015) and lymph node metastasis (odds ratio, 0.300 [95 % confidence interval, 0.119-0.754) were not used in the analysis of factors associated with overall tumor progression. p=0.010) was a risk factor for overall tumor progression. Overall tumor progression was independent of baseline hepatitis B virus deoxyribonucleic acid level, age, sex, eastern cooperative oncology group, hepatitis B e-antigen, type of programmed cell death protein 1 inhibitor, and type of hepatic arterial chemotherapy (p>0.05).
Keywords
Primary liver cancer, radical surgery, chronic hepatitis B, chemotherapy, tumor
Primary liver cancer is one of the most common malignant tumor diseases. It ranks 5th in the global cancer incidence rate and 3rd in the mortality rate. It is a serious hidden danger to human health[1]. In clinical practice, due to atypical early symptoms, most patients have already entered the mid to late stage of diagnosis, and only about 1/3rd can still undergo radical surgery. Therefore, for patients with advanced liver cancer who have lost the opportunity for surgery, hepatic arterial chemotherapy, immunotherapy, and molecular targeted therapy have become their preferred treatment methods.
In clinical practice, Transarterial Chemoembolization (TACE) and Hepatic Arterial Infusion Chemotherapy (HAIC) can create surgical resection opportunities for patients with advanced unrespectable liver cancer and prolong their survival. For patients with a risk of recurrence after surgery, research has confirmed that TACE treatment has the effect of reducing the probability of recurrence and prolonging survival for postoperative patients[2,3]. The median survival period after TACE is (16-45) mo in the early stage (Barcelona Clinic Liver Cancer (BCLC) 0-A), (15.6-26.3) mo in the middle stage (BCLC B), and (6.8-13.6) mo in the late stage (BCLC C)[4]. Programmed Cell Death Protein-1 (PD-1) inhibitors are currently a hot topic in cancer research. The survival benefits of PD-1 inhibitors for unrespectable liver cancer patients are borderless, with an average Overall Survival (OS) of 13.9-15.6 mo, and their treatment-related toxic effects are relatively low[5].
Chronic hepatitis B is a chronic progressive disease that, if not treated in a timely manner, can lead to cirrhosis, liver cancer, and even liver failure and death[6]. Existing studies have shown that the higher the viral load level of Hepatitis B Virus (HBV) infected individuals, the greater the risk of poor prognosis in disease progression. Therefore, using antiviral drugs to control HBV-Deoxyribonucleic Acid (DNA) levels can reduce the risk of disease progression and improve the long-term prognosis of patients. Related studies have found that common treatment measures for liver cancer, such as surgery, hepatic artery chemoembolization, radiofrequency ablation, and chemotherapy, can cause varying degrees of HBV reactivation. Although HBV reactivation may automatically improve in some patients, in many cases, it inevitably leads to delayed chemotherapy plans and interruption of cytotoxic treatment plans. In more severe cases, anticancer treatment is often terminated early; this can affect the long-term prognosis of individual cancer treatment. In addition, in the most severe cases, HBV reactivation can directly lead to fatal liver failure. Therefore, preventive use of antiviral drugs can significantly reduce the incidence of HBV reactivation events, improve liver function, and increase patient survival rate.
Research has shown that hepatic arterial chemotherapy, as a commonly used treatment method for liver cancer patients, can easily lead to reactivation of HBV-DNA in HBV related liver cancer patients, and should be highly valued in clinical practice. Effective antiviral therapy should be used to treat HBV related liver cancer patients receiving hepatic arterial chemotherapy, which can inhibit and prevent HBV reactivation, improve liver function, and reduce the incidence of adverse events such as liver failure and even death.
At the same time, studies have found that in patients with hepatitis B, HBV specific Cluster of Differentiation (CD)-8+ T lymphocytes express PD-1 molecules highly and are inversely proportional to their replication ability. Antiviral therapy can inhibit HBV replication and also reduce PD-1 expression[7-10]. Blocking the PD-1 signaling pathway can inhibit the replication of HBV virus, which has been confirmed in clinical studies[11].
Animal experiments have shown that, compared with the HBV negative control group, the positive group animals have high expression of PD-1 molecules on HBV specific CD8+ T lymphocytes. Blocking the PD-1 signaling pathway can promote anti HBV specific CD8+ T lymphocyte response, thereby promoting virus clearance. More importantly, research on patients with chronic HBV infection has also confirmed its correlation with humans. The HBV-specific T cells present in the peripheral blood of patients with chronic HBV infection express high levels of PD-1, which impairs their function. However, after the infection is resolved, the T cells found in the patient's body function is intact, and the expression of PD-1 is significantly reduced[8].
Although there are some case reports indicating that some patients who have resolved HBV infection have experienced HBV reactivation during PD-1 inhibitor treatment. However, studies have shown that PD-1 inhibitors are effective and safe treatment methods for advanced liver cancer patients, and HBV reactivation or HBV related hepatitis associated with PD-1 inhibitors is mild and controllable[12-14].
