- *Corresponding Author:
- H. N. Shivakumar
Department of Pharmaceutics, Al-Ameen College of Pharmacy, Hosur Road, Bangalore-560 027, Department of Pharmaceutical Technology, K. L. E. S’s College of Pharmacy, Rajajinagar 2nd block, Bangalore-560010, India
E-mail: shivakumarhn@yahoo.co.in
| Date of Submission | 07 March 2005 |
| Date of Revision | 27 July 2005 |
| Date of Acceptance | 12 May 2006 |
| Indian J Pharm Sci,2006, 68 (3): 301-307 |
Abstract
Carbamazepine was complexed with β -cyclodextrin in an attempt to enhance the solubility features of the drug. Phase solubility studies revealed a linear relationship between carbamazepine solubility and b-cyclodextrin concentration. The value of the stability constant (405.42 M-1sub ) calculated from the phase solubility diagram indicated that the complexes were adequately stable. Carbamazepine-β -cyclodextrin complex prepared by kneading method was used to produce dispersible tablets. A 2 3sub factorial design was employed to investigate the effect of factors such as amount of binder, hardness and type of disintegrant on the tablet disintegration time and dissolution rate. Mathematical models containing only the significant factors influencing each response were generated using multiple linear regression and analysis of variance. The three main factors studied had a significant influence on both the response parameters. In addition to the main factors, the two-way interaction factors also showed a significant effect on the release rate. Type of disintegrant emerged as the main effect with the highest statistical significance affecting both the responses. Two formulations with a combination of factors within the experimental domain were developed and evaluated to validate the mathematical models. The predicted values were found to agree with the experimental values, confirming the forecasting ability of multi-linear regression and ANOVA.
Carbamazepine is an anticonvulsant drug widely used in the treatment of simple and complex seizures, trigeminal neuralgia, and bipolar affective disorder. The drug is practically insoluble in water (<200 μg/ml), and its absorption is dissolution rate limited [1]. Carbamazepine is reported to form inclusion complex with β-cyclodextrin (β-CD), which could improve its biological performance [2].Cyclodextrins are a group of structurally related saccharides that are formed by enzymatic cyclization of starch by a group of amylases termed glycosyl transferases. β-CD is extensively used to trap certain drug molecules inside its cavity and thereby modify their physicochemical and biological activity [3]. Methods such as kneading, neutralization, freeze drying, spray drying, and solvent evaporation have been cited in literature for preparation of drug-β-CD complexes [3].
As carbamazepine is the drug of choice in treatment of paediatric seizures [4], a dispersible tablet of the drug is highly recommended. In this context, an attempt has been made to complex carbamazepine and β-CD with the aim to enhance the drug solubility. The work was also focused to quantify the effect of various formulation and processing variables on the disintegration and dissolution rates of dispersible tablets containing carbamazepine β-CD complex using a 23 factorial design.
Materials and Methods
Carbamazepine was a gift sample from Micro Nova Laboratories Pvt. Ltd., Bangalore. β-cyclodextrin was generously gifted by M/s Cerestar Inc., Chicago, USA. All the other chemicals and reagents purchased from S. D. Fine Chemicals, Mumbai, were of laboratory grade.
Phase solubility studies
Phase solubility studies were carried out at room temperature (25°) in triplicate according to the method reported by Higuchi and Conners[5]. Excess amount of carbamazepine was added to distilled water containing various concentrations of β-CD (0.5-3 mM) in a series of stoppered conical flasks and shaken for 48 h on a rotary flask shaker. The suspensions were filtered through Whatman No. 1 filter paper and assayed for carbamazepine using a UV/Vis spectrophotometer (Shimadzu, UV 1601) at 285 nm against blanks prepared using same concentration of β-CD in distilled water.
Preparation of carbamazepine β-cyclodextrin complex
Solid complexes of carbamazepine-β-CD were prepared in 1:2 molar ratio following kneading method [3]. β-CD was mixed in a glass mortar along with water to obtain a homogeneous paste. The drug was slowly added to the paste and the mixture triturated for 1 h. During the process, the water content of the paste was empirically adjusted to maintain the consistency of the paste. The paste was dried at 45° for 48 h, pulverized and passed through sieve #100.
