- *Corresponding Author:
- Y. H. Sun
Department of Oncology, The First Affiliated Hospital of Hunan University of Traditional Chinese Medicine, Changsha, Hunan 410000, China
E-mail: 33468088@163.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “176-183” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the reversal effect of Fuzheng oral liquid on anlotinib resistance in non-small cell lung cancer cells and its mechanism is the objective of the study. 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide assay and flow cytometric assay were performed to evaluate the effects of anlotinib on the proliferation and apoptotic cycles of adenocarcinomic human alveolar basal epithelial cell line A549 cells, respectively. Western blot was performed to detect the expression levels of unfolded protein response, endoplasmic reticulum-associated degradation, E-cadherin, vimentin, beta-catenin, transcription factor 4 and Axin in anlotinib-resistant cell line A549/AL3818 cells. Anlotinib-resistant A549 cells were successfully constructed and named as A549/AL3818. Fuzheng oral solution had proliferation inhibition, apoptosis induction and cycle blocking effects on A549/AL3818 cells. High dose of Fuzheng oral liquid was able to inhibit unfolded protein response and endoplasmic reticulum-associated degradation, expression in A549/ AL3818 cells and suppress wingless-related integration signaling pathway and epithelial-mesenchymal transition progression in A549/AL3818 cells. The inhibitory effect of Fuzheng oral liquid on winglessrelated integration signaling pathway and epithelial-mesenchymal transition progression was the same as that of paired box protein 6. Fuzheng oral liquid inhibited epithelial-mesenchymal transition progression through regulating unfolded protein response-endoplasmic reticulum-associated degradation signaling pathway, thereby reversing the resistance of non-small cell lung cancer cells to anlotinib.
Keywords
Fuzheng oral liquid, non-small cell lung cancer, epithelial-mesenchymal transition, wingless-related integration signaling pathway, anlotinib
In recent years, molecularly targeted drugs have made remarkable achievements in the treatment of Non-Small Cell Lung Cancer (NSCLC), especially Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor (EGFR-TKI). In particular, the clinical application of EGFR-TKI has led to breakthroughs in the treatment of NSCLC, significantly improving the outcome of advanced NSCLC[1]. However, the emergence of acquired drug resistance has improved the 5 y survival rate to only 19.3 %[2]. Therefore, the acquired drug resistance of NSCLC targeted therapies has once again put the current lung cancer treatment in a lost way and it is imperative to discover new, highly efficient and less toxic targeted therapeutic strategies.
Anlotinib (AL 3818) is a new small molecule multi-target TKI, developed independently in China. Anlotinib resistance in NSCLC cells is the reason for the decrease in response rate and survival rate of clinical NSCLC patients treated with anlotinib[3].
However, the mechanism of resistance involved in the treatment of NSCLC with anlotinib is very complex. Epithelial-Mesenchymal Transition (EMT) is involved in resistance to antitumor chemotherapeutic agents, leading to altered cell polarity, loss of intercellular adhesion and destruction of tumor basement membrane and extracellular matrix, ultimately leading to ineffective chemotherapeutic agents[4].
In view of the evolution of the disease mechanism before and after targeted therapy for NSCLC, the group believes that "deficiency of positive qi and transmission of residual toxins" is the basic disease mechanism of acquired drug resistance to targeted drugs, and "supporting positive and detoxifying toxins" is the main treatment to improve drug resistance to targeted therapy for NSCLC. Fuzheng oral liquid is an in hospital preparation of Chinese herbs, Jun Zi Tang, Sha Shen Mai Dong Tang and Tao Hong Si Wu Tang, which is clinically safe and effective in long-term application.
Although it has been reported that Fuzheng oral liquid has a reverse effect on the resistance of NSCLC to chemotherapy, its mechanism is still unclear[5]. This study investigated the mechanism of reversal of resistance to anlotinib by Fuzheng oral liquid in human NSCLC cells.
Materials and Methods
Drugs and cells:
The NSCLC cell line A549 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 with 10 % fetal bovine serum and 1 % penicillin in a saturated humidity incubator at 37°, 5 % Carbon dioxide (CO2). The cells were in log phase.