Related studies have shown that the combination of PD-1 inhibitors and HAIC can improve patient prognosis and prolong patient survival. And studies have shown that TACE can increase the expression level of PD-1, which is closely related to the therapeutic effect of TACE and patient prognosis. These provide a theoretical basis for the use of PD-1 inhibitors in liver cancer patients undergoing hepatic arterial chemotherapy. TACE is commonly used in the clinical practice of hepatic arterial chemotherapy, but there are currently few published clinical studies on the combination of PD-1 inhibitors and hepatic arterial chemotherapy. Among them, hepatic arterial chemotherapy is limited to HAIC and does not involve the discussion of HBV reactivation. Therefore, we will conduct an analysis of PD-1 inhibitors on HBV reactivation and efficacy in HBV related liver cancer patients who have undergone hepatic arterial chemotherapy.
Materials and Methods
Research object:
In this experiment, patients with HBV related liver cancer who received hepatic artery chemotherapy in Lianyi Hospital from January 2018 to mid-January 2022 were selected.
Inclusion criteria: Hepatitis B surface Antigen (HbsAg) positive and/or HBV DNA >20 IU/ml and/or previous diagnosis of hepatitis B was clear; phase II and III liver cancer confirmed by histology/imaging; Eastern Cooperative Oncology Group (ECOG)-PS ≤2; having good organ and bone marrow function; follow up time exceeding 12 w; receive at least one course of hepatic arterial chemotherapy.
Exclusion criteria: Presence of other malignant tumors; having a history of autoimmune diseases; lack of HBV DNA and liver function testing before initial treatment and during follow-up.
Grouping: Patients who use hepatic artery chemotherapy alone; patients who use a combination of hepatic arterial chemotherapy and PD-1 inhibitors[15-20].
Observation indicators:
Hepatitis episodes related to HBV reactivation refer to Alanine Transaminase (ALT) elevation ≥3 times the baseline level and absolute value >100 U/l. HBV reactivation refers to patients with persistent stability of HBV DNA, where HBV DNA elevation is ≥2 log10 IU/ml, or baseline HBV DNA negative individuals transition from negative to positive and ≥100 IU/ml, and those without baseline HBV DNA have HBV DNA ≥20 000 IU/ml.
Statistical processing:
Establish a database and use Statistical Package for the Social Sciences (SPSS) 25.0 statistical software for analysis. p<0.05 indicates statistically significant differences.
Results and Discussion
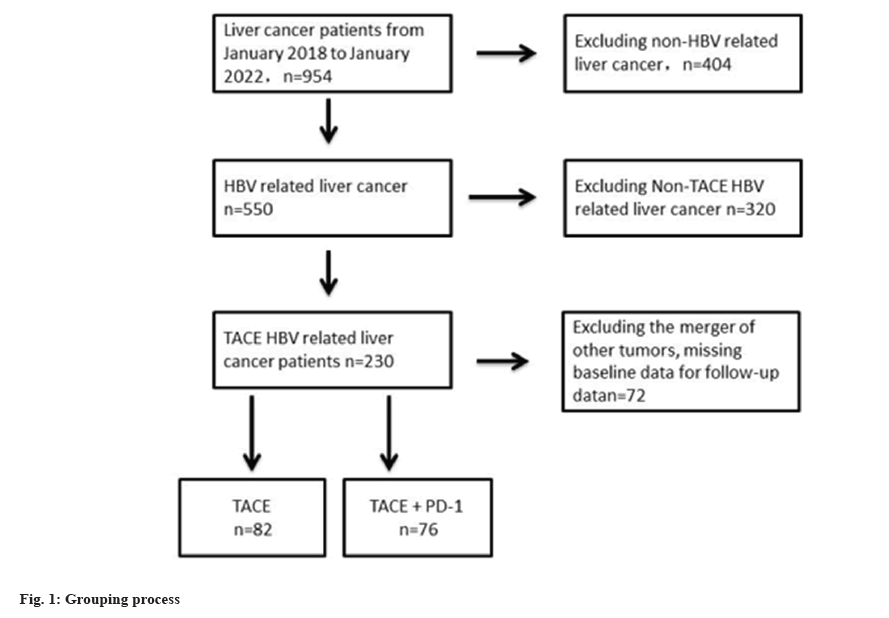
From January 2018 to January 2022, 158 HBV related liver cancer patients who received hepatic arterial chemotherapy met the inclusion criteria. 82 patients who received hepatic arterial chemotherapy alone, and 76 patients who received combination therapy with PD-1 inhibitors were shown in fig. 1.
