- *Corresponding Author:
- Jinyan Chen
Department of Anesthesiology, General Hospital of Western Theater, Sichuan 610083,China
E-mail: 18087991383@163.com
| Date of Received | 14 December 2022 |
| Date of Revision | 20 April 2023 |
| Date of Acceptance | 07 November 2023 |
| Indian J Pharm Sci 2023;85(6):1632-1639 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The diabetic peripheral neuropathy pain model was constructed after 95 Sprague Dawley rats had been randomly divided among blank reference and model groups. Aescin low-dose group (0.5 mg/kg), aescin medium-dose group (1.0 mg/kg), aescin high-dose group (1.5 mg/kg), and positive control group (0.25 mg/ kg mecobalamin) were then randomly assigned to the simulation group following successful modeling. At the conclusion of the study, the mean sciatic nerve conduction velocity was determined, blood glucose in addition to the amount of inflammatory factors tumor necrosis factor-alpha, interleukin-6, and interleukin-1 were determined, and sciatic nerve cells from mice in all groups were collected for Western blot to identify the protein levels of high mobility group box 1 and receptor for advanced glycation end products. The mean sciatic nerve conduction velocity of the medium and high-dose groups of aescin was higher than those of the model control (p<0.05); rat blood glucose content, the contents of inflammatory factors interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha as well as the protein expression of high mobility group box 1 and receptor for advanced glycation end products in sciatic nerve tissue were lower and the body mass was higher than that of the model control group (p<0.05). Comparing the high-dose aescin group as well as the positive control group, there had been no discernible change in the aforementioned indices (p>0.05). Aescin is able to effectively improve the inflammatory response in the body by regulating high mobility group box 1-receptor for advanced glycation end products levels and slow down diabetic peripheral neuropathy caused by the inflammatory response.
Keywords
Glycation, aescin, diabetic peripheral neuropathy, cerebral infarction, amputation, pain
Diabetic Peripheral Neuropathy (DPN), clinically characterized mainly by sensory and motor nerve symptoms[1], has an insidious onset and unclear pathogenesis, its early detection is relatively difficult, and by the late stage, it will cause the patient's limbs to suffer from recurrent infections, ulceration of the foot, gangrene and other serious complications, and more seriously, it will make patients have the risk of amputation and threaten human health. According to studies, the prevalence of diabetes is around 3 % in the general population, whereas its neuropathy consequences are around 60 %, which means that 50 % are DPN[2]. Highly Mobile Group Box 1 (HMGB1), a nuclear transcriptional protein associated with the inflammatory process, alongside the Receptor For Advanced Glycation End Products (RAGE), an association recognition receptor present in the immune system of the human body, have both been implicated in lung injury, nephritis, type 2 diabetes, ischemia-reperfusion injury, and a number of additional illnesses brought on by inflammatory or infectious conditions[3-5]. It has been proposed that in HMGB1 involved in body cell signal transduction, RAGE is involved as one of the receptors, and the combination of the two can activate relevant signal transduction pathways in cells to promote the body inflammatory response[6]; inflammatory reactions are brought on by the ability of HMGB1 to associate with RAGE on the outermost layer of inflammatory cells outside the cell, which encourages the production of inflammatory factors including Tumour Necrosis Factor-Alpha (TNF-α), Interleukin (IL), and IL-6, among others[7]. Aescin has anti-inflammatory and anti-tumor effects[8], and its main component, sodium aescin, can inhibit the release of inflammatory factors and even kill cancer cells through a variety of mechanisms in turn to prevent or delay the occurrence of chronic diseases such as tumors, and aescin is clinically used alone or in combination with other drugs for anti-tumor therapy[8-10]. In addition, as a Chinese herbal extract, aescin is also commonly used clinically to treat diabetic complications such as diabetic retinopathy and diabetes with acute cerebral infarction[11-12], but the mechanism of aescin on DPN is still under investigation.
Materials and Methods
Experimental animals:
Under licence number SCXK (Shanghai)-2013-0016, 95 male Specific Pathogen-Free (SPF)-grade Sprague Dawley (SD) rats were obtained from Shanghai Sippe-Bk Lab Animal Co., Ltd. The experimental alimentary surroundings were set to 24°±2°, humidity (55 %), and a 1:1 day/night cycle. The experimental animal ethics commission of our medical facility gave its approval to this work.