3-(4, 5-Dimethylthiazolyl-2)-2,5-Diphenyltetrazolium Bromide (MTT) assay to assess cell proliferation:
A549 cells were inoculated into 96-well plates and cultured overnight to about 80 % fusion. The medium containing different concentrations of anlotinib (0.25, 0.5, 1, 2, 4, 8 μg/ml) was replaced and incubated for 48 h. After that, the medium containing MTT (0.5 mg/ ml) was replaced and continued to be incubated for 4 h and subsequently removed. The medium was spiked with 200 μl of Dimethyl Sulfoxide (DMSO) and the Optical Density (OD) 490 values were detected. The cell growth inhibition rate was calculated according to the following formula: Inhibition rate=(OD490Control-OD490Treatment)/OD490Control×100 %.
Flow cytometry detection of apoptosis:
Groups of cells were collected in 1.5 ml centrifuge tubes and 10 μl of fluorescently labeled Annexin V and 5 μl of Propidium Iodide (PI) were added to each tube and incubated for 10 min at room temperature. Approximately 200 μl of cell suspension was added to a flow tube containing 2 ml of Phosphate Buffered Saline (PBS) and detected by flow cytometry (BD).
Flow cytometry detection in cell cycle of cells:
Treated cells were rinsed with PBS and fixed in 70 % ethanol at 4°. After fixation overnight, they were rinsed again with PBS and stained with appropriate amounts of PI (Sigma, United States of America (USA)) and Ribonuclease (RNase) A (MBI Fermentas) for 30 min [40×PI (2 mg/ml):100×RNase (10 mg/ml):1×PBS]. Cell cycles were analyzed using a BD FACSCalibur™ flow cytometry system (BD Biosciences) and data were analyzed using CellQuest Pro software (BD Biosciences).
Protein immunoblotting assay:
Total tissue or cellular proteins were extracted using the nuclear and cytoplasmic protein extraction kit (Beyotime, China). 40 μg of protein was subjected to Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), transferred membrane, Tris Buffered Saline+Tween 20 (TBST) containing 5% skimmed milk powder (triple buffered saline/0.1 % Tween-20, pH 7.4) blocked for 1 h at room temperature and then blocked separately with Unfolded Protein Response (UPR) (1:1000), Endoplasmic Reticulum Associated Degradation (ERAD) (1:1000), E-cadherin (1:1000), Vimentin (1:1000), beta (β)-catenin (1:1000), Transcription Factor 4 (TCF4) (1:1000), Axin (1:1000) and Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) (1:1000) (Abcam, USA) primary antibodies and horseradish peroxidase conjugated rabbit Immunoglobulin G (IgG) antibody (1:5000, Abcam, USA) as secondary antibodies. Enhanced Chemiluminescence (ECL) was used for color development.
Transfection:
The plasmid Cloning Deoxyribonucleic Acid Paired box protein 6 (pCDNAPax-6), Pax-6 small interfering Ribonucleic Acid (siRNAs), pCDNA UPR and pCDNA ERAD plasmids were purchased from Shanghai Jima Biotechnology Co. The relevant plasmids were transfected into A549 cells using Lipofectamine™ 2000 (Invitrogen) transfection reagent and after 48 h, the transfected cells were collected for further assay and analysis.
Chromatin Immunoprecipitation (ChIP):
Each group of cells were fixed with 0.8 % methylal, collected and then fragmented by ultrasonication. Pax-6 antibody or IgG antibody was added to the mixture and incubated overnight at 4°. Subsequently, protein A/G agarose bead precipitation complex (Abcam) was added to elute the protein/DNA complexes. The reaction system was supplemented with Proteinase K and incubated at 65° for 2 h. UPR enrichment was determined by quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR).
Real-time RT-PCR:
Total RNA was collected from ChIP assay samples using RNA extraction kit (Takara) and extracted RNA was quantified using a NanoDrop spectrophotometer (NanoDrop Technologies). After reverse transcription, SYBR® Premix Ex Taq™ (Takara) and Applied Bio-Rad CFX96 sequence detection system (Applied Biosystems) were used for real-time PCR detection and relative expression levels were calculated using the 2−ΔΔCt method. UPR primers: Forward-TGATAAAATTTTTGATTTTTACGT. GAPDH: Forward-CAATGACCCCTTCATTGACC and reverse-GAGAAGCTTCCCGTTCTCAG.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 20.0 software was used for statistical analysis. One-way Analysis of Variance (ANOVA) was used to compare differences between groups for continuous variables and Least Significant Difference (LSD) test was used for normally distributed data, p<0.05 was considered a statistically significant difference.