A total of 158 patients were enrolled, of which 82 were treated solely with hepatic arterial chemotherapy and 76 were treated with a combination of hepatic arterial chemotherapy and PD-1 inhibitor. There was no statistically significant difference in general status such as gender (χ²=1.114, p=0.291) and age (χ²=0.171, p=0.679) between the two groups of patients. The proportion of patients who received a combination of hepatic arterial chemotherapy and PD-1 inhibitors had portal vein cancer thrombi and distant metastasis was higher (17.07 % and 44.74 %, p<0.001; 2.44 % and 13.16 %, p<0.05), and there was a statistical difference between the two groups as shown in Table 1.
| TACE (n=82) | TACE+PD-I inhibitor (n=76) | p | ||
|---|---|---|---|---|
| Age (year) | <50 | 24 (29.3) | 20 (26.3) | 0.679 |
| ≥50 | 58 (70.7) | 56 (73.7) | ||
| Gender | Male | 70 (85.37) | 60 (78.95) | 0.291 |
| Female | 12 (14.63) | 16 (21.05) | ||
| AFP (ng/ml) | <5 | 22 (26.8) | 10 (13.2) | 0.088 |
| 5400 | 32 (39.0) | 32 (42.1) | ||
| ≥400 | 28 (34.1) | 34 (44.7) | ||
| HBeAg | + | 22 (26.8) | 14 (18.4) | 0.208 |
| - | 60 (73.2) | 62 (81.6) | ||
| HcvAb | + | 2 (2.44) | 2 (2.63) | 0.939 |
| - | 80 (97.56) | 74 (97.37) | ||
| Baseline HBV-DNA (IU/ml) | <500 | 28 (34.15) | 30 (39.47) | 0.488 |
| ≥500 | 54 (65.85) | 46 (60.53) | ||
| Anti-virus | Yes | 40 (48.78) | 26 (34.21) | 0.064 |
| No | 42 (51.22) | 50 (65.79) | ||
| Cirrhosis | Yes | 52 (63.41) | 54 (71.05) | 0.307 |
| No | 30 (36.59) | 22 (28.95) | ||
| Portal vein tumor thrombus | No | 68 (82.93) | 42 (55.26) | <0.001 |
| Yes | 14 (17.07) | 34 (44.74) | ||
| Lymph node metastasis | No | 72 (87.80) | 64 (84.21) | 0.514 |
| Yes | 10 (12.20) | 12 (15.79) | ||
| Distant metastasis | No | 80 (97.56) | 66 (86.84) | 0.025 |
| Yes | 2 (2.44) | 10 (13.16) | ||
| Number of tumors | diffuse | 24 (29.27) | 12 (15.79) | 0.249 |
| 1 indivual | 26 (31.71) | 28 (36.84) | ||
| 2 indivual | 4 (4.88) | 4 (5.26) | ||
| >3 indivual | 28 (34.15) | 32 (42.11) | ||
| Hepatic arterial chemotherapy cycle | 1 | 32 (65.00) | 44 (57.89) | 0.284 |
| 2 | 16 (20.00) | 22 (28.95) | ||
| 3 | 6 (7.50) | 2 (2.63) | ||
| ≥4 | 6 (7.50) | 8 (10.53) | ||
| Operation | Non | 68 (82.93) | 44 (57.89) | <0.001 |
| Other | 8 (9.76) | 6 (7.89) | ||
| Liver radiofrequency ablation | 6 (7.32) | 26 (34.21) | ||
| ECOG | 0 | 14 (17.07) | 22 (28.95) | 0.06 |
| 1 | 28 (34.15) | 30 (39.47) | ||
| 2 | 40 (48.78) | 24 (31.58) | ||
| Child-Pugh | Grade A | 56 (68.29) | 44 (57.89) | 0.175 |
| Grade B | 26 (31.71) | 32 (42.11) |
Table 1: Basic clinical characteristics of patients.
Among 158 patients, 10 (6.33 %) experienced HBV reactivation, including 8 patients treated with a combination of hepatic arterial chemotherapy and PD-1 inhibitors, and 2 patients treated solely with hepatic arterial chemotherapy (Odds Ratio (OR), 0.213 [95 % Confidence Interval (CI), 0.044-1.035], p=0.055).
Among these 10 patients, the HBV DNA of 4 patients increased by more than 2 logs (100 times) compared to baseline, and the baseline HBV DNA levels of 6 patients were lower than the detectable value, with HBV DNA ≥3 logs (1000) IU/ml. The highest level of HBV DNA at diagnosis of HBV reactivation is 1.47×10^ 7 IU/ml, with the highest ALT level of 894.99 U/l.
Among 10 patients with HBV reactivation, 8 were males and 2 were females; four patients were diagnosed with HBV related hepatitis, with a sudden increase in HBV DNA levels followed by an ALT outbreak; all patients recovered from HBV related hepatitis after treatment, and there were no deaths related to HBV reactivation or HBV related hepatitis.
Among 158 patients, 14 (8.86 %) showed a 3-fold increase in ALT with an absolute value greater than 100 U/l. Among patients treated with combination of hepatic artery chemotherapy and PD-1 inhibitors, 20 (OR, 0.576 [95 % CI, 0.267-1.244], p=0.160) showed no statistically significant difference between the two groups as shown in Table 2.
| TACE (n=82) | TACE+PD-1 inhibitor (n=76) | OR (95 % CI) | p | ||
|---|---|---|---|---|---|
| HBV reactivation | Yes | 2 (1.27) | 8 (5.06) | 0.213 | 0.055 |
| No | 80 (43.04) | 68 (41.77) | (0.044-1.035) | ||
| ALT elevation | Yes | 14 (8.86) | 20 (12.66) | 0.576 | 0.16 |
| No | 68 (43.04) | 56 (35.44) | (0.267-1.244) | ||
Table 2: The effect and comparison of treatment methods on the incidence of HBV reactivation and ALT elevation.
