- *Corresponding Author:
- G. Lopez-Angulo
School of Chemical and Biological Sciences, Autonomous University of Sinaloa, University City s/n, Culiacan, Sinaloa 80010, Mexico
E-mail: gabylopez@uas.edu.mx
| Date of Received | 25 February 2021 |
| Date of Revision | 12 April 2022 |
| Date of Acceptance | 02 April 2023 |
| Indian J Pharm Sci 2023;85(2):479-490 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Leaf extracts of Echeveria subrigida show antioxidant, antibacterial, anti-mutagenic, and α-glucosidase inhibitory activities. The inhibitory α-glucosidase is associated with the content of quercetin-3-O-glucoside and isorhametin-3-O-glucoside. The aims of this study were to analyze the effect on mice glycemia and in vitro kinetics of α-glucosidase inhibition of the hydroalcoholic extract of Echeveria subrigida standardized in the content of isorhametin-3-O-glucoside. The high-performance liquid chromatography method was validated to quantitate isorhametin-3-O-glucoside. Hydroalcoholic extract of Echeveria subrigida identity was characterized by its organoleptic, physicochemical, pharmacological and toxicological parameters. The effects on glycemia were carried out in 6 w old male Balb-C mice and using the following treatments; hydroalcoholic extract of Echeveria subrigida at 50, 100 and 200 mg/kg b.w.; positive control glibenclamide or acarbose at 10 mg/kg b.w. The inhibition kinetics of hydroalcoholic extract of Echeveria subrigida was determined by the Lineweaver-Burk plots. Hydroalcoholic extract of Echeveria subrigida had 4.87±0.14 mg isorhametin-3-O-glucoside/g, complied with the World Health Organization parameters for standardized extracts and induced a mixed inhibition on α-glucosidase. Hydroalcoholic extract of Echeveria subrigida (200 mg/kg b.w) and glibenclamide treatments showed similar percent hypoglycemia, 49.1 % and 52 %, respectively. The anti-hyperglycemic assay showed that similar percent reductions in glucose-levels were found in the treatments with hydroalcoholic extract of Echeveria subrigida (100 mg/kg, 29.32 %; 200 mg/kg, 28.99 %) and acarbose (10 mg/kg, 19.87 %). The standardized hydroalcoholic extract of Echeveria subrigida showed hypoglycemic and anti-hyperglycemic activities, stability, and innocuity, suggesting its potential to prevent/treat diabetes mellitus.
Keywords
Antidiabetic activity, ethanolic extract, standardized extract, Echeveria subrigida, high-performance liquid chromatography method validation
Diabetes Mellitus (DM) is characterized by the loss of glucose homeostasis and alteration of insulin signaling and metabolism of carbohydrates and lipids, leading to progressive systemic disorders (hyperlipidemia, hyperglycemia, nephropathy, hepatic damage, vascular dysfunction). DM affects more than 425 million people worldwide, and about 90 % of them have type 2 DM (DM2), which is associated with obesity and a sedentary lifestyle[1]. Different drugs are used to treat DM2 (insulin secretagogues, insulins, inhibitors of glucosidases)[2], but many diabetic patients use traditional/complementary medicine[3].
Different plant natural products contain compounds with hypoglycemic properties (flavonoids, alkaloids, steroids, peptides) and potential to be developed as new anti-diabetic drugs. Flavonoids (flavones and flavonols) are potent inhibitors of α-glucosidase, showing different inhibition kinetics[4]. Plant extracts (Cinnamomum zeylanicum, Stevia rebaudiana) have anti-diabetic properties, showing inhibitory activity of α-glucosidase and suppression of the hyperglycemic response in diabetic rats[5]. In fact, several plant standardized extracts have been evaluated to treat DM (Cleome droserifolia, Quassia amara L., Withania somnifera)[6,7]. A standardized extract contains constant levels of active ingredients, induces predictable pharmacologic and physiologic effects and shows identity characteristics and therapeutic efficacy. Thus, the method of quantification used in the standardization process must be validated and the physical, chemical and pharmacological properties of the standardized extract must be established[8].
Echeveria subrigida (E. subrigida) (BL Rob. & Seaton) Rose (Crassulaceae) is a plant native to Mexico. Its leaves contain active metabolites, nutritional components (ascorbic acid, tocopherol, β-carotene), organic acids (malic, malonic, succinic), fatty acids (linoleic, palmitic), carbohydrates (fructose, altrose, sucrose), terpenes (α-amirine, campesterol, germanicol, sitosterol), and phenolics (flavonoids, tannins), among others[9-11]. Leaf extracts of E. subrigida show relevant activities for the human being like antibacterial against human pathogens, antioxidant, antimutagenic and Inhibitory of α-Glucosidase (IαG). The methanol extract of E. subrigida leaves has higher IαG activity than acarbose[10], and the flavonoids isorhamnetin-3-Oglucoside (I3G), quercetin-3-O-glucoside (Q3G), and proanthocyanidins are the main compounds responsible for the IαG activity[12]. These studies support the potential of E. subrigida extracts to treat DM2, but the employed extracts were obtained with methanol and not standardized, besides, the in vivo effect on glycemia and the inhibitory mechanism of α-glucosidase have not been established. Consequently, the aims of this study were to standardize a Hydroalcoholic Extract of E. subrigida (HE-Es) in its content of I3G, to evaluate the extract quality properties (pharmacognosy, physicochemical, and toxicological), to establish its effect on the basal and postprandial levels of glucose on normoglycemic Balb-C mice, and to determine its in vitro inhibition kinetics on α-glucosidase.
Materials and Methods
Plant material:
Leaves of E. subrigida Rose were collected in October 2018 near the town of El Palmito, Concordia, Sinaloa, Mexico 2000 m a.s.l.; 23°34′06″ N, 105°50′53″O). Dr. Rito Vega Aviña authenticated the plant material and deposited one specimen (11742) in the herbarium of the School of Agronomy of the Autonomous University of Sinaloa. The leaves were washed (93.8±0.2 % moisture), cut, freeze-dried (VirTis 25EL, VirTis Co. U.S.), and milled to obtain a fine powder (mesh 40). The powder was stored at 20° in darkness until its use.
Animals:
Balb-C mice were obtained from Bioinvert company (Bioinvert, SA of CV, Mexico) and maintained at 24±2°, 50 % relative humidity, and light/dark cycles of 12 h. Water and feed (Nutricubos, Purina S.A. of C.V., Mexico) were provided ad libitum. The animal management was carried out according to the Official Mexican Standard NOM-062-ZOO-1999 and permission was obtained from the Institutional Ethical Committee constituted for the purpose.