Drugs and reagents:
Sodium aescin for injection (Saudi Food and Drug Authority's (SFDA's) Approval No. H20003369) manufactured by Wuhan Aimin Pharmaceutical; mecobalamin (SFDA Approval No. H20052325) from Yangtze River Pharmaceutical Group, streptozotocin (Lot No. S0130) from Sigma; IL-6, IL-1 β, and TNF-? kit provided by Nanjing Jiancheng Bioengineering Institute (Lot No. H007-1-1, H002, H052-1); HMGB1, RAGE and β-actin antibody from Abcam (Lot No. ab12856, ab216329, ab8226); protein assay (Bicinchoninic Acid (BCA)) reagent provided by Shanghai Qiyuan Biology (Lot No. QYS-237013); Radioimmunoprecipitation Assay (RIPA) protein lysate (Lot No. BB-3201) supplied by BestBio, Shanghai, China; Enhanced Chemiluminescence (ECL) chromogenic reagent (Lot No. rbr01135) provided by Shanghai Rong Bai Biology; Hematoxylin and Eosin (H&E) staining reagent (Lot No. AC11546) provided by Shanghai ACMEC Biochemical.
Experimental instruments:
Gel Doc2000 gel imaging system from Bio-Rad; Nikon SMZ745 light microscope provided by Shanghai Puhe Bio; Allegra X-15R tabletop refrigerated centrifuge provided by UniQuantus Biotech, Beijing; Spectra Max 340PC384 light absorption microplate reader supplied by Molecular Devices; PowerLab multi-lead physiology recorder supplied by AD Instruments, Australia.
Model building and grouping:
Diabetes rat model establishment: The mice were randomized randomly among two groups following 1 w of adaptation feeding: a blank control group (n=15) and a model group (n=80). By administering 60 mg/kg of streptozotocin sodium citrate buffer (pH=4.5) intraperitoneally to the model group, the diabetes model was produced[13]. For the rats in the blank control group, an equivalent dose of water was administered. Following 72 h, the rats showed indications of diabetes which means polyphagia, polydipsia and polyuria, as well as their fasting blood glucose level was detected reaching in excess of 16.00 mmol/l in the vein of the tail blood. The diabetes model was effectively developed in all 80 rats.
Pain model establishment: Before and (1-6) w after modeling, rats in the model group were acclimated to the metal cage for 10 min, the rat paw withdrawal response threshold was determined by stimulating the rat hind paw plantar using a von Frey filament with a different label, appearing obvious paw withdrawal was scored as positive, at the 4th w the threshold was reduced compared with the basal value von Frey label at 2 and above, which was the rat pain model established successfully[13]. Using the up-and-down technique, we found that the 50 % paw withdrawing threshold=10 log(x)+kd. Among them, five rats died during the pain model establishment, and a total of 75 diabetes rats successfully established the pain model.
Grouping and administration: 75 rats from the model group who had successfully modeled were randomly assigned to the model control group, which included the aescin medium, low, as well as high-dose categories, and the positive control group, each containing 15 rats. Based on past academics' research[14], intraperitoneal injection was used to provide aescin at doses of 0.5 mg/kg for the low-dose group, 1.0 mg/kg for the medium-dose group, and 1.5 mg/kg for the high-dose group; the positive control group received 0.25 mg/kg mecobalamin via gavage[15]; for 8 w, an identical volume of normal saline was intraperitoneally administered into the control group with no treatment as well as the model control group. Throughout the period of rising, all animals had unrestricted possession of food and water.
Blood glucose and body mass assay:
After 72 h of streptozotocin injection and immediately after the end of administration, the body mass of rats in each group in the two periods was weighed. After 72 h of modeling and 12 h of fasting after the completion of drug administration, their tail vein blood was sampled, and the fasting blood glucose of the two periods was measured.