Among 158 patients, 10 (6.33 %) experienced HBV reactivation, with 8 patients receiving baseline antiviral treatment and 2 patients not receiving antiviral treatment (OR, 0.328 [95 % CI, 0.067-1.598], p=0.168). There was no statistically significant difference between the two groups.
Among 158 patients, 18 patients received baseline antiviral treatment, and 16 patients without antiviral treatment showed a 3-fold increase in ALT with an absolute value greater than 100 U/l (OR, 1.316 [95 % CI, 0.613-2.822], p=0.481), with no statistically significant difference between the two groups as shown in Table 3.
| Baseline anti-virus | |||||
|---|---|---|---|---|---|
| Yes (n=66) | No (n=92) | OR (95 % CI) | p | ||
| HBV reactivation | Yes | 2 (1.27) | 8 (5.06) | 0.328 | 0.168 |
| No | 64 (40.51) | 84 (53.16) | (0.067-1.598) | ||
| ALT elevation | Yes | 16 (10.13) | 18 (11.39) | 1.316 | 0.481 |
| No | 50 (31.65) | 74 (46.84) | (0.613-2.822) | ||
Table 3: The impact and comparison of baseline antiviral status on the incidence of HBV reactivation and ALT elevation.
Patients who only use hepatic artery chemotherapy have a higher number of tumor progression compared to those who use a combination of hepatic artery chemotherapy and PD-1 inhibitors. Overall tumor progression (OR, 2.400 [95 % CI, 1.183-4.871], p=0.015), tumor growth (OR, 2.296 [95 % CI, 1.098-4.803], p=0.027), and increased lesion volume (OR, 2.401 [95 % CI, 1.017-5.670], p=0.046). The differences in the number of lesions increased (OR, 3.614 [95 % CI, 1.443-9.053], p=0.006), new tumor metastasis (OR, 2.742 [95 % CI, 1.127-6.672], p=0.026), and new distant metastasis (OR, 3.281 [95 % CI, 1.226-8.779], p=0.018) were all statistically significant as shown in Table 4.
| TACE (n=82) | TACE+PD-1 inhibitor (n=76) | OR (95 % CI) | p value | ||
|---|---|---|---|---|---|
| Survival rate (6 mo) | Survival | 75 (91.4) | 74 (97.4) | 3.453 (0.694-17.172) | 0.13 |
| Death | 7 (8.6) | 2 (2.6) | |||
| Survival rate (12 mo) | Survival | 50 (61.1) | 61 (80.3) | 1.888 (0.908-3.925) | 0.089 |
| Death | 32 (38.9) | 15 (19.7) | |||
| Overall progression of tumors | Yes | 32 (20.25) | 16 (10.13) | 2.400 (1.183-4.871) | 0.015 |
| No | 50 (31.65) | 60 (37.97) | |||
| Tumor growth | Yes | 28 (17.72) | 14 (8.86) | 2.296 (1.098-4.803) | 0.027 |
| No | 54 (34.18) | 62 (39.24) | |||
| Increase in volume | Yes | 20 (12.66) | 9 (5.70) | 2.401 (1.017-5.670) | 0.046 |
| No | 62 (39.24) | 67 (42.40) | |||
| Increased number of lesions | Yes | 22 (13.92) | 7 (4.43) | 3.614 (1.443-9.053) | 0.006 |
| No | 60 (37.97) | 69 (43.67) | |||
| New tumor metastasis | Yes | 20 (12.66) | 8 (5.06) | 2.742 (1.127-6.672) | 0.026 |
| No | 62 (39.24) | 68 (43.04) | |||
| Newly added portal vein cancer thrombus | Yes | 2 (1.27) | 2 (1.27) | 0.925 (0.127-6.735) | 0.939 |
| No | 80 (50.63) | 74 (44.30) | |||
| Remote metastasis | Yes | 18 (11.39) | 6 (3.80) | 3.281 (1.226-8.779) | 0.018 |
| No | 64 (40.51) | 70 (44.30) | |||
| New lymph node metastasis | Yes | 10 (6.33) | 4 (2.53) | 2.5 (0.749-8.339) | 0.136 |
| No | 72 (45.57) | 72 (45.57) | |||
Table 4: The impact and comparison of treatment methods on tumor progression.