Reagents and solvents:
Solvents were High-Performance Liquid Chromatography (HPLC) grade (TEDIA, U.S.). Bacteriological media were from Becton Dickinson: agars, soy trypticase, Baird-Paker, salt and mannitol, MacConkey, citrate deoxycholate, red violet bile, Sabouraund; and broths, soy trypticase, tetrathionate brilliant green bile, peptone water, lactose and Mossel. The following reagents were from Sigma Aldrich (St. Louis, MO, U.S.): quinine chlorohydrate, ethylenediaminetetraacetic acid, ferric ammonium sulfate, catechin, Triton X-100, chlorohydric acid, α-glucosidase from Saccharomyces cerevisiae (EC number 3.2.1.20), p-Nitrophenyl Glucopyranoside (p-NPG), p-Nitrophenol (p-NP), acarbose and glibenclamide.
Preparation of the HE-Es:
HE-Es was prepared by maceration in 80 % ethanol of the E. subrigida powder (1:10 w/v) with continuous stirring (150 rpm/3 d). The solvent was changed daily and the hydroalcoholic phases were mixed. Ethanol was eliminated under reduced pressure at 40° (BÜCHI Labortechnick AG, Switzerland), vacuum oven heating at 40° (Prendo SS-250, Mexico), and freeze-drying. The residue (HE-Es) was stored at -20°, under N2 atmosphere and in darkness until use.
Standardization and quality control of the material/ extract:
The quantitative analysis of the active principles in HE-Es and the determination of the identity parameters (i.e., organoleptic, physicochemical, pharmacologic, and toxicologic) of the E. subrigida powder and HE-Es were carried out as described in the quality control methods for medicinal plant materials of the World Health Organization[13].
Standardization of the HPLC method:
HPLC conditions: The HPLC analyses were performed using an HPLC-DAD 1100 system (Agilent Technologies, U.S.) provided with an ACE EXCEL C18-Amide column (150×30 mm×3 μm) (Advanced Chromatography Technologies, U.K.). The mobile phase consisted of 1 % formic acid (A) and acetonitrile (B). 0.5 % B, linear gradient to 30 % B in 10 min, isocratic 10 min, linear gradient to 60 % B in 10 min, and isocratic 5 min. The separation conditions were as follows; running time 35 min, flow 0.4 ml/min, injection volume 15 μl, and detection at 355 nm[10].
Validation of the analytical method:
The parameters of the HPLC method (suitability, specificity, accuracy, precision and linearity) were validated as recommended by the United State Food and Drug Administration (FDA)[14]. The marker compound was I3G, and some parameters were also evaluated for Q3G.
Preparation of the sample and standard:
HE-Es was dissolved in MeOH (40 mg/ml). A C18 cartridge (CHROMAFIX, U.S.) was conditioned with 2 ml 50 % MeOH and 500 μl of the HE-Es were passed through the cartridge. Phenolics were eluted with 4 ml of MeOH into a 5 ml volumetric flask, and the volume was made up with MeOH. The solution was passed through a PVDF filter (17 mm, 0.45 μm, TITAN, U.S.) before HPLC analysis. Stock solutions of the standards (i.e., I3G, Q3G, and quercetin) were prepared in MeOH (1 mg/ml) and stored in amber vials at 4°. For analysis, dilutions in MeOH (1-100 μg/ml) were prepared from the stock solutions.
System suitability:
The system suitability was established by repeated injections (n=6) of 15 μg/ml I3G. The following parameters were calculated, Relative Standard Deviation (% RSD) for the retention time and peak area, the capacity factor (k'), the theoretical plate number (N) and the asymmetry factor (T). Peak resolution was determined for the HE-Es.
Specificity:
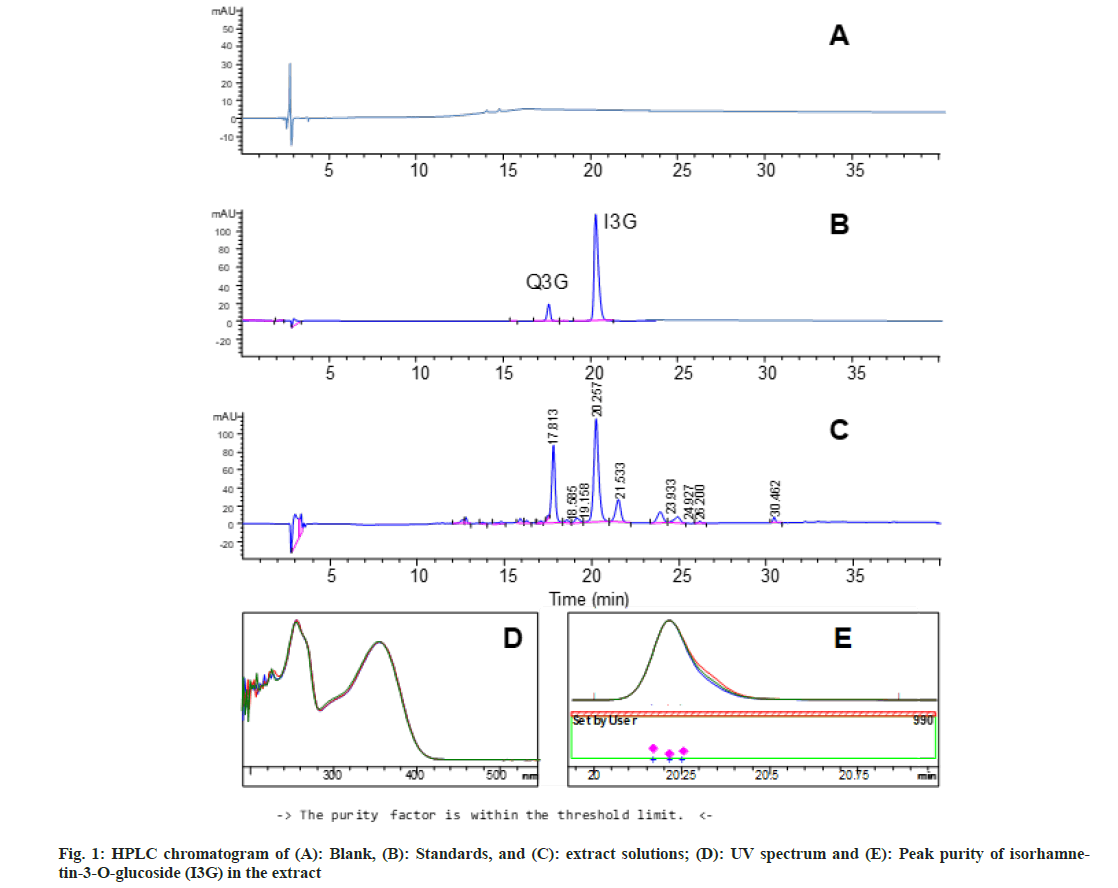
Solutions of the blank, standard (I3G), and HE-Es were analyzed by the established HPLC method. The method is specific if there is no signal in the blank or sample which interferes with the marker compound; besides, the Resolution Factor (Rs) (>1.5) and the peak purity are important parameters for the method specificity.