Results and Discussion
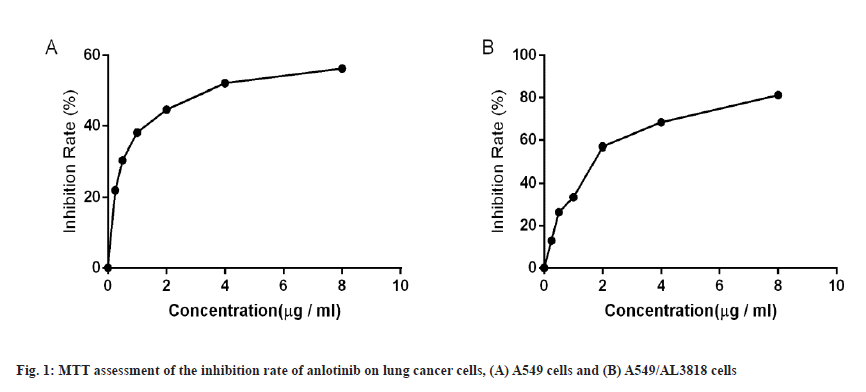
To investigate the effect of Fuzheng oral liquid on chemotherapy resistance and anlotinib-resistant A549 cell line was constructed in this study. The half-maximal Inhibitory Concentration (IC50) value of anlotinib inhibition of A549 was assessed by MTT and the inhibition rate of anlotinib on A549 cells was increased significantly as the concentration increased from 0.25 μg/ml to 1 μg/ml and became flat when the concentration of anlotinib exceeded 4 μg/ml. The IC50 value calculated from the inhibition curve was 1.39 μg/ ml as shown in fig. 1A.
A549 cells were incubated in complete medium at a concentration of 1 μg/ml of anlotinib. The survival status of the cells was observed every 12 h. When the cell death rate reached 50 %, the medium was immediately removed and replaced with fresh culture medium without anlotinib. The cell survival status was checked every 12 h until the cells were normalized and stable, and then placed in 1 μg/ml of anlotinib medium again. Repeat the procedure until the cells resume stable proliferation in the drug medium and their growth rate was not significantly different from that in drug-free culture, and then assess the inhibition rate of anlotinib by MTT. When the concentration of anlotinib increased from 0.25 μg/ml to 2 μg/ml, the inhibition rate was increased significantly and became stable when the concentration of anlotinib was greater than 4 μg/ml. According to the inhibition curve, at this time, the IC50 value of anlotinib on cells was 6.53 μg/ ml, which was 4.69 times higher than the inhibition of A549 by anlotinib (fig. 1B). The results indicated that an anlotinib-resistant cell line strain was successfully constructed and named as A549/AL3818.
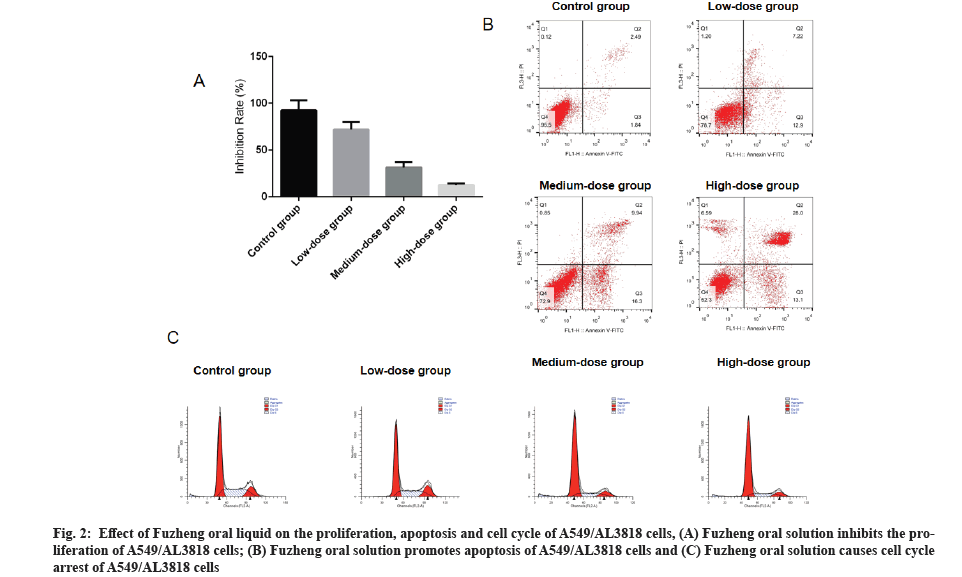
Effect of Fuzheng oral solution on proliferation, apoptosis and cell cycle of A549/AL3818 pretreated with anlotinib was described below. A549/AL3818 cells pretreated with 1.39 μg/ml anlotinib was added with three concentrations of Fuzheng oral solution, which were low (5 ml Fuzheng oral solution plus 95 ml Polyethylene-Glycol modified (PEGylated) preparation), medium (10 ml Fuzheng oral solution plus 90 ml PEGylated preparation), and high (20 ml Fuzheng oral solution plus 80 ml PEGylated preparation). The cell viability was decreased significantly with increasing concentration of Fuzheng oral solution and the results showed that Fuzheng oral solution was able to inhibit the proliferation of A549/AL3818 cells in a dose-dependent manner (fig. 2A).