The number of tumor progression in patients receiving baseline antiviral therapy was lower compared to those without antiviral therapy (OR, 0.459 [95 % CI, 0.214-0.984], p=0.045), and there was a statistically significant difference between the two groups as shown in Table 5.
| Baseline anti-virus | |||||
|---|---|---|---|---|---|
| Yes (n=66) | No (n=92) | OR (95 % CI) | p value | ||
| Survival rate (6 mo) | Survive | 63 (95.45) | 86 (93.48) | 0.683 (0.164-2.834) | 0.599 |
| Die | 3 (4.55) | 6 (6.52) | |||
| Survival rate (12 mo) | Survive | 44 (66.67) | 73 (79.35) | 1.921 (0.936-3.942) | 0.075 |
| Die | 22 (33.33) | 19 (20.65) | |||
| Overall progression of tumors | Have | 18 (11.39) | 30 (18.99) | 0.775 (0.387-1.533) | 0.472 |
| None | 48 (30.38) | 62 (39.24) | |||
| Tumor growth | Have | 12 (7.60) | 30 (18.99) | 0.459 (0.214-0.984) | 0.045 |
| None | 54 (34.17) | 62 (39.24) | |||
| Increase in volume | Have | 5 (3.16) | 24 (15.19) | 0.232 (0.083-0.646) | 0.005 |
| None | 61 (38.61) | 68 (43.04) | |||
| Increased number of lesions | Have | 9 (5.70) | 20 (12.66) | 0.568 (0.241-1.343) | 0.198 |
| None | 57 (36.08) | 72 (45.57) | |||
| New tumor metastasis | Have | 12 (7.59) | 12 (7.59) | 1.481 (0.620-3.541) | 0.377 |
| None | 54 (34.18) | 80 (50.63) | |||
| Newly added portal vein cancer thrombus | Have | 4 (2.53) | 0 (0) | / | / |
| None | 62 (39.24) | 92 (58.23) | |||
| Remote metastasis | Have | 12 (7.59) | 12 (7.59) | 1.481 (0.620-3.541) | 0.377 |
| None | 54 (34.18) | 80 (50.63) | |||
| New lymph node metastasis | Have | 3 (1.90) | 11 (6.96) | 0.351(0.094-1.310) | 0.119 |
| None | 63 (39.87) | 81 (51.27) | |||
Table 5: The impact and comparison of baseline antiviral status on tumor progression.
Failure to use PD-1 receptor inhibitors (OR, 2.400 [95 % CI, 1.183-4.871], p=0.015) and lymph node metastasis (OR, 0.300 [95 % CI, 0.119-0.754], p=0.010) are risk factors for overall tumor progression. The overall progression of the tumor was not related to baseline HBV DNA levels, age, gender, ECOG, HBeAg, PD-1 inhibitor type, and hepatic arterial chemotherapy type (p>0.05) as shown in Table 6[21-25].
| Overall tumor progression | |||||
|---|---|---|---|---|---|
| No. | No. of progress | No. | No. of progress | ||
| Age (year) | <50 | 44 | 10 | 0.588 (0.263-1.316) | 0.197 |
| ≥50 | 114 | 38 | 1 | ||
| Gender | Male | 130 | 40 | 1.111 (0.451-2.734) | 0.819 |
| Female | 28 | 8 | 1 | ||
| AFP (ng/ml) | <5 | 32 | 6 | 0.684(0.427-1.095) | 0.113 |
| 5-400 | 64 | 20 | / | ||
| ≥400 | 72 | 22 | / | ||
| HbeAg | - | 122 | 36 | 1.194 (0.540-2.644) | 0.661 |
| + | 36 | 12 | 1 | ||
| Baseline HBV-DNA | <500 IU/ml | 58 | 18 | 1.050 (0.521-2.118) | 0.892 |
| ≥500 IU/ml | 100 | 30 | 1 | ||
| Baseline anti-virus | Yes | 66 | 18 | 0.775 (0.387-1.553) | 0.472 |
| No | 92 | 30 | 1 | ||
| Portal vein tumor thrombus | Yes | 48 | 14 | 0.920 (0.438-1.933) | 0.827 |
| No | 110 | 34 | 1 | ||
| lymph node metastasis | Yes | 22 | 12 | 0.300 (0.119-0.754) | 0.01 |
| No | 136 | 36 | 1 | ||
| metastasis | Yes | 12 | 2 | 2.300 (0.484-10.921) | 0.295 |
| No | 146 | 46 | 1 | ||
| Number of tumors | Diffuse | 36 | 16 | 1.283(0.963-1.710) | 0.089 |
| 1 | 54 | 16 | / | ||
| 2 | 8 | 0 | / | ||
| ≥3 | 60 | 16 | / | ||
| PD-1 inhibitor used | No | 82 | 32 | 2.400 (1.183-4.871) | 0.015 |
| Yes | 76 | 16 | 1 | ||
| PD-1 inhibitor type | Karelizumab | 48 | 14 | 2.225 (0.894-5.534) | 0.085 |
| Xindilizumab | 12 | 0 | / | ||
| Trepril monoclonal antibody | 16 | 2 | / | ||
| HBV reactivation | Yes | 10 | 4 | 1.576 (0.424-5.860) | 0.497 |
| No | 148 | 44 | 1 | ||
| Operation | None | 112 | 36 | 1.191 (0.771-1.842) | 0.431 |
| Other | 14 | 4 | / | ||
| ECOG | Liver radiofrequency ablation | 32 | 8 | / | |
| 0 | 36 | 12 | 1.194 (0.540-2.644) | 0.661 | |
| ≥1 | 122 | 36 | 1 | ||
Table 6: Analysis of relevant factors for overall tumor progression.