Evaluation of the complexes
An accurately weighed amount of the complex was dissolved in methanol and assayed for carbamazepine spectrophotometrically at 285 nm against blanks prepared using same concentration of β-CD in the methanol. Thin layer chromatography (tlc) has been used to a very limited extent to support the formation of inclusion comlex [6]. The complex and the reference standard solutions were spotted on tlc plates that were activated by heating at 105° for 1 h. The plates were developed in a chamber saturated with the solvent system comprising of ammonia and methanol in a ratio of 1.5:100. The plates were exposed to vapours of nitrous oxide and sprayed with naphthylethylene diamine. The Rf value of the complex was determined and compared with that of the reference standard.
Infrared spectrophotometry (IR) has been employed as a useful tool to identify the drug excipient interaction[7] . Samples were analyzed by potassium bromide pellet method in an IR spectrophotometer (Shimadzu, FTIR 8700) in the region between 4600-400 cm-1. Complex formation was evaluated by comparing the IR spectra of the solid complex and of the drug.
Differential scanning calorimetry (DSC) has been one of the most widely used calorimetric techniques to study the solid state interaction of drug with β CD [8]. Samples of the solid complex, pure drug, and β CD were taken in a flat-bottomed aluminium pans and heated over a temperature range of 50-250 at a constant rate of 10°/min with purging of nitrogen (50 ml/min) using alumina as a reference standard in a differential scanning calorimeter (DSC-7, Perkin Elmer).
Powder X-ray diffraction technique (PXRD) has been extensively utilized along with DSC to study the interaction between drug and β CD [8]. The diffraction studies were carried out in a powder X-ray diffractometer (STOESTADI-P) germanium monochromated Cu Kα radiation in transmission mode. The samples were rotated during data collection to reduce orientation effects. PXRD pattern of solid complex, pure drug, and β CD were recorded between 2θ = 5 to 50° at 40 kV and 30 mA.
Formulation of tablets
Dispersible tablets of carbamazepine-β-CD complex were prepared by conventional wet granulation technique. The solid complex was dry-mixed with starch/sodium starch glycollate (SSG) and granulated with aqueous solution of polyvinyl pyrollidone (PVP K-30) and passed through sieve #16. The granules obtained were dried at 60° for 1 h and passed through the same sieve to break the lumps. The granules retained on sieve #40 were blended with starch/SSG, magnesium stearate, and talc and compressed into tablets using 12 mm flat punches to a hardness of 3.56.5 kg/sq cm in a rotary tablet press (Rimek minipress model RSB-4).
Statistical design
An 8 run 23 factorial design [9] consisting of 3 factors at 2 levels was set up to investigate the effect of different variables on tablet disintegration and dissolution rate. The amount of binder (A) and type of disintegrant (C) were investigated as the formulation variables, whereas tablet hardness (B) was studied as the processing variable. The disintegration time (DT) and release at the end of 1 h (rel 60) were evaluated as the responses (dependent variables). The levels and the variation intervals for the 8 formulations are outlined in Table 1.
| Formulation Code | Factor levels* | Disintegration timea (sec) | Release in1 ha (%) | ||
|---|---|---|---|---|---|
| Binder (% w/w) | Hardness (Kg/sq cm) | Disintegrant type | |||
| 1 | -1 (2) | -1 (4) | -1 (Starch) | 144.3±5.08 | 78.68±3.92 |
| a | +1 (4) | -1 (4) | -1 (Starch) | 150.7±4.03 | 76.92±4.16 |
| b | -1 (2) | +1 (6) | -1 (Starch) | 156.0±4.43 | 73.66±3.76 |
| c | -1 (2) | -1 (4) | +1 (SSG) | 108.0±4.10 | 95.76±3.78 |
| ab | +1 (4) | +1 (6) | -1 (Starch) | 168.7±5.50 | 74.58±3.88 |
| ac | +1 (4) | -1 (4) | +1 (SSG) | 120.3±5.12 | 90.76±3.90 |
| bc | -1 (2) | +1 (6) | +1 (SSG) | 126.7±4.63 | 89.82±4.14 |
| abc | +1 (4) | +1 (6) | +1 (SSG) | 132.0±4.94 | 87.62±4.14 |
*The parentheses in the data represent the decoded factor levels. aThe data represents Mean values ± Standard deviation of three determinations
Table 1: Selected Factor Combinations And Responses For Tablets Prepared According To 23 Factorial Design
Analysis of data
The response parameters were fitted into a first-order polynomial model (Y= b0+b1X1+b2X2+b3X3+----) by performing a multiple linear regression analysis on Design expert 6.0.5 trial (Statease statistical software package). ANOVA was performed on the response parameters to identify the statistically significant effects and generate a predictor equation comprising only the significant main and interaction effects.