Fig. 2: Effect of Fuzheng oral liquid on the proliferation, apoptosis and cell cycle of A549/AL3818 cells, (A) Fuzheng oral solution inhibits the proliferation of A549/AL3818 cells; (B) Fuzheng oral solution promotes apoptosis of A549/AL3818 cells and (C) Fuzheng oral solution causes cell cycle arrest of A549/AL3818 cells.
The apoptosis rates of A549/AL3818 cells in the control, low, medium and high dose groups were 12.96 %, 26.25 %, 38.73 % and 52.53 %, respectively, indicating that Fuzheng oral solution was able to induce apoptosis in A549/AL3818 cells (fig. 2B). In addition, the percentage of resting phase (G0)/Growth 1 phase (G1) increased and the percentage of Growth 2 phase (G2)/Mitotic phase (M) decreased greatly as the concentration of Fuzheng oral solution increased, and the results indicated that the Fuzheng oral solution caused A549/AL3818 cells to undergo G0/G1 phase block (fig. 2C).
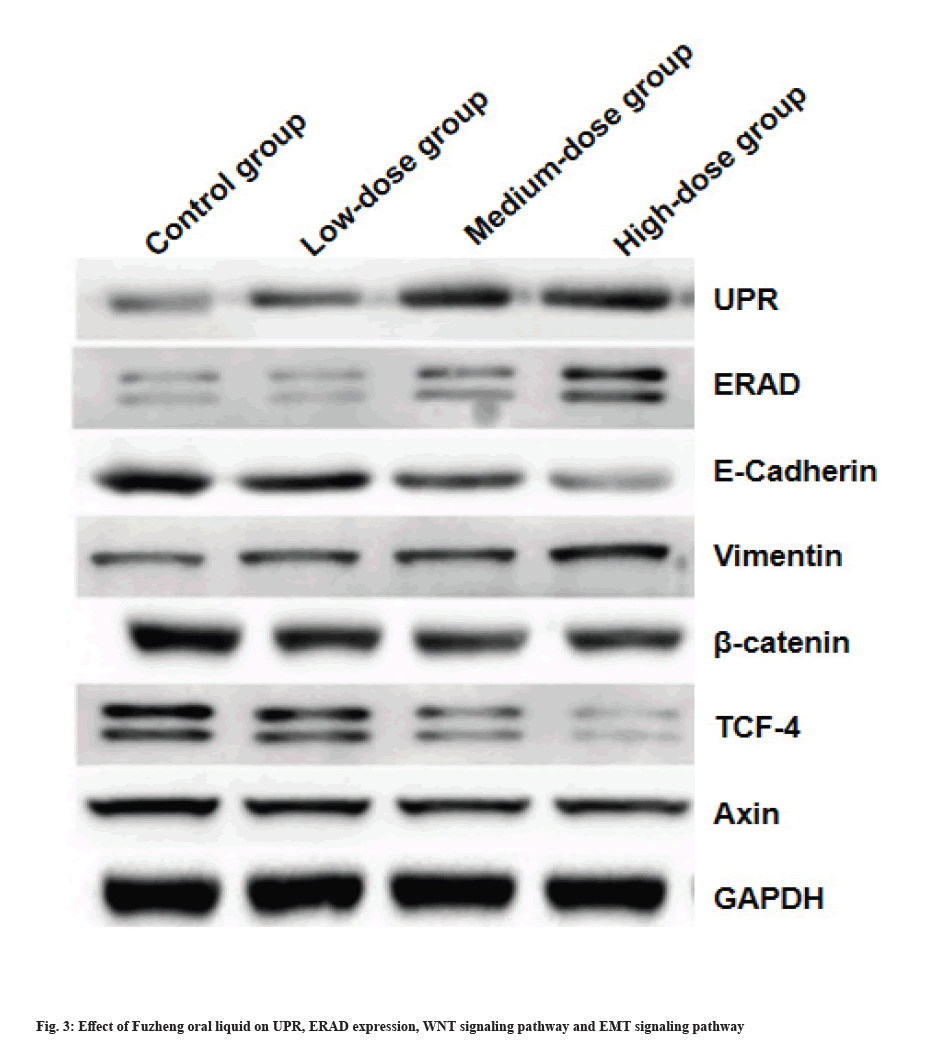
Effect of Fuzheng oral liquid on UPR and ERAD expression, Wingless-Related Integration (WNT) signaling pathway and EMT signaling pathway was explained here. The expression levels of UPR, ERAD and vimentin increased significantly with increasing concentration of Fuzheng oral solution, while E-cadherin, β-catenin, TCF4 and Axin were significantly down-regulated. Except for TCF4, the expression of other proteins showed a dose-dependent relationship with Fuzheng oral solution (fig. 3).