The etiology of liver cancer is complex, highly malignant, and difficult to treat. Although significant progress has been made in recent years, the current use of various treatment methods and drugs has limited effectiveness in prolonging the survival period of patients with advanced liver cancer. Currently, the overall 5 y survival rate of liver cancer patients in China is <15 %[25-30]. Although early diagnosis is crucial for the effective treatment and prolongation of life expectancy of liver cancer, due to the clinical asymptomatic nature of early liver cancer and the lack of liver cancer monitoring programs in many regions of the world, most patients have entered the mid to late stage once diagnosed, losing the opportunity for curative treatment such as radical resection and liver transplantation. Therefore, systemic therapies such as hepatic arterial chemotherapy, radiation therapy, molecular targeted therapy, and immune checkpoint inhibitor therapy have become the standard treatment methods for patients with advanced liver cancer who have lost surgical opportunities[31-34].
In clinical practice, TACE and HAIC are often used as treatment methods for advanced liver cancer, which can reduce tumor recurrence and prolong patient survival. TACE has become the standard for treating patients with large or multiple tumors, or small tumors that cannot be removed or percutaneous ablation[35-40]. The median survival period of patients after TACE treatment is 16-45 mo in the early stage (BCLC 0-A), 15.6-26.3 mo in the middle stage (BCLC B), and 6.8-13.6 mo in the late stage (BCLC C)[41-46]. The median survival period after HAIC is (2.8-15.9) mo [47-50].
A study targeting patients with unrespectable advanced liver cancer suggests that as the number of TACE treatments increases[51-54], the proportion of patients with complete and partial remission gradually decreases, and the proportion of patients with disease progression gradually increases. Therefore, it is urgent to seek new combined treatment options.
Related studies have shown that the combination of PD-1 inhibitors and HAIC can improve patient prognosis and prolong survival. The most commonly used hepatic arterial chemotherapy in clinical practice is TACE[55-57]. However, this study did not include patients treated with TACE. This is the first study to compare the efficacy of simple hepatic arterial chemotherapy (including TACE and HAIC) and hepatic arterial chemotherapy (including TACE and HAIC) combined with PD-1 inhibitors in the treatment of HBV reactivation and efficacy analysis in HBV related liver cancer.
As a local method, hepatic arterial chemotherapy can control intrahepatic lesions. Unfortunately, it is not as effective in controlling extrahepatic metastasis. Anti PD-1 therapy can stimulate the systemic immune response, which may compensate for the limitations of single therapy for hepatic arterial chemotherapy. The results of this experiment demonstrate that the combination of PD-1 inhibitors can reduce the risk of distant metastasis in patients (OR, 3.281 [95 % CI, 1.226-8.779], p=0.018), and the combination of the two has positive significance in delaying intrahepatic growth of tumors (OR, 2.296 [95 % CI, 1.098-4.803], p=0.027). Lymph node metastasis (OR, 0.300 [95 % CI, 0.119-0.754], p=0.010) is also a risk factor for tumor progression, which may be related to patients with lymph node metastasis often reaching advanced stages of cancer, making disease progression more difficult to control.
The risk of HBV reactivation is high in patients with HBV associated liver cancer, which has been identified as an adverse prognostic factor for overall survival[58]. According to reports, the mortality rate associated with HBV is 20 %-30 %[59]. In addition to the direct cause of death, the deterioration of liver function caused by HBV reactivation may lead to treatment interruption in liver cancer patients, which has a negative impact on their prognosis. Therefore, during the treatment of HBV related liver cancer patients, preventive antiviral therapy is recommended regardless of HBV DNA levels[60]. This experiment also demonstrated that baseline antiviral therapy is effective in delaying tumor progression (OR, 0.459 [95 % CI, 0.214-0.984], p=0.045).
Virological factors, anti-tumor cytotoxic drugs, anti-rejection therapy after tissue and organ transplantation, immunosuppressive therapy for non-tumor diseases, surgery, and other factors may all cause HBV reactivation. TACE, as a common treatment method for advanced liver cancer patients who have lost surgery opportunities, can severely inhibit the immune function of primary liver cancer patients, thereby breaking the original balance of immune function within the body. The rapid replication of HBV cells leads to reactivation of HBV. Although studies have confirmed that blocking the PD-1 signaling pathway can inhibit the replication of HBV virus, there are still reports of HBV reactivation in patients using PD-1 inhibitors. The results of this study indicate that the incidence of HBV reactivation and ALT elevation is low in both groups of patients, and no significant differences were observed. This indicates that on the basis of using hepatic arterial chemotherapy for liver cancer, the combination of PD-1 inhibitors does not increase the risk of HBV reactivation in patients.