Evaluation of the tablets
Uniformity of weight was determined by following the official method [10]. Disintegration time was recorded by following the IP procedure10 using USP XXIII disintegration tester (Model ED-2, Electrolab, Mumbai).
Hardness of the tablets was evaluated using a Monsanto hardness tester [11] (Tab Machines, Mumbai). The friability of tablets for each batch was determined using automated USP friabilator [11] (Model EF-2, Electrolab, Mumbai). The in vitro drug release studies from the tablets were carried out in USP XXIII dissolution apparatus-II (Model TDT06T, Electrolab, Mumbai) using distilled water containing 0.1% sodium lauryl sulphate [12].
Results and Discussion
In the present work, complexation of carbamazepine with β cyclodextrin was tried in an attempt to improve its solubility and dissolution rate. The Phase solubility studies revealed a linear relationship between the aqueous drug solubility with increase in β-CD concentration (R2=0.9946). The phase solubility diagram can be classified as AL type, according to Higuchi and Connors. The extent of complexation is characterized by the apparent 1:1 stability constant Ks, which was calculated based on the solubility diagram. The Ks value was found to be 405.42 M-1 indicating that the complex formed was adequately stable. The results obtained from tlc studies were not conclusive as the Rf values of the complex and the reference standard were not significantly different. Therefore, further characterization of the complex was carried out by IR, DSC and PXRD to study the solid state interaction of drug with β CD. The IR spectra of carbamazepine showed a characteristic peak at 3462 cm-1 (-NH valence vibration), 1676 cm-1 (-CO-R vibration), 1598 cm-1 (-C=Cand -C=O vibration), and 1384 cm-1 (-NH deformation)13. β-CD spectrum presents a large band between 3400 and 3900 cm-1 and peaks at 2923 cm-1, 1639 cm-1, 1417 cm-1 and 1157 cm-1. In the IR spectra of the solid complex, the βCD main bands were found to overlap with the characteristic drug peaks, which can be attributed to low drug content in the solid complexes (9.43%w/w); however, the Carbamazepine characteristic peak at 1680 cm-1 could be clearly detected in the IR spectra of the solid complex, which confirmed the formation of the inclusion complex.
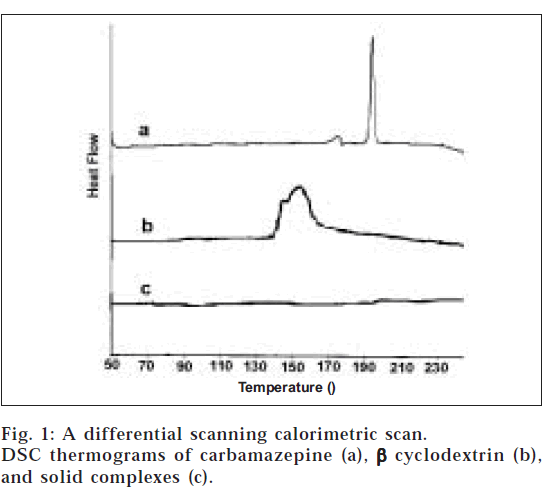
DSC thermograms of carbamazepine, β-CD, and the solid complex are portrayed in fig. 1. The thermogram of the pure drug presents a sharp endothermic peak at 193.3°, corresponding to its melting transition temperature [14]. The broad band (140-170°) observed with β CD thermogram corresponds to its dehydration. The solid complex did not display any such characteristic peak corresponding to carbamazepine melting which can be ascribed to the formation of carbamazepine-β-CD inclusion complex.