Linearity:
Linearity was measured by three injections of five different concentrations (6.25-100 μg/ml) of the marker compounds I3G and Q3G (1-25 μg/ml). Calibration curves were obtained for these compounds (R2>0.99) and used to calculate the Limits Of Detection (LOD) and Limits Of Quantitation (LOQ).
Accuracy:
The accuracy was calculated from the Recovery Percentage (% R) of a compound similar to the analyte. The sample (40 mg/ml) was mixed with different concentrations of the Internal Standard (IS) quercetin (80, 100, and 120 % of the analyte concentration in the sample). The mixtures were processed as established in the section “Preparation of the sample and standard”, and three independent injections per IS concentration were analyzed. The quantity of recovered IS was calculated using the IS calibration-curve (5-40 μg/ml). Results were reported as the average of % R for the three concentrations. The average recovering is acceptable if it ranges from 98 % to 102 %.
Precision:
The intraday precision (repeatability) was calculated from nine measurements (three concentrations and three repetitions each) of I3G and Q3G; they were carried out the same day using the previously described HPLC conditions. The interday precision was calculated using new solutions prepared at the same concentrations, which were analyzed on different days by a different analyst. The method’s precision was reported as the average RSD, RSD ≤2 % is considered acceptable.
Quantitation of the active compounds by HPLC:
Flavonoids I3G and Q3G were purified and characterized as previously described[12], and calibration curves were prepared. Flavonoids were quantitated by HPLC as described in the previous section. Results were reported as mg of I3G and Q3G per g of HE-Es (mg/g of HE-Es).
Evaluation of quality parameters:
Organoleptic and physicochemical parameters: The organoleptic (color, odor, odor type, texture and particle size) and physicochemical (total ash, acid-insoluble ash, water-soluble ash and moisture) parameters of E. subrigida powder and HE-Es were determined as identity characteristics[13]. Color was also determined using a colorimeter (Chroma Meter CR-200, Minolta, Japan).
Pharmacological parameters: The bitterness of the HE-Es was measured by comparison with diluted solutions of quinine chlorohydrate. The assay was carried for only one person. Stock solutions of the quinine chlorohydrate standard (10 μg/ml) and sample (1 mg/ml) were prepared in drinking water (20-25°), as well as the nine dilutions indicated in the method[13]. The measurement was carried out by alternating the tasting of different quinine chlorohydrate solutions (4.2-5.8 μg/ml) and HE-Es starting with dilution five to continue with lower or higher dilutions depending on the bitterness. The standard concentration corresponding with the HEEs bitterness was established as follows, lateral and superior parts of the tongue were exposed to 10 ml of each concentration solution for 30 s, the solution was spat out and the mouth was rinsed with drinking water between tastings. The bitterness was reported as units of quinine; one unit corresponded to a solution containing 1 in 2000 parts of quinine chlorohydrate and was calculated using the equation.
Bitterness in units/g=(2000×C)/(A×B),
where A=Concentration of the extract dilution (mg/ ml), B=Volume (ml) per 10 ml used to prepare the dilution of the sample’s minimal bitterness concentration, and C=Quantity of the quinine chlorohydrate contained in the dilution of the initial test’s minimal bitterness concentration.
Hemolytic properties: Hemolytic activity was determined as described by Dima et al.[15] with some modifications. Blood samples of two healthy individuals were obtained in tubes added with ethylenediaminetetraacetic acid. After centrifugation (200 g/5 min) (EBA 20 Hettich Zentrifugen, Germany), the pellet was recovered, washed and centrifuged three times with Phosphate Buffered Saline ((PBS) 0.1 M, pH 7.4), resuspended in PBS, and incubated at 37° for 5 min. Then, 500 μl of the suspended cells were mixed with 500 μl of HE-Es (1 mg/ml), incubated at 37° for 30 min, and centrifuged at 200 g for 10 min. The free hemoglobin in the supernatant was measured at 540 nm in a microplate reader (Multiskan Bichromatic, Fisher Scientific, U.S.). Samples were analyzed by triplicate. The controls were Triton X-100 (1 % v/v) (positive) and PBS (negative).
The hemolysis percentage was calculated using the equation;
Percentage hemolysis=(Abs of sample/Abs of the positive control)×100
Bitterness properties: Condensed tannins were quantitated as described in the laboratory manual “Quantification of Tannins in Tree Foliage”[16]. The reaction mix consisted of 0.125 ml of HE-Es (20 mg/ml), 0.750 ml of acidified butanol (butanol:HCl 95:5 v/v), and 25 μl of ferric reagent (2 % of sulfate ammonium ferric in 2 N HCl, 1:1 v/v). The mixture was heated at 98±2° for 60 min in a heating block (Fisher Scientific, IN, U.S.) and then cooled to room temperature; a blank without sample maintained at room temperature was included for colour correction. The mixtures (100 μl) were transferred to a 96 well microplate and the absorbance was measured at 540 nm in a microplate reader. A calibration curve of catechin (0-5 mg/ml in MeOH) was used for quantification, and the results were reported as Catechin Equivalents (CE) (mg CE/g HE-Es).
Swelling index: The swelling index is the volume increase (ml) obtained after swelling 1 g of plant material under specific conditions. HE-Es (1 g), 1 ml of ethanol, and 25 ml of water were added into a 50 ml graduated cylinder; the initial volume was registered. The components were mixed with a stirring rod (10 s) every 10 min for 1 h and left to stand for 3 h. The final volume was registered, including any mucilaginous component if present. The assay was carried out by triplicate, and the average of the difference in volume was calculated.
Foam index: HE-Es (1 g) was mixed with 100 ml of water. The mixture was boiled for 30 min, cooled, filtered and the volume was made up to 100 ml. Dilutions were prepared in graduated cylinders (1 to 10) in a final volume of 10 ml, the components were mixed vigorously for 15 s and left to stand for 15 min. The height (cm) of foam formed was measured, and the foam index was calculated as follows; foam index=1000/a; where a is the volume (ml) of the decoction used to prepare the dilution in the cylinder where the height was 1 cm.
Toxicological parameters:
Microbial contamination: The microbial contamination was determined as described in the “Quality Control Methods for Medicinal Plant Materials”[13]. The total count of bacteria and fungi and the presence of pathogens as enterobacteria and other Gram (-) (Escherichia coli, Salmonella spp., Pseudomonas aeruginosa, and Staphylococcus aureus) were determined.