This study has some limitations. Firstly, this is a retrospective study. Prospective cohort studies are needed to increase data. Secondly, the total number of patients in this experimental study is relatively small, and it is necessary to expand the sample size to increase the credibility of the experimental results.
Authors’ contribution:
Wenqian Qi and Yiting Liu have contributed equally to this study.
Funding:
This work was supported by Jilin Provincial Department of Science and Technology Project 20230203185 and Jilin Provincial Department of Finance SY2021SCZ2.
References
- Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol 2017;3(12):1683-91.
[Crossref] [Google Scholar] [PubMed]
- Liang Y, Luo X, Wang D. The effect of entecavir combined with sorafenib on the efficacy of TACE treatment for hepatocellular carcinoma patients. Medical 2019;14(6):11-25.
- Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37(3):212-20.
[Crossref] [Google Scholar] [PubMed]
- Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology 2014;60(5):1697-707.
[Crossref] [Google Scholar] [PubMed]
- Finn RS, Ryoo BY, Merle P, Bouattour M, Lim HY, Breder V, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol 2020;38(3):193–202.
[Crossref] [Google Scholar] [PubMed]
- Chinese Medical Association Hepatology Branch, Chinese Medical Association Infectious Disease Branch Guidelines for the Prevention and Treatment of Chronic Hepatitis B (updated in 2015). J Clin Hepatobiliary Dis 2015;31(12):1941-60.
- Huang G, Lai EC, Lau WY, Zhou WP, Shen F, Pan ZY, et al. Posthepatectomy HBV reactivation in hepatitis B–related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg 2013;257(3):490-505.
[Crossref] [Google Scholar] [PubMed]
- Tang TJ, Kwekkeboom J, Mancham S, Binda RS, de Man RA, Schalm SW, et al. Intrahepatic CD8+ T-lymphocyte response is important for therapy-induced viral clearance in chronic hepatitis B infection. J Hepatol 2005;43(1):45-52.
[Crossref] [Google Scholar] [PubMed]
- Fioravanti J, Di Lucia P, Magini D, Moalli F, Boni C, Benechet AP, et al. Effector CD8+ T cell-derived interleukin-10 enhances acute liver immunopathology. J Hepatol 2017;67(3):543-8.
[Crossref] [Google Scholar] [PubMed]
- Benechet AP, Iannacone M. Determinants of hepatic effector CD8+ T cell dynamics. J Hepatol 2017;66(1):228-33.
[Crossref] [Google Scholar] [PubMed]
- Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1: PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol 2007;178(5):2714-20.
[Crossref] [Google Scholar] [PubMed]
- Mei J, Li SH, Li QJ, Sun XQ, Lu LH, Lin WP, et al. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma 2021;8:167-76.
[Crossref] [Google Scholar] [PubMed]
- Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16(10):589-604.
[Crossref] [Google Scholar] [PubMed]
- Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol 2019;70(2):284-93.
[Crossref] [Google Scholar] [PubMed]
- Shi J, Zhang J, Wang F. Immunopathogenesis and antiviral treatment strategies of HBV infection. Chin J Viral Dis 2017;7(3):161-6.
- Rehermann B, Thimme R. Insights from antiviral therapy into immune responses to hepatitis B and C virus infection. Gastroenterology 2019;156(2):369-83.
[Crossref] [Google Scholar] [PubMed]
- Li X, Xu D. Research progress on the immunopathological pathogenesis of HBV related chronic and acute liver failure. J Pract Liver Dis 2015;18(3):317-20.
- Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, et al. Incubation phase of acute hepatitis B in man: Dynamic of cellular immune mechanisms. Hepatology 2000;32(5):1117-24.
[Crossref] [Google Scholar] [PubMed]
- Xiaojing L, Minghui C. Study on the role of NK cells in HBV infection. Chin J Exp Clin Virol 2016;30(5):439-43.
- Sun Li, Gao H, Zhao P. Research progress on the metabolic activity of natural killer cells in patients with chronic hepatitis B. J Med People's Liberation Army 2017;42(7): 656-60
- Li Z, Chen G, Cai Z, Dong X, Qiu L, Xu H, et al. Genomic and transcriptional profiling of tumor infiltrated CD8+ T cells revealed functional heterogeneity of antitumor immunity in hepatocellular carcinoma. Oncoimmunology 2019;8(2):e1538436.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Wang Q, Zhao P, Hu X, Jiang Y. Effects of entecavir on peripheral blood lymphocyte profiles in chronic hepatitis B patients with suboptimal responses to adefovir. Clin Exp Pharmacol Physiol 2014;41(7):514-23.
[Crossref] [Google Scholar] [PubMed]
- Bell CC, Faulkner L, Martinsson K, Farrell J, Alfirevic A, Tugwood J, et al. T-cells from HLA-B* 57: 01+ human subjects are activated with abacavir through two independent pathways and induce cell death by multiple mechanisms. Chem Res Toxicol 2013;26(5):759-66.