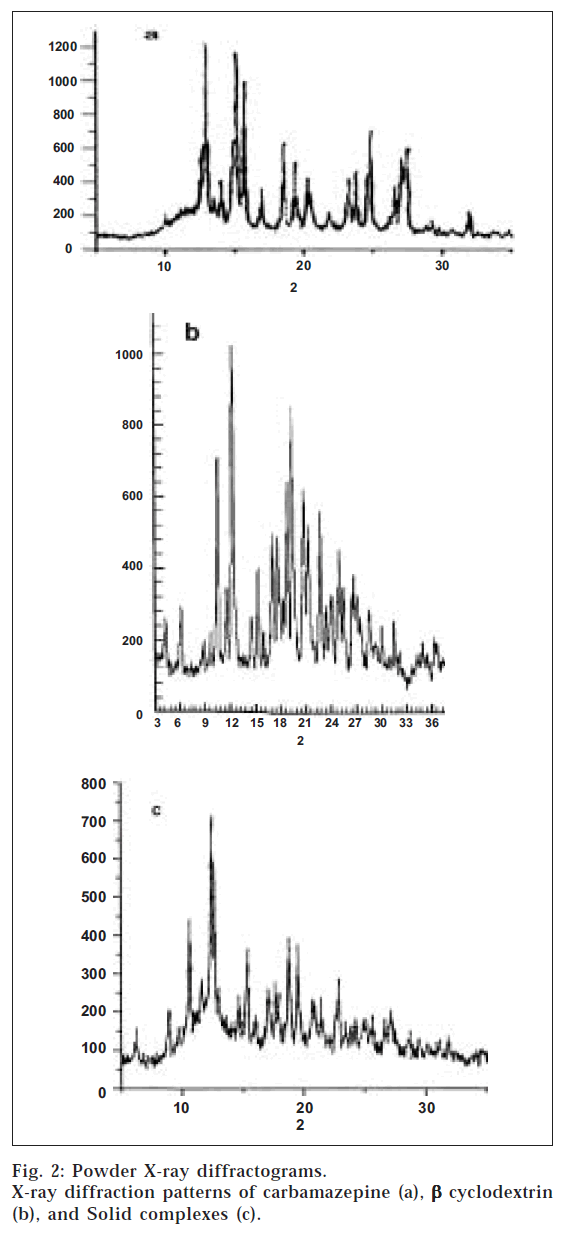
PXRD patterns for carbamazepine, β-CD, and the solid complexes are shown in fig. 2. The crystalline nature of carbamazepine was clearly demonstrated by its characteristic PXRD pattern containing well-defined peaks. The drug characteristic peaks were observed at 15, 15.5, 24, and 27.5 2 θ values with intensities of 1100, 1000, 650, and 550, respectively. The PXRD pattern of βT CD also exhibited well-defined peaks at 12.5 and 19 2 θ values with intensities of 1000 and 820, respectively. The diffractogram of the complex displayed well-defined peaks at 12.5, 15, 19, and 22.5 2 θ values with intensities of 700, 350, 400, and 250, respectively. Even though the PXRD diffractogram of the solid complexes displayed characteristic pattern corresponding to the crystalline drug, the peak intensities were reduced, indicating the decrease in the drug crystallinity. This decrease in the drug crystallinity was responsible for the increased solubility of the solid complex when compared to that of the pure drug. The increase in solubility with decrease in drug crystallinity has been cited in literature [15]. The IR, DSC and PXRD studies collectively suggest a stronger interaction between the drug and β-CD in the solid complex due to formation of inclusion complex.
Several formulation and processing variables are found to affect the properties of tablets. The effect of several factors and their interactions can be determined simultaneously by factorial design experiments. In the present work, factorial design model was employed to quantify the effect of amount of binder (A), tablet hardness (B), and the type of disintegrant (C) on the disintegration time and release rate from tablets. Table 1 portrays the effect of the three factors as well as their interaction effects on the tablet disintegration time and drug release along with their level of significance.
With the aim to develop a tablet formulation with minimum disintegration time and maximum dissolution rate, preliminary trials were undertaken by varying the type of disintegrant and the amount of binder at different values of compressional force. The responses obtained were found to be a linear function of the factors selected. Based on the results obtained, the lower and higher levels of PVP K30 as well as the tablet hardness were fixed for the two disintegrants studied.