Aflatoxins: Aflatoxins were measured by High Performance Thin Layer Chromatography (HPTLC, CAMAG, Muttenz, Switzerland) as recommended in the application note A-12.4 of the CAMAG Company (Switzerland). The HE-Es (1 g) was dissolved in 75 % MeOH and filtered (Nylon 25 mm, 0.45 μm, Millipore, U.S.). The filtrate was fractionated by washing three times with 10 % NaCl and hexane. The aqueous phase was recovered, and the aflatoxins were obtained by washing twice with dichloromethane; the organic phase was recovered and concentrated using a rotary evaporator (BÜCHI R-124 Labortechnick, Germany). The sample was resuspended in 5 ml MeOH-water (60:40 v/v) and diluted with 5 ml of water. The solution was passed through an immunoaffinity column Easi- Extract Aflatoxin (10 cm×1 cm) (R-Biopharm Ag, Germany). The column was washed twice with PBS and the aflatoxins were eluted with 2 ml MeOH. The solvent was evaporated with N2(g) and the residue was suspended in 200 μl of acetonitrile. A calibration curve was prepared with aflatoxin standards: B1, G1, B2, and G2 (Sigma-Aldrich, U.S.). The sample (10 μl) and standard (2, 5, 7.5, and 10 μl) were automatically applied (Linomat 5 HPTLC, CAMAG, Muttenz, Switzerland) onto a silica gel 60 F254 plate (20×10 cm) (Merck, Germany). The plate was developed with chloroform-acetone-water (140:20:0.3 v/v/v) in a chamber previously saturated with MgCl2 for 20 min. After plate development and solvent evaporation, the chromatogram was obtained at 366 nm with the software visionCATS2.5.
Inhibitory activity of α-glucosidase and inhibition kinetics:
Percentage inhibition=[(AbsC−AbsM)/AbsC]×100
where AbSC is the absorbance for the control reaction, and AbSM is the absorbance of the sample. The IC50 value was calculated with the inhibition percentages.
Determination of the enzyme-inhibition kinetics: The kinetics of α-glucosidase inhibition was determined with the Lineweaver-Burk method[17]. The enzyme activity was measured using different concentrations of p-NPG (0.625, 1.25, 2.5, and 5 mM) and HE-Es (0, 1×IC50, and 2×IC50). The reaction conditions are indicated in the previous paragraph, but the absorbance measurements were taken every minute for 5 min. The reaction was monitored by the p-NP formation, using a calibration curve (0.1875- 6 mM). A double reciprocal curve (1/activity vs. 1/ substrate concentration) was graphed to obtain the constants of Michaelis-Menten (Km) and maximal Velocity (Vmax).
Hypoglycemic activity of HE-Es on normoglycemic mice: The hypoglycemic activity was determined as reported[18] with some modifications. Five groups (n=6) of Balb-C mice were formed. group 1, negative control (saline solution); group II, positive control (glibenclamide 10 mg/kg b.w.); groups III, IV and V, HE-Es (50, 100, and 200 mg/kg b.w., respectively). The basal glucose level was measured after 8 h fasting; immediately, treatments were administered orally and glucose levels were measured every h for 5 h. Blood was obtained by tail puncture, and glucose was measured with a glucometer (Accu-Chek® Roche, Mexico). Glucose levels were reported as mg/dl of blood.
Antihyperglycemic activity of the HE-Es on normoglycemic mice (oral glucose tolerance test): Oral glucose tolerance test was carried out as previously described[5] with some modifications. Five groups (n=6) of Balb-C mice were formed. Group I, negative control (saline solution); group II, positive control (acarbose 10 mg/kg b.w.); groups III, IV, and V, HE-Es (50, 100, and 200 mg/kg b.w., respectively). The basal glucose level was determined after 8 h fasting, and treatments were administered orally immediately. A half-hour later, mice received sucrose (2 g/kg b.w.), and the glucose levels were measured after 30, 60, 90, and 120 min. Blood was obtained by tail puncture, and glucose was measured with a glucometer (Accu-Chek® Roche, Mexico). Glucose levels were expressed in mg/ml of blood and as the incremental area under the blood glucose response curve (iABC) (mg.min/dl).
Statistical analysis:
The results were reported as the mean±Standard Error (SE) or Standard Deviation (SD). The hypoglycemic and antihyperglycemic activities were analyzed by one-way analysis of variance to establish the differences among and within treatments. Means were contrasted by the Dunnett test with a significance level of p≤0.05. The incremental area under the blood glucose response curve was calculated with the trapezoidal rule. Data was analyzed with the software Graphpad Prism 8.1.
Results and Discussion
The extraction yield for the HE-Es was 21.80±0.85 % d.w. The HPLC method was suitable to quantitate the marker compound I3G in the HE-Es and complied with the FDA criteria[14] (Table 1). The method was specific since the other peaks did not interfere with that of I3G (fig. 1A-fig. 1C); the resolutions (R) with peaks adjacent to that of I3G were 1.6 and 2.3 (Table 1). Besides, the peak purity of I3G was above the limit (>990) (fig. 1D and fig. 1E). The HPLC method showed good accuracy, repeatability (% RSD<2 %), linearity (R2>0.99), and its LOQ (I3G=0.25 μg/ml and Q3G=0.21 μg/ml) was lower than the content of marker compounds in the HE-Es solution (I3G=19.3 μg/ml, Q3G=12.04 μg/ml) (Table 2). According to the described characteristics, the developed HPLC method is adequate to standardize the HE-Es[14]. The main monomeric flavonoids with α-glucosidase inhibitory activity in HE-Es are I3G and Q3G[12]. The validated HPLC analysis of the HE-Es showed that 1 g of HE-Es contained 4.82±0.15 mg of the marker compound I3G and 3.01±0.03 mg of Q3G; these values were similar to those reported in a methanol extract of E. subrigida[9]. In this regard, different plant extracts have been associated with biological activities and standardized in the content of flavonoids. For example the methanol extract of Balanites aegyptiaca, standardized in the content of rutin (1.3 mg/g) and isorhamnetin (0.04 mg/g), shows antidiabetic activity, reducing the glucose absorption by C2C12 muscle cells[7]; on the other hand, the ethanol extract of Carthamus tinctorius, standardized in kaempferol-3-O-rutinoside (56 mg/g) and anhydrosafflor yellow B (57 mg/g), has neuroprotective activity in mice with Parkinson[19].