Motor Nerve Conduction Velocity (MNCV) assay:
While wrapping up the experiments, rats were fixed by referring to references[16], the sciatic nerve was exposed after depilation, the stimulating electrode was placed at the right sciatic notch, the recording electrode was clamped between the second toe of the left foot and the ankle, and the MNCV was measured using a Power Lab multi-guide with three measurements of the mean.
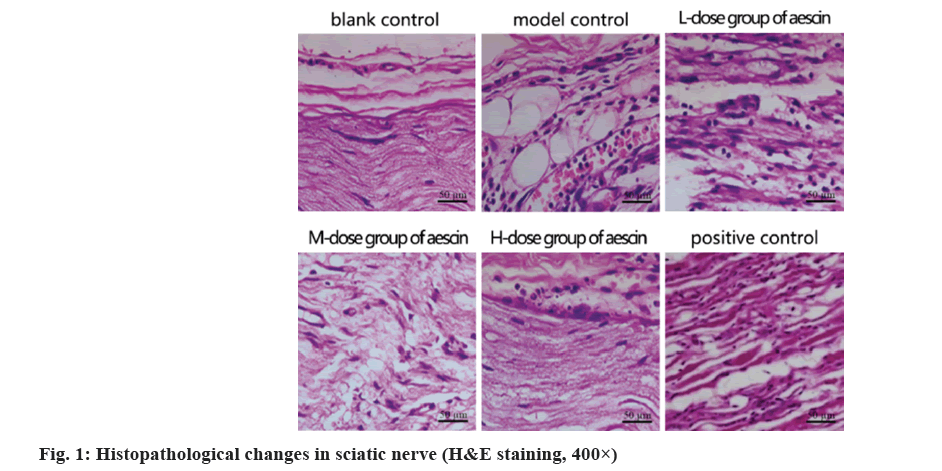
Observation of pathological changes in sciatic nerve tissues by H&E staining:
Tissues representing the correct sciatic nerves of the rats in all groups were collected at the conclusion of the study, fixed in formaldehyde and dehydrated in graded alcohol, sectioned (5 µm), stained as directed and viewed under a microscope.
Detection of inflammatory factors IL-1β, IL-6 and TNF-α:
At the end of the experiment, rats in each group were bled via the ophthalmic vein, and the supernatant was obtained after centrifugation, i.e., the actual contents or elements of TNF-α, IL-6 and IL-1β in serum belonging from every single group were detected following the instructions.
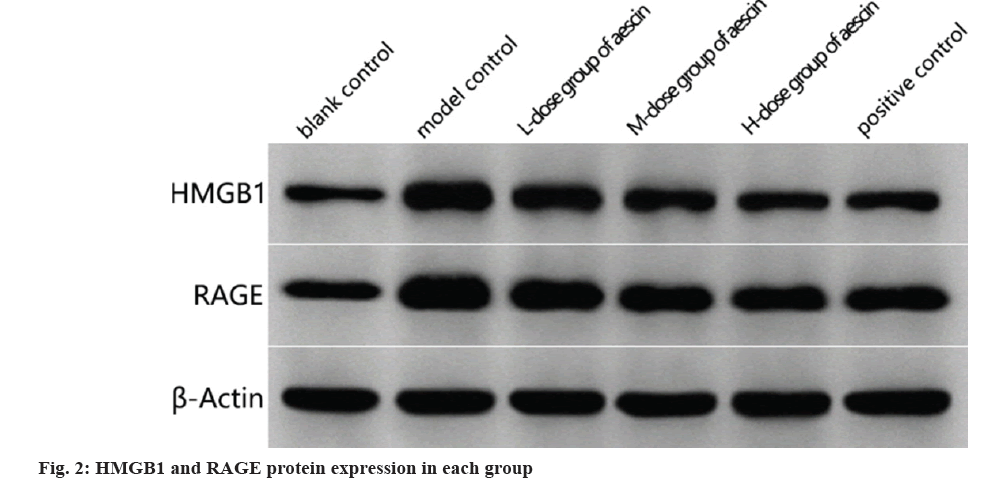
Western blot analysis of HMGB1 and RAGE proteins in sciatic nerve tissues:
Sciatic nerve tissue from each group was collected and total protein was detected by the BCA method, denatured, equal protein samples were loaded, electrophoresed on Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), Polyvinylidene Difluoride (PVDF) membranes were moved and blocked with 5 % Bovine Serum Albumin (BSA) for 1 h, and HMGB1 was then applied, hatred and a private allusion. The primary antibodies against-actin were diluted and incubated at 4° continuously; secondary antibodies were incorporated following a Phosphate Buffered Saline (PBS) wash, and the mixture was incubated at the ambient temperature for 60 min; after PBS washing and color development with color development solution, the gel imaging system was applied to scan, HMGB1 and RAGE levels of expression of proteins were determined using gray-value analyses.