[Crossref] [Google Scholar] [PubMed]
- Sherman AC, Trehanpati N, Daucher M, Davey RT, Masur H, Sarin SK, et al. Augmentation of hepatitis B virus-specific cellular immunity with programmed death receptor-1/programmed death receptor-L1 blockade in hepatitis B virus and HIV/hepatitis B virus coinfected patients treated with adefovir. AIDS Res Hum Retroviruses 2013;29(4):665-72.
[Crossref] [Google Scholar] [PubMed]
- Yang HC, Kao JH. Persistence of hepatitis B virus covalently closed circular DNA in hepatocytes: Molecular mechanisms and clinical significance. Emerg Microbes Infect 2014;3(1):1-7.
- Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology 2015;479:672-86.
[Crossref] [Google Scholar] [PubMed]
- Werle–Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004;126(7):1750-8.
[Crossref] [Google Scholar] [PubMed]
- Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol 2016;64(1):S71-83.
[Crossref] [Google Scholar] [PubMed]
- Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy: Report of a prospective study. Gastroenterology 1991;100(1):182-8.
[Crossref] [Google Scholar] [PubMed]
- Terrault NA, Lok AS, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67(4):1560-99.
[Crossref] [Google Scholar] [PubMed]
- Riveiro-Barciela M, Gubern P, Roade L, Abrisqueta P, Carreras MJ, Farriols A, et al. An electronic alert system increases screening for hepatitis B and C and improves management of patients with haematological disorders. Sci Rep 2020;10(1):3038.
- Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug-induced hepatic injuries: A French population-based study. Hepatology 2002;36(2):451-5.
[Crossref] [Google Scholar] [PubMed]
- Norvell JP, Blei AT, Jovanovic BD, Levitsky J. Herpes simplex virus hepatitis: An analysis of the published literature and institutional cases. Liver Transpl 2007;13(10):1428-34.
[Crossref] [Google Scholar] [PubMed]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007;27(4):670-84.
[Crossref] [Google Scholar] [PubMed]
- Goodman A, Patel SP, Kurzrock R. PD-1–PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol 2017;14(4):203-20.
[Crossref] [Google Scholar] [PubMed]
- Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010;138(2):682-93.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology 2008;134(7):1938-49.
[Crossref] [Google Scholar] [PubMed]
- Boni C, Fisicaro P, Valdatta C, Amadei B, di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81(8):4215-25.
[Crossref] [Google Scholar] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci 2002;99(19):12293-7.
[Crossref] [Google Scholar] [PubMed]
- Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci 2004;101(49):17174-9.
[Crossref] [Google Scholar] [PubMed]
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009;458(7235):206-10.
[Crossref] [Google Scholar] [PubMed]
- Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77(1):68-76.
[Crossref] [Google Scholar] [PubMed]
- Guo J, Wang S, Han Y, Jia Z, Wang R. Effects of transarterial chemoembolization on the immunological function of patients with hepatocellular carcinoma. Oncol Lett 2021;22(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1α and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res 2016;8(4):297-302.
[Crossref] [Google Scholar] [PubMed]
- European Association for the Study of the Liver. Electronic address: Easloffice@ easloffice. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236.
- Sarin S, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int 2016;10:1-98.
[Crossref] [Google Scholar] [PubMed]
- Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int 2017;11:317-70.
[Crossref] [Google Scholar] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492-502.
[Crossref] [Google Scholar] [PubMed]
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19(7):940-52.
[Crossref] [Google Scholar] [PubMed]
- Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, et al. Hepatitis B virus–specific and global T-cell dysfunction in chronic hepatitis B. Gastroenterol 2016;150(3):684-95.
[Crossref] [Google Scholar] [PubMed]
- Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol 2017;43(3):604-11.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Zhong Y, Peng S, Zhou X, Gan X. Efficacy and safety of PD1/PDL1 blockades vs. docetaxel in patients with pretreated advanced non-small-cell lung cancer: A meta-analysis. Onco Targets Ther 2018;11:8623-32.
[Crossref] [Google Scholar] [PubMed]
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515(7528):558-62.
[Crossref] [Google Scholar] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372(4):311-9.
- Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep 2018;20:1-7.
- Jang JW. Hepatitis B virus reactivation in patients with hepatocellular carcinoma undergoing anti-cancer therapy. World J Gastroenterol 2014;20(24):7675.
[Crossref] [Google Scholar] [PubMed]
- Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: A prospective study of 626 patients with identification of risk factors. J Med Virol 2000;62(3):299-307.
[Crossref] [Google Scholar] [PubMed]
- Jiang S, Li W, Lv Y, Wei J, Liu Y. The efficacy of entecavir combined with transcatheter arterial chemoembolization in patients with liver cancer complicated with hepatitis B and the impact of hepatitis B virus reactivation. Oncology 2018;8(2):215-8.
- Peck-Radosavljevic M, Kudo M, Raoul JL, Lee HC, Decaens T, Heo J, et al. Outcomes of patients (pts) with hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE): Global OPTIMIS final analysis.
- Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Jpn J Clin Oncol 2018;48(2):103-14.
[Crossref] [Google Scholar] [PubMed]