| Parameter | I3G (Mean) | HE-Es | Acceptance requirements | |
|---|---|---|---|---|
| Retention time (RT) | Mean | 20.238 | - | |
| % RSD | 0.19 | ≤2 % | ||
| Peak area | Mean | 4007.91 | - | |
| % RSD | 1.93 | ≤2 % | ||
| Retention factor (kʹ) | 5.55 | - | >1 | |
| Column plate number (N) | 87,097 | - | >3000 | |
| Tailing factor (T) | 1.09 | <2 | ||
| Resolution (Rs)1 | - | 1.6, 2.3 | >1.5 | |
| Note: 1: Resolution calculated with respect to the peaks adjacent to the marker compound (isorhamnetin-3-O-glucoside, I3G) in the hydroalcoholic extract of Echeveria subrigida (HE-Es) | ||||
Table 1: System Suitability
| Parameters | I3G | Q3G |
|---|---|---|
| Accuracy | ||
| % Recovery2 (Mean) | 95.3±0.91 | |
| Precision (% RSD) | ||
| Repeatability (n=6) | 1.59 | 1.73 |
| Intermediate precision | 1.43 | 1.57 |
| Linearity | ||
| Range (µg/ml) | 6.25–100 | 1.05–25 |
| Regression equation | y=102.17x+219.6 | y=100.97x+30.95 |
| Coefficient of determination (R2) | 0.9976 | 0.999 |
| Limit of detection (µg/ml) | 0.083 | 0.068 |
| Limit of quantification (µg/ml) | 0.25 | 0.21 |
| Note: 1: I3G is isorhamnetin-3-O-glucoside and Q3G is quercetin-3-O-glucoside and 2Recovery percentage of quercetin internal standard, the mean of the three added concentrations is presented | ||
Table 2: Method Validation Parameters1
The organoleptic characteristics of the E. subrigida powder and HE-Es showed clear differences in color, particle texture, and odor (Table 3). The aromaticfruity odor of the HE-Es could be associated with esters, aldehydes, and ketones identified in E. subrigida[11]; these compounds were previously shown to provide a fruity odor[20]. The physicochemical analysis showed higher ash content in E. subrigida powder than in the HE-Es, mostly water-soluble in both materials, and hygroscopic characteristics for the HE-Es. The higher ash values in the flour can be associated with the loss of inorganic material during the extraction with ethanol (Table 3). In general, the ash represents the content of inorganic salts (silicates, oxalates, phosphates) in the plant material, whereas the acid-insoluble ash corresponds to the content of silica and calcium oxalate[21]. The consumption of acid-insoluble ash must be limited since it contributes to renal oxalate deposition (Chaplin 1977). The ash levels of the HE-Es were lower than in leaf extracts of Kalanchoe pinnata (total ash=10.2 % and acid-insoluble ash=6.8 %)[22] and in the range of values of medicinal-plant powders of Western Himalaya (total ash=2.7-12.2 % and acid-insoluble ash=0.5-2.3 %)[23] and Nigeria (total ash=11.0-18.7 % and acid-insoluble ash=1.0-8.4 %)[24]. E. subrigida powder and HE-Es showed low moisture (Table 3), decreasing the risk of chemical and microbiological degradation; however, the HE-Es was hygroscopic and must be stored hermetically in refrigeration[13,25].
| Parameter | Flour | HE-Es |
|---|---|---|
| Organoleptic | ||
| Color1 | Opaque light green | Mustard |
| (L=68.2, a=-10.35, b=20.48) | (L=46.47, a=3.29, b=14.2) | |
| Particle size (mm) | 0.4 | 0.4 |
| Texture | Granular/soft | Granular/hard |
| Odor (strength/type) | Distinct/aromatic | Strong/fruity |
| Physicochemical2 | ||
| Ashes (%) | ||
| Total | 15.02±0.4 | 3.44±0.3 |
| Acid insoluble | 1.40±0.1 | 2.01±0.02 |
| Water soluble | 11.99±0.19 | 3.24±0.23 |
| Weight loss by drying (%) | 3.95±0.2 | 7.32±0.2 |
| Toxicological (colonies/g) | ||
| Bacteria | 150 | 0 |
| Fungus | 8 | 0 |
| Note: 1: Color parameters in parenthesis were determined with a Minolta colorimeter and 2: Values are the mean±SD of three determinations | ||
Table 3: Quality Control Parameters of Echeveria SUBRIGIDA Flour and its Hydroalcoholic Extract (He-Es)
The organoleptic and physicochemical HEEs properties could be useful as HE-Es’ quality parameters (identity and purity) and to detect adulteration and inadequate manipulation of the plant materials[13,21,25]. The toxicological analyses showed a low total count of bacteria and fungi (Table 3) and absence of pathogenic bacteria (Salmonella spp., Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli) and the analyzed toxins (B1, B2, G1, and G2). These values were within the norm, suggesting that HE-Es consumption is safe[13,25]. HE-Es showed no bitterness, no hemolysis, no astringency, and low content of tannins and foaming index (Table 4).
| Parameter | HE-Es1 | Control |
|---|---|---|
| Bitterness | 0 units/g | Quinine hydrochloride |
| (2000 units/g) | ||
| Hemolysis | 0 % | Triton X100 |
| (100 %) | ||
| Astringency (tannins)2 | 1.9±0.4 mg EC/g HE-Es | 5 mg/mL Catechin |
| Swelling index | 0 ml | - |
| Foaming index | £100 | - |
| Note: 1: Values are the results of three determinations and 2: mean±S.D | ||
Table 4: Pharmacological Parameters of the Hydroalcoholic Extract of Echeveria Subrigida (He-Es)
These parameters complied with norms established for natural products[13]; thus, it is suggested that HEEs consumption will not induce adverse effects such as hemotoxicity[13,15]. The detection of tannins in HEEs (1.9±0.4 mg CE/g) (astringency) agreed with its previous identification in the methanol extract of E. subrigida[10,12]; these compounds and polyphenols have also been identified in other Crassulaceae plants[26]. Consumption of tannins is associated with positive effects in human health (anti-inflammatory, antioxidant, anticonvulsant, antitumoral)[27], and the levels of these compounds in the HE-Es could contribute to its biological properties. On the other hand, the null HE-Es’ swelling index suggested the absence of mucilaginous substances[13]. Besides, the HE-Es’ foaming index was lower than 100, corresponding to low content of saponins and absence of hemolytic activity. The absence of saponins in the methanol extract of E. subrigida[10] supported our results.
The IC50 value for the α-glucosidase inhibitory activity of HE-Es (52.9±2.40 μg/ml) was lower than that of acarbose (858.3±35.49 μg/ml), showing a Coefficient Relative to Acarbose (RCAca) of 0.062. Several phenolics show low RCAca values (0.00944- 0.2), being remarkably active quercetin and epigallocatechin gallate, and authors suggest that extracts/supplements containing these compounds could be useful in DM2 treatment[28,29].
HE-Es was a reversible inhibitor of α-glucosidase; the slope of the graph [p-NP] vs. time in the presence of inhibitor was lower than that in control (without inhibitor) and decreased with the increment of HEEs concentration. The Lineweaver-Burk analysis showed that the HE-Es was a mixed inhibitor inducing variations in the Vmax and Km parameters[29]. In the reactions with inhibitor (HE-Es), the Vmax (mM/min) values were 0.327 (50 μg/ml) and 0.156 (100 μg/ml), and the Km (mM) values were 1.293 (50 μg/ml) and 0.866 (100 μg/ml); the values of both parameters were lower than those of α-glucosidase without inhibitor (Vmax=0.504 mM/min; Km=1.745 mM). Mixed inhibition has been reported for quercetin, Q3G, and rutin[30], as well as for tannins (epicatechin gallate and type A trimmers of epicatechin-(4β,8)- epicatechingallate)[4,31]. I3G is a less active inhibitor of α-glucosidase[4] and its inhibitory kinetics has not been reported; however, I3G showed mixed inhibition of tyrosinase[32].