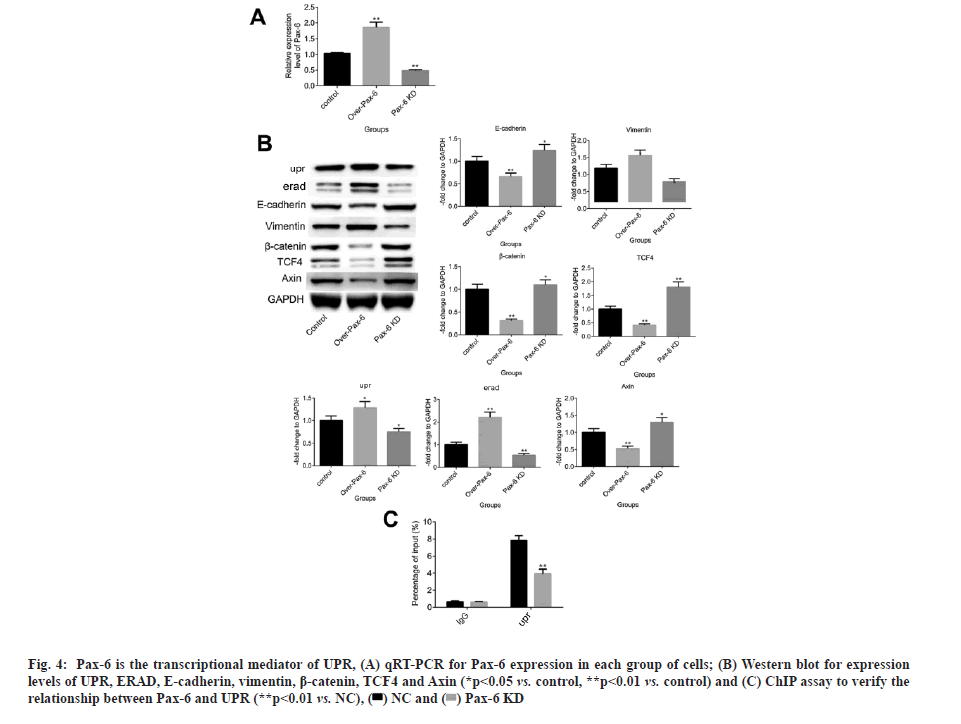
Pax-6 is a transcriptional mediator of the UPR and it is a key transcription factor for Cytosine and Guanine separated by only one phosphate group (CpG) island methylation[6]. To investigate the transcriptional effects of Pax-6 on UPR, A549/AL3818/Pax-6 OE cell line with Pax-6 overexpression and A549/AL3818/Pax-6 KD cell line with Pax-6 knockdown were constructed. qRT-PCR results showed that A549/AL3818/Pax-6 OE and A549/AL3818/Pax-6 KD cell lines were successfully constructed. AL3818/Pax-6 KD cell lines were successfully constructed. Protein immunoblotting experiments showed that UPR, ERAD and vimentin expression were significantly upregulated and E-cadherin, β-catenin, TCF4 and Axin expression were significantly downregulated in A549/AL3818/Pax-6 OE cells compared with the control. The opposite was observed in A549/AL3818/Pax-6 KD cells results (fig. 4A and fig. 4B). The results suggest that Pax-6 can inhibit the WNT signaling pathway and thus prevent the progression of EMT. ChIP assay experiments showed that the proportion of UPR efflux was significantly reduced in Pax-6 KD A549 cells. The results suggest that Pax-6 is a potential transcriptional mediator of UPR (fig. 4C).