Triplicate results from the analysis of data obtained for each response parameter were fitted into a linear polynomial equation of the form Y = b0+b1X1+b2X2+b3X3+b12X1 X2+b13X1 X3+b23X2X3+ b123X1 X2X3----(1), where Y is the level of the response parameter, b is the regression coefficient for the first-order polynomial, and X is the level of the independent variable. ANOVA was performed to eliminate the non-significant terms from the equation and generate a simplified predictor equation containing only the significant terms. From this simplified mathematical model, the respective responses can be calculated without any further trials. In addition, the equation provides predictions of the behaviour of different formulations based on various combinations of the above mentioned factors.
The fitted linear model for disintegration time and rel 60 are represented as Eqns. 2 and 3, respectively. DT = 138.33+4.58X1+7.5 X2–16.58 X3—(2) (R2 = 0.9921, P <0.01), rel60 = 83.48–1.00 X1–2.06 X2+7.51 X3+0.69 X1X2–0.79 X1X3–0.21 X2X3—(3) (R2 = 1.000, P < 0.01).
From Eqn. 2, it was seen that all the three main effects A, B, and C had a significant effect on the DT. The type of the disintegrant (C) was the main effect with the highest statistical significance (F = 391). This can be attributed to the inclusion of SSG in the formulations that has resulted in appreciable lowering of the DT. The activity of SSG is due to the capillary action resulting in expansion and subsequent disintegration of the compressed tablet. SSG is super disintegrant, which expands in volume by 200300% in water when compared to that of starch, which expands by 10-25% [16].
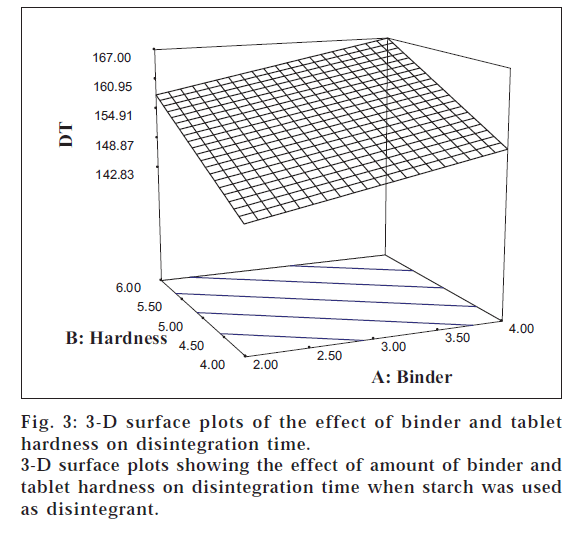
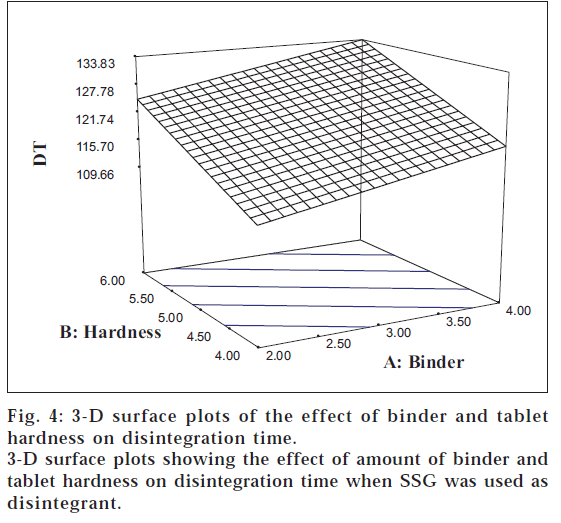
The results of ANOVA indicated that tablet hardness (F = 79.98) had a significant positive effect on the tablet DT. The 3-D surface plots (figs. 3 and 4) portray a linear relationship between tablet hardness and DT for the two disintegrants studied. A similar linear relationship between the increase in tablet DT with increase in the crushing strength has been cited in literature [17].
It is clear from Eqn. 2 that the amount of binder also had a significant positive impact on the DT (F = 29.86). The 3-D plots illustrate that DT augments with increasing levels of PVP K30 in case of both the disintegrants. The linearity of the contour lines reveals the fact that a low DT can be obtained using low levels of the binder at low hardness levels. All the three main effects were found to exert their influence on the DT independently without producing any interactions (C>B>A). This was well supported by the earlier reports that DT was found to be independent of compressional force when SSG was used as disintegrant [16].