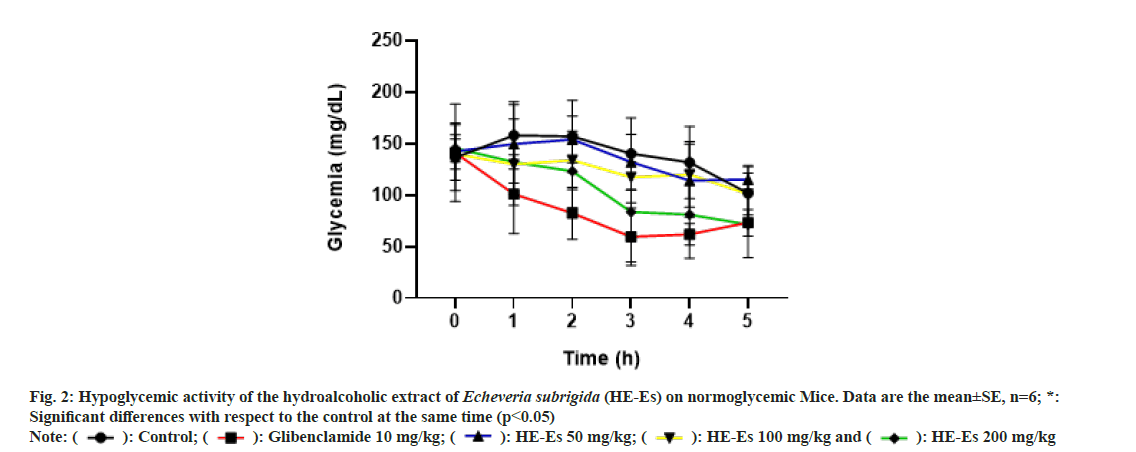
The blood glucose levels in normoglycemic mice treated with HE-Es, 50 and 100 mg/kg b.w., induced a non-significant hypoglycemic effect (p>0.05) (fig. 2); probably, these doses are too low to overcome the physiological contra-regulatory mechanisms[33]. On the other hand, the treatment with 200 mg/kg b.w. of HE-Es induced a hypoglycemic effect after 3 h (p<0.05), which was similar to that produced by glibenclamide (10 mg/kg b.w.) (p>0.05) (fig. 2). The curves of hypoglycemic activities of the treatments HE-Es (200 mg/kg b.w.) and glibenclamide (10 mg/ kg b.w.) showed similar decreasing tendencies and reached similar values at 5 h (fig. 2): the reductions in the blood glucose levels of treated mice were 49.1 % for HE-Es (200 mg/kg b.w.), 52 % for glibenclamide, and 25 % for the control. Thus, the HE-Es’ hypoglycemic-effect was not considered severe in normoglycemic mice, similar results have been reported with other plant extracts[18]; consequently, phytochemicals of E. subrigida have similar efficacy to glibenclamide. The glibenclamide induces β-pancreatic cells to release insulin and decreases liver gluconeogenesis[34]. In this regard, flavonoids (quercetin, isorhamnetin, tannins) also stimulate insulin secretion or trigger an insulin-like effect[18,35,36]. Thus, the HE-Es’ hypoglycemic-effect could be associated with its content of flavonoids I3G and Q3G, but further studies are needed to support this hypothesis.
Fig. 2: Hypoglycemic activity of the hydroalcoholic extract of Echeveria subrigida (HE-Es) on normoglycemic Mice. Data are the mean±SE, n=6; *:
Significant differences with respect to the control at the same time (p<0.05)
Note: ( ): Control; (
): Control; ( ): Glibenclamide 10 mg/kg; (
): Glibenclamide 10 mg/kg; ( ): HE-Es 50 mg/kg; (
): HE-Es 50 mg/kg; ( ): HE-Es 100 mg/kg and (
): HE-Es 100 mg/kg and ( ): HE-Es 200 mg/kg
): HE-Es 200 mg/kg
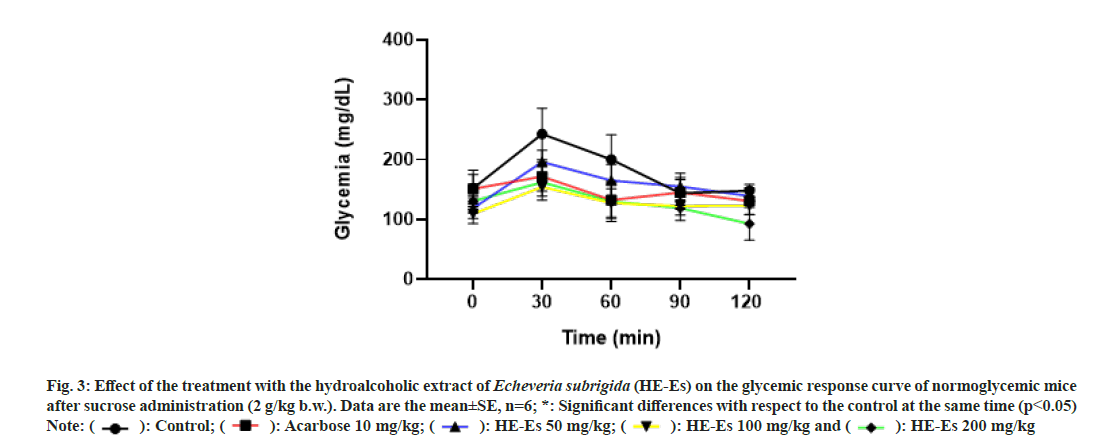
HE-Es induced antihyperglycemic activity in Balb-C mice, the oral tolerance curves were similar to that of acarbose (fig. 3). The HE-Es treatments avoid the increase in blood glucose levels at 30 min (p<0.05), the level of control mice increased an average of 90.8±7.9 mg/dl, but this effect was significantly reduced (p<0.05) in HE-Es treated mice: 77.2±9.5 mg/dl (50 mg/kg b.w.), 49.2±8.7 mg/dl (100 mg/kg b.w.), and 31.4±13.2 mg/dl (200 mg/kg b.w.) (fig. 3). Mice treated with acarbose increased the blood glucose an average of 20.7±12.2 mg/dl. This pattern of values could suggest the mechanism of action of HE-Es, HE-Es was a potent inhibitor of α-glucosidase and could induce a quick decrease in the postprandial hyperglycemia[37]. Strong inhibitory activity in vitro was also registered for the methanol extract of E. subrigida (IC50=25.21 μg/ml)[10], an activity that is associated with the content of flavonoids (I3G, Q3G, and proanthocyanidins)[12].