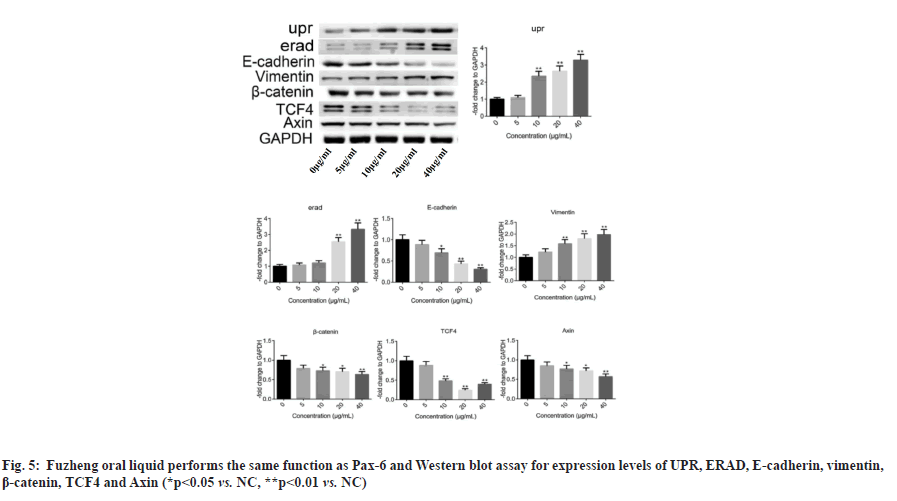
Fig. 4: Pax-6 is the transcriptional mediator of UPR, (A) qRT-PCR for Pax-6 expression in each group of cells; (B) Western blot for expression levels of UPR, ERAD, E-cadherin, vimentin, β-catenin, TCF4 and Axin (*p<0.05 vs. control, **p<0.01 vs. control) and (C) ChIP assay to verify the relationship between Pax-6 and UPR (**p<0.01 vs. NC), .
.
Fuzheng oral liquid can perform the same function as Pax-6. To further investigate the anti-drug resistance effect of Fuzheng oral solution on A549 cells, A549/AL3818 cells with Pax-6 overexpression (A549/AL3818/Pax-6 OE), A549 cells with UPR overexpression (A549/UPR OE) and A549 cells with ERAD overexpression (A549/ERAD OE) were constructed in this study. Empty plasmid (NC) was used as the control and A549 cells cultured with 40 μg/ml Fuzheng oral solution were used as the parallel group. Compared with NC, the expression levels of UPR,ERAD and vimentin were increased and E-cadherin, β-catenin, TCF4, and Axin were significantly down-regulated in the four groups (fig. 5), which indicated that the biological functions of Pax-6, UPR, ERAD and Fuzheng oral solution on WNT signaling pathway and EMT progression were the same.
NSCLC is a common respiratory tract tumor and is one of the top three malignancies worldwide. In China, nearly 250 000 people are newly diagnosed with NSCLC each year, accounting for 1/5th of all newly diagnosed NSCLC patients worldwide[7]. Currently, chemotherapy is still the main effective treatment for NSCLC in clinical practice and anlotinib is mostly used to treat patients with progressive NSCLC. However, the efficiency of anlotinib in progressive NSCLC is still only 10 %-15 %[8]. Resistance of NSCLC cells to anlotinib is the main factor leading to treatment failure with anlotinib in NSCLC patients. Therefore, there is an urgent need to clarify the mechanism of drug resistance and to find new strategies to address this problem. In this study, Fuzheng oral solution has been reported to effectively inhibit tumor cell proliferation and metastasis[9], and we used it to explore the anti-drug resistance effect in A549/AL3818 cells. The results of MTT assay, apoptosis assay and cell cycle assay showed that Fuzheng oral solution enhanced the inhibitory effect of anlotinib on the growth of A549/ AL3818 cells.