Since the absorption of carbamazepine was dissolution rate limited, drug release at the end of the first hour (rel 60) was studied as one of the responses. The studies revealed a rank order correlation between the tablet DT and the drug release at the end of the first hour. The results of ANOVA revealed the fact that all the three main factors that influenced the tablet DT also had a significant impact on the drug release. Eqn. 3 shows that the two-way interactions also had a significant effect on the release rate (C>B>A>AC>AB>BC). As observed earlier, the type of disintegrant emerged as the main effect with highest statistical significance (F = 2.51*105) to influence the drug release. Tablets formulated using SSG disintegrated quickly and showed faster release when compared to tablets with starch.
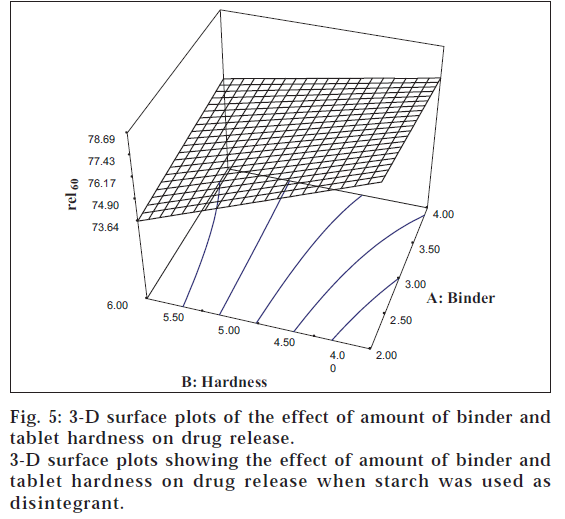
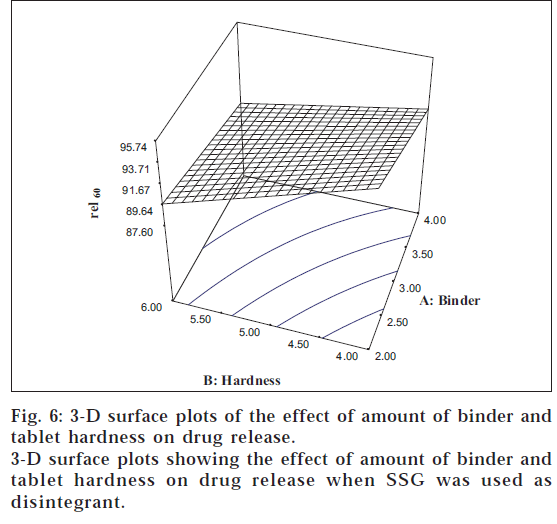
The tablet hardness showed a significant negative influence on the drug release (F = 18769). The 3-D surface plots (figs. 5 and 6) portray an inverse relationship between rel60 and tablet hardness for both the disintegrants investigated. Eqn. 3 depicts the fact that the amount of binder (F = 4489) also had a significant negative influence on the drug release.
The contour lines showed that maximum release can be obtained using low level of binder coupled with low hardness levels. All the two-way interactions (AB, BC, and AC) were found to have a significant influence on the drug release. The interaction terms AC (F = 2809) and BC (F = 205.44) had a negative effect on the release, which can be ascribed to the negating influence of the main effects A and B, respectively. The interaction term AB (F = 2085) had a positive influence on the drug release, whereas the three-way interaction (ABC) failed to show any significant effect.
The mathematical models representing the response parameters were validated by preparing formulations with combination of factors within the experimental domain, and the value for each response was determined experimentally as well as theoretically from the respective mathematical equations. Table 2 enlists the value of the observed response variables, theoretical and the predicted values along with the percent prediction error. The prediction error for the response variables ranged between 0.81 and 3.58% with a mean±SD of the absolute error as 2.09±1.14. The low magnitude of error reflects the ability of multiple linear regression and ANOVA to predict the performance of the optimized formulations.