Fig. 3: Effect of the treatment with the hydroalcoholic extract of Echeveria subrigida (HE-Es) on the glycemic response curve of normoglycemic mice
after sucrose administration (2 g/kg b.w.). Data are the mean±SE, n=6; *: Significant differences with respect to the control at the same time (p<0.05)
Note: ( ): Control; (
): Control; ( ): Acarbose 10 mg/kg; (
): Acarbose 10 mg/kg; ( ): HE-Es 50 mg/kg; (
): HE-Es 50 mg/kg; ( ): HE-Es 100 mg/kg and (
): HE-Es 100 mg/kg and ( ): HE-Es 200 mg/kg
): HE-Es 200 mg/kg
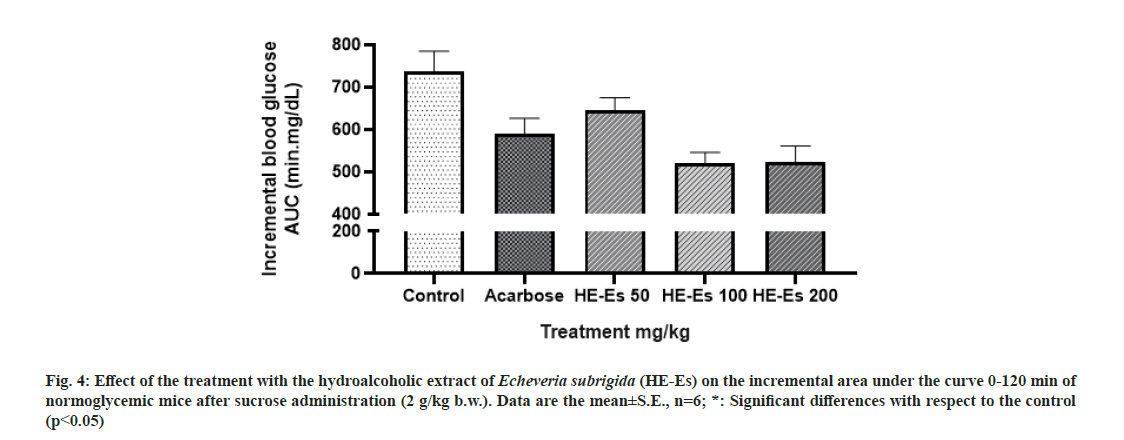
Comparing with the complete glycemic response (Area under curve) of treated control animals, a significant percent reduction in glucose (p<0.05) was only observed in mice treated with acarbose (10 mg/kg b.w., 19.87 %) and HE-Es at 100 mg/kg b.w. (29.32 %) and 200 mg/kg b.w. (28.99 %) (fig. 4). These glycemic responses of mice were similar to that reported for quercetin (300 mg/kg b.w.), which decreased 30.3 % the postprandial glucose level on Wistar rats treated with maltose (2 g/kg b.w.) [38]. The stem extract of Acalypha indica Linn (600 mg/kg b.w.) induces a 52 % decrease in rats’ blood glucose level[39]. Thus, the standardized HE-Es could be considered as an effective antihyperglycemic agent based on the tested concentrations. In this regard, different standardized extracts have shown positive effects on glucose metabolism. The Ficus deltoidea extract, standardized in the content of C-glycosylflavones, shows antidiabetic effect on rats with Streptozotocin (STZ) induced diabetes[40]. The extract of Ficus carica standardized in abscisic acid improves the glycemic and insulinemic responses of healthy human adults[41].
Fig. 4: Effect of the treatment with the hydroalcoholic extract of Echeveria subrigida (HE-Es) on the incremental area under the curve 0-120 min of normoglycemic mice after sucrose administration (2 g/kg b.w.). Data are the mean±S.E., n=6; *: Significant differences with respect to the control (p<0.05)
Different mechanisms could mediate the hypoglycemic and antihyperglycemic activities of HE-Es, inhibition of intestinal glucose absorption, inhibition of liver gluconeogenesis, stimulation of tissue glycolysis, stimulation of insulin release, and induction of insulin-like effects[35,36]. However, further studies must be carried out to demonstrate how the HE-Es is regulating the glucose levels.
The developed HPLC method to standardize the HEEs complies with established norms to quantitate the marker compound (isorhamnetin-3-glucoside, I3G). The standardized HE-Es extract ([I3G]=4.81 mg/g) showed hypoglycemic and antihyperglycemic activities, as well as physicochemical, pharmacological, and toxicological characteristics that support its antidiabetic potential, stability, and innocuity. The standardized HE-Es is a potential antidiabetic phytopharmaceutical.
Author’s contribution:
B. Heredia-Mercado and F. Delgado-Vargas contributed equally to this work
Acknowledgements:
Authors acknowledge the financial support (grant number A1-S-24537) and scholarship to Belinda Heredia-Mercado provided by the National Council for Science and Technology of Mexico (CONACyT), as well as the plant identification by Dr. Rito Vega-Aviña.
Conflict of interest:
The authors declared no conflict of interest.
References
- International Diabetes Federation. IDF Diabetes Atlas. Brussels, Belgium: International Diabetes Federation; 2019.
- Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes 2016;7(17):354-95.
[Crossref] [Google Scholar] [PubMed]
- Robinson MM, Zhang X. The world medicines situation 2011, traditional medicines: Global situation, issues and challenges. World Health Organization. 3rd ed. Geneva: 2011. p. 14.
- Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci Hum Wellness 2014;3(3-4):136-74.
- Mohamed Sham Shihabudeen H, Hansi Priscilla D, Thirumurugan K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metabol 2011;8(1):46.
[Crossref] [Google Scholar] [PubMed]
- Husain GM, Singh PN, Singh RK, Kumar V. Antidiabetic activity of standardized extract of Quassia amara in nicotinamide–streptozotocin‐induced diabetic rats. Phytother Res 2011;25(12):1806-12.
[Crossref] [Google Scholar] [PubMed]
- Motaal AA, Shaker S, Haddad PS. Antidiabetic activity of standardized extracts of Balanites aegyptiaca fruits using cell-based bioassays. Pharmacogn J 2012;4(30):20-4.
- Pradhan N, Gavali J, Waghmare N. WHO (World Health Organization) guidelines for standardization of herbal drugs. Int Ayurvedic Med J 2015;3(8):2238-43.
- López-Angulo G, Montes-Avila J, Díaz-Camacho SP, Vega-Aviña R, López-Valenzuela JÁ, Delgado-Vargas F. Comparison of terpene and phenolic profiles of three wild species of Echeveria (Crassulaceae). J Appl Bot Food Qual 2018;91:145-54.
- López-Angulo G, Montes-Avila J, Díaz-Camacho SP, Vega-Aviña R, Ahumada-Santos YP, Delgado-Vargas F. Chemical composition and antioxidant, α-glucosidase inhibitory and antibacterial activities of three Echeveria DC. species from Mexico. Arabian J Chem 2019;12(8):1964-73.
- López-Angulo G, Montes-Avila J, Díaz-Camacho SP, Vega-Aviña R, Báez-Flores ME, Delgado-Vargas F. Bioactive components and antimutagenic and antioxidant activities of two Echeveria DC. species. Ind Crops Prod 2016;85:38-48.