Multiple factors have been reported to contribute to resistance to anlotinib in NSCLC, including the DNA repair system, dysfunction of the uptake transporter and the inherent organophosphorus system of NSCLC cells[10]. Currently, EMT is reported to be associated with NSCLC resistance to anlotinib[11,12]. EMT occurs around tumor cells at lymphatic vessels and vascular junctions, and contributes to the invasion of tumor cells into lymphatic vessels or blood vessels or distant organs through bypass reconstruction. EMT progression is induced by activation of the WNT signaling pathway, which is activated by a variety of growth factors, such as Transforming Growth Factor-β (TGF-β), Epidermal Growth Factor (EGF), and Platelet-Derived Growth Factor (PDGF)[13,14]. In this study, we found that the expression of the WNT signaling pathway downstream proteins, TCF-4 and Axin in A549/AL3818 cells was down-regulated in a dose-dependent manner after intervention with different concentrations of Fuzheng oral solution. The tumor cell epithelium-related genes, E-cadherin and β-catenin were also expressed in a dose-dependent manner, while the mesenchymal-related gene vimentin was expressed in a dose-dependent manner, suggesting that Fuzheng oral solution could inhibit the WNT signaling pathway and reverse the progression of EMT. In addition, MTT experiments showed that Fuzheng oral solution enhanced the inhibitory effect of anlotinib on the growth of A549/AL3818 cells. Therefore, the enhanced inhibitory effect of Fuzheng oral solution on anlotinib was closely related to EMT progression and WNT signaling pathway was involved in the inhibition of EMT progression by Fuzheng oral solution, and the regulatory mechanism of WNT signaling pathway by Fuzheng oral liquid still needs to be further investigated.
Cancer cells are often exposed to hypoxia, nutrient deficiencies, oxidative stress and other metabolic dysregulation, resulting in endoplasmic reticulum stress and UPR activation. The UPR is a complex signaling pathway that regulates multiple gene expression at the transcriptional and translational levels and is a fundamental mechanism for maintaining cellular homeostasis[15,16]. Depending on the duration and extent of endoplasmic reticulum stress, the UPR can provide survival signals by activating the adaptive and anti-apoptotic pathway, ERAD or by inducing the cell death program Endoplasmic Reticulum Stress Induced Apoptosis (ERSIA). The UPR-regulated ERAD plays an important role in the proliferation, metastasis and drug resistance mechanisms of various tumor cells. In this study, we found that Fuzheng oral liquid promoted UPR and ERAD expression, suggesting that the anticancer effect of Fuzheng oral liquid was associated with upregulation of UPR. Pax-6 is a key transcriptional mediator of CpG island methylation. The present study showed that Pax-6 is a transcriptional mediator of UPR expression. In this study, we found that overexpression of UPR or ERAD and Fuzheng oral liquid intervention both upregulated Pax-6 expression and inhibited EMT progression. In addition, this study also found that Fuzheng oral solution and Pax-6 have the same effect on UPR.
In this study, we found that Fuzheng oral solution enhanced the growth inhibitory effect of anlotinib on A549/AL3818 cells. Mechanistic studies showed that Fuzheng oral solution inhibited WNT signaling pathway and reversed EMT progression. Both Fuzheng oral solution and Pax-6 promoted UPR expression, which in turn upregulated ERAD expression and inhibited WNT signaling pathway. In future studies, the transcription-mediating effect of Fuzheng oral liquid on UPR and the demethylation function of UPR on ERAD should be investigated in depth to better understand the relationship between the anti-drug resistance effect of Fuzheng oral liquid and the UPR-ERAD-WNT signaling pathway. In conclusion, the findings of this study suggest that Fuzheng oral solution reverses EMT progression through modulating the UPR-ERAD-WNT signaling pathway, thereby reversing anlotinib resistance in A549/AL3818 cells.
Funding:
This study was supported by Hunan Provincial Clinical Medical Technology Innovation Guidance Project (No. 2020SK51405); Hunan Provincial Natural Science Foundation (No. 2021JJ30523) and Hunan University of Traditional Chinese Medicine Key Project (No. 2020XJJJ033).