| Formulation | Responses | Predicted | Observed | % |
|---|---|---|---|---|
| value | value | error | ||
| F 1 | DT | 154.91 | 160.66 | 3.58 |
| rel60 | 37.98 | 38.72 | 1.91 | |
| F 2 | DT | 121.75 | 124.33 | 2.07 |
| rel60 | 45.49 | 45.86 | 0.81 |
The tablets F 1 and F2 were compressed using PVP K-30 (3% w/w) as a binde to a hardness of 5 kg/sq cm. F1 was prepared employing starch as a disintegrant, whereas F2 was prepared using sodium starch glycollate as a disintegrant
Table 2: Experimental And Theoretical Values Of The Responses Predicted For The Tablet Formulations
In the present work, an enhancement of solubility of carbamazepine was obtained by its complexation with β-CD.incorporation of carbamazepine previously complexed with β-CD influenced its solubility and dissolution rate from tablets. The results obtained justify the use of 23 factorial studies to quantify the effect of several formulation and processing variables as well as their interactions on the tablet properties, which would minimize the number of experimental trials and also reduce the cost of formulation development. Since carbamazepine is a drug of choice for children, the dispersible tablets developed would be invaluable for paediatric administration.
Acknowledgements
The authors are grateful to Prof. B. G. Shivananda, Principal, Al-Ameen College of Pharmacy, for providing facilities to carry out the research work. They are also thankful to Micro Nova Laboratories Pvt. Ltd., Bangalore, for providing the gift sample of carbamazepine.
References
- Levy, R., Wilensky, A.J., and Anderson, G.D., In: Evans, W.E., Schentag, J.J., and Jusko, W.J. Eds., Applied Pharmacokinetics, Principles of Therapeutic Drug Monitoring, 3rd Edn., Applied Therapeutics, Vancouver, 1992, 1.
- El-Nahhas, A.S, Pharmazie, 1996, 51, 960.
- Loftsson, T. and Brewster, M., Pharma. Manager, 1997, 3, 22.
- White, H. S., In: Gennaro, A. R., Ed., Remington: The Science and Practice of Pharmacy, 19th Edn., Mack Publishing Company, Pennsylvania, 1995, 1173.
- Higuchi, T. and Connors, K.A., Adv. Anal. Chem. Instr. 1965, 4, 117.
- Aithal, K. S., Udupa, N. and Srinivasan. K. K., Indian Drugs, 1995, 32, 293.
- Mohamed, K. G. and Moji, C.A., Pharm. Dev. Tech., 2001, 6, 315.
- Mohamed, K. G. and Moji, C.A., Pharm. Dev. Tech., 2001, 6, 305.
- Bolton, S., In; Pharmaceutical Statistics, 3rd Edn., Marcel Dekker Inc., New York, 1997, 326.
- Indian Pharmacopeia, 4th Edn., Controller of Publication, Ministry of Health and Family Welfare, 1996 A-80.
- Banker, G.S. and Anderson, N.R, In: Lachmann, L., Liberman, H.A. and Kanig, J.L., Eds., The Theory and Practice of Industrial Pharmacy, 3rd Edn., Varghese Publishing House, Mumbai, 1987, 293.
- The United States Pharmacopoeia, 19th Edn., The United States Pharmacopoeial Convention, Rockville, 1975, 706.
- Rustichelli, C., Gamberini, G., Ferioli, V., Gamberini, M.C., Ficarra, R. and Tommasini, S., J. Pharm. Biomed. Anal., 2000, 23, 41.
- Kinalekar, M.S., Kulkarni, S.R. and Vavia, P.R., J. Pharm.Biopharm. Anal. 2000, 22, 661.
- Eugene, F,F. and Timothy, A.H., In: Lachmann, L., Liberman, H.A. and Kanig, J.L., Eds., The Theory and Practice of Industrial Pharmacy, 3rd Edn., Varghese Publishing House, Mumbai, 1987, 171.
- Garnet, E.P., Baley, G.J., Mccurdy, V.E. and Banker, G.S., In: Liberman, H.A. Lachmann, L. and Schwartz, J.B., Eds., Pharmaceutical Dosage forms: Tablets, Vol. 1, Marcel Dekker Inc., New York, 1989, 75.
- Marshall, K., In: Lachmann, L., Liberman, H.A. and Kanig, J.L., Eds., The Theory and Practice of Industrial Pharmacy, 3rd Edn., Varghese Publishing House, Mumbai, 1987, 66.