- López-Angulo G, Miranda-Soto V, López-Valenzuela JA, Montes-Avila J, Díaz-Camacho SP, Garzón-Tiznado JA, Delgado-Vargas F. α-Glucosidase inhibitory phenolics from Echeveria subrigida (BL Rob & Seaton) leaves. Nat Prod Res 2022;36(4):1058-61.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. Quality control methods for herbal materials, Geneva, Switzerland. World Health Organization; 2011.
- FDA. Analytical Procedures and Methods Validation for Drugs and Biologics. MD, U.S.: U.S. Department of Health and Human Services; 2015.
- Dima J, Raghda L, Jalil GA. Evaluation of hemolytic and anti-hemolytic activity of the aerial parts of Sonchus oleraceus extracts. Int J Pharm Sci Nanotechnol 2017;10(3):3745-51.
- FAO. Quantification of tannins in tree foliage. In: AGRICULTURE FIDONTIFA, editor. FAO/IAEA Working document. Austria, Vienna FAO; 2000. p. 31.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56(3):658-66.
- Kifle ZD, Yesuf JS, Atnafie SA. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of Hagenia abyssinica (rosaceae). J Exp Pharmacol 2020;12:151-67.
[Crossref] [Google Scholar] [PubMed]
- Ren R, Shi C, Cao J, Sun Y, Zhao X, Guo Y, et al. Neuroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of Parkinson’s disease. Sci Rep 2016;6(1):22135.
[Crossref] [Google Scholar] [PubMed]
- Licon CC, Bosc G, Sabri M, Mantel M, Fournel A, Bushdid C, et al. Chemical features mining provides new descriptive structure-odor relationships. PLoS Comput Biol 2019;15(4):e1006945.
[Crossref] [Google Scholar] [PubMed]
- Rather AA, Jain K. Pharmacognostic and physicochemical standardization of Nigella sativa and Allium cepa seeds. Pharma Biosci J 2017;5(6):35-40.
- Singh SK, Patel JR, Dangi A. Physicochemical, qualitative and quantitative determination of secondary metabolites and antioxidant potential of Kalanchoe pinnata (Lam.) Pers. leaf extracts. J Drug Deliv Ther 2019;9(1):220-4.
- Krishna AB, Manikyam HK, Janoti DS. Comparative physicochemical Ash study of some medicinal plants species of Western Himalaya. Int J Pharmacogn Phytochem Res 2016;8(3):442-5.
- Uba A, Baburo SI. Physico-chemical parameters and heavy metals determination in selected medicinal plants sold in ‘Yar Marina market, Sokoto, Nigeria. Chem Mater Res 2016;8(9):37-41.
- Mukhi S, Bose A, Panda P, Rao MM. Pharmacognostic, physicochemical and chromatographic characterization of Samasharkara Churna. J Ayurveda Integr Med 2016;7(2):88-99.
[Crossref] [Google Scholar] [PubMed]
- Eid O, Gonaid M. Crassulaceae (chemistry and pharmacology)-A review. Future J Pharm Sci 2018;4(2):234-40.
- Hussain G, Huang J, Rasul A, Anwar H, Imran A, Maqbool J, et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: An updated review. Molecules 2019 ;24(12):2213.
[Crossref] [Google Scholar] [PubMed]
- Tang H, Huang L, Sun C, Zhao D. Exploring the structure–activity relationship and interaction mechanism of flavonoids and α-glucosidase based on experimental analysis and molecular docking studies. Food Funct 2020;11(4):3332-50.
- Zhu J, Chen C, Zhang B, Huang Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit Rev Food Sci Nutr 2020;60(4):695-708.
[Crossref] [Google Scholar] [PubMed]
- Li YQ, Zhou FC, Gao F, Bian JS, Shan F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J Agric Food Chem 2009;57(24):11463-8.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Wen L, Lu Q, Liu R. Interaction mechanism between α-glucosidase and A-type trimer procyanidin revealed by integrated spectroscopic analysis techniques. Int J Biol Macromol 2020;143:173-80.
[Crossref] [Google Scholar] [PubMed]
- Si YX, Wang ZJ, Park D, Jeong HO, Ye S, Chung HY, et al. Effects of isorhamnetin on tyrosinase: Inhibition kinetics and computational simulation. Biosci Biotechnol Biochem 2012;76(6):1091-7.
[Crossref] [Google Scholar] [PubMed]
- Gebreyohannis T, Shibeshi W, Asres K. Effects of solvent fractions of Caylusea abyssinica (Fresen.) Fisch. and Mey. on blood glucose levels of normoglycemic, glucose loaded and streptozotocin-induced diabetic rodents. J Nat Remedies 2014;14(1):67-75.
- Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front Endocrinol 2017;8:6.
[Crossref] [Google Scholar] [PubMed]
- Alema NM, Periasamy G, Sibhat GG, Tekulu GH, Hiben MG. Antidiabetic activity of extracts of Terminalia brownii Fresen. Stem bark in mice. J Exp Pharmacol 2020;12:61-71.
[Crossref] [Google Scholar] [PubMed]
- Kifle ZD, Enyew EF. Evaluation of in vivo antidiabetic, in vitro α-amylase inhibitory, and in vitro antioxidant activity of leaves crude extract and solvent fractions of Bersama abyssinica fresen (melianthaceae). J Evid Based Integr Med 2020;25:2515690X20935827.
[Crossref] [Google Scholar] [PubMed]
- DiNicolantonio JJ, Bhutani J, O'Keefe JH. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015;2(1):e000327.
[Crossref] [Google Scholar] [PubMed]
- Hussain SA, Ahmed ZA, Mahwi TO, Aziz TA. Effect of quercetin on postprandial glucose excursion after mono-and disaccharides challenge in normal and diabetic rats. J Diabetes Mellitus 2012;2(1):82-7.
- Priya CL, Rao KB. Postprandial antihyperglycemic and antioxidant activities of Acalypha indica Linn stem extract: An in vivo study. Pharmacogn Mag 2016;12(4):S475.
[Crossref] [Google Scholar] [PubMed]
- Farsi E, Ahmad M, Hor SY, Ahamed MB, Yam MF, Asmawi MZ. Standardized extract of Ficus deltoidea stimulates insulin secretion and blocks hepatic glucose production by regulating the expression of glucose-metabolic genes in streptozitocin-induced diabetic rats. BMC Complement Altern Med 2014;14(1):220.
[Crossref] [Google Scholar] [PubMed]
- Atkinson FS, Villar A, Mulà A, Zangara A, Risco E, Smidt CR, et al. Abscisic acid standardized fig (Ficus carica) extracts ameliorate postprandial glycemic and insulinemic responses in healthy adults. Nutrients 2019;11(8):1757.
[Crossref] [Google Scholar] [PubMed]