Conflict of interests:
The authors declared no conflict of interest.
References
- Liu L, Wang C, Li S, Bai H, Wang J. Tumor immune microenvironment in epidermal growth factor receptor-mutated non-small cell lung cancer before and after epidermal growth factor receptor tyrosine kinase inhibitor treatment: A narrative review. Transl Lung Cancer Res 2021;10(9):3823-39.
[Crossref] [Google scholar] [PubMed]
- Tripathi SK, Biswal BK. SOX9 promotes epidermal growth factor receptor-tyrosine kinase inhibitor resistance via targeting β-catenin and epithelial to mesenchymal transition in lung cancer. Life Sci 2021;277:119608.
[Crossref] [Google scholar] [PubMed]
- Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The alter 0303 phase 3 randomized clinical trial. JAMA Oncol 2018;4(11):1569-75.
[Crossref] [Google scholar] [PubMed]
- Wu SJ, Arundhathi A, Wang HC, Chen CY, Cheng TM, Yuan SS, et al. Migration and invasion of NSCLC suppressed by the downregulation of Src/focal adhesion kinase using single, double and tetra domain anti-CEACAM6 antibodies. Transl Oncol 2021;14(7):101057.
[Crossref] [Google scholar] [PubMed]
- Chen JF. Efficacy of Fuzheng Hua phlegm anti-cancer formula combined with chemotherapy in the treatment of advanced drug-resistant non-small cell lung cancer. Chin J Traditi Chin Med Sci Technol 2017;24(4):478-9.
- Jin M, Gao D, Wang R, Sik A, Liu K. Possible involvement of TGF?β?SMAD?mediated epithelial?mesenchymal transition in pro?metastatic property of PAX6. Oncol Rep 2020;44(2):555-64.
[Crossref] [Google scholar] [PubMed]
- Daxing ZH, Xiaoming QI, Qinghua ZH. Combined double sleeve lobectomy and superior vena cava resection for non-small cell lung cancer with persistent left superior vena cava. Zhongguo Fei Ai Za Zhi 2015;18(11):718-20.
[Crossref] [Google scholar] [PubMed]
- Wen YX, Liang LJ, Chen T, Jiang XD. Advances in clinical application of anlotinib. Chin J Oncol Prev Treat 2019;26(14):979-85.
- Wang XZ, Ray HY, Du H. Clinical study of Fuzheng oral liquid combined with chemotherapy in the treatment of advanced lung cancer. Chin J Integr Tradit Chin West Med 1999;19(10):622.
- Xiang F, Yu W, Shen Y, Wu C, Wang Y. Effects of neoadjuvant chemotherapy on the quantitative expression of P-gp, LRP, MRP, GST-π in NSCLC and its clinical significance. Zhongguo Fei Ai Za Zhi 2007;10(5):398-405.
[Crossref] [Google scholar] [PubMed]
- Ntzifa A, Strati A, Kallergi G, Kotsakis A, Georgoulias V, Lianidou E. Gene expression in circulating tumor cells reveals a dynamic role of EMT and PD-L1 during osimertinib treatment in NSCLC patients. Sci Rep 2021;11(1):1-2.
[Crossref] [Google scholar] [PubMed]
- Li XF, Shen WZ, Jin X, Ren P, Zhang J. Let-7c regulated epithelial-mesenchymal transition leads to osimertinib resistance in NSCLC cells with EGFR T790M mutations. Sci Rep 2020;10(1):1-9.
- Ying Q, Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: An update. Ren Fail 2017;39(1):474-83.
[Crossref] [Google scholar] [PubMed]
- Hernández-Martínez R, Ramkumar N, Anderson KV. p120-catenin regulates WNT signaling and EMT in the mouse embryo. Proc Natl Acad Sci USA 2019;116(34):16872-81.
[Crossref] [Google scholar] [PubMed]
- Gu LF, Chen JQ, Lin QY, Yang YZ. Roles of mitochondrial unfolded protein response in mammalian stem cells. World J Stem Cells 2021;13(7):737-52.
[Crossref] [Google scholar] [PubMed]
- Khateb A, Ze’ev AR. Unfolded protein response in leukemia: From basic understanding to therapeutic opportunities. Trends Cancer 2020;6(11):960-73.
[Crossref] [Google scholar] [PubMed]