Statistical methods:
Employing Statistical Package for the Social Sciences (SPSS) 26.0 as the statistical software, the researchers expressed their findings as the power source mean standard deviation, compared metrology data across categories employing the F-test if appropriate (otherwise, they employed the rank sum test), as well as utilized the variations among groups to make pair-wise contrasts employing the Student–Newman–Keuls (SNK)-q test. The threshold for statistical significance was established at 5 %.
Results and Discussion
The model control group had higher blood glucose concentrations than the blank control group; however, there was a statistically significant difference between the model and blank control groups in terms of body mass. Each dosage of aescin resulted in larger body mass and lower blood glucose levels in comparison to the model control group (p<0.05), as well as a dose effect (p<0.05) as shown in Table 1.
| Group | Blood glucose content (mmol/l) | Body mass (g) |
|---|---|---|
| Blank control | 4.25±1.63 | 393.87±11.23 |
| Model control | 23.14±5.23a | 232.75±7.81a |
| Aescin low-dose | 18.96±3.72ab | 298.07±9.85ab |
| Aescin medium-dose | 15.22±3.23abc | 312.37±6.11abc |
| Aescin high-dose | 10.23±2.06abcd | 327.64±9.32abcd |
| Positive control | 10.36±2.09abcd | 325.92±9.34abcd |
| F | 66.281 | 492.461 |
| p | 0.000 | 0.000 |
Note: Compared with the blank control group, ap<0.05; compared with the model control group, bp<0.05; compared with the aescin low-dose group, cp<0.05 and compared with the aescin medium-dose group, dp<0.05
Table 1: Changes in Blood Glucose and Body Mass of Rats in Each Group (x?±s, n=15)
The MNCV was greater in the blank control group of rats compared to the model control group (p<0.05). Although there were statistically significant differences between the MNCVs of the low-dose aescin group and the model control group (p<0.05), the MNCVs of the medium- and high-dose aescin group remained both larger than those of the model control group. Comparing the positive control group and the aescin high-dose group, the results showed no discernible change in MNCV (p>0.05) are shown in Table 2.
| Group | MNCV (m/s) |
|---|---|
| Blank control | 52.23±3.16 |
| Model control | 40.12±2.99a |
| Aescin low-dose | 41.37±3.11a |
| Aescin medium-dose | 43.34±2.23ab |
| Aescin high-dose | 45.14±3.74abc |
| Positive control | 45.19±3.78abc |
| F | 26.467 |
| p | 0.000 |
Note: Compared with the blank control group, ap<0.05; compared with model control, bp<0.05 and compared with the aescin low-dose group, cp<0.05
Table 2: Effects of Aescin on MNCV in DPN Rats (x?±s, n=15)
Sciatic nerve filaments with apparent morphology and normal cellular morphology throughout the blank control group; decreased fiber quantity and scattered dispersion with enlarged cells in the simulated control group. In contrast, the total amount of fibers dropped in the low- and medium-dose groups, as well as the cells in those groups were somewhat enlarged. The morphology of the positive control group and the group receiving high doses was intact, the fiber distribution appeared more regular, while the cells displayed no signs of degradation or necrosis as shown in fig. 1.
Rats in the model control group had substantially higher blood levels of TNF, IL-6, and IL-1 than rats in the blank control group (p<0.05). Medicine had an impact (p<0.05) on the blood levels of the aforementioned aggressive indicators in the aescin-treated rats compared to the model control group. No statistically significant differences were found (p>0.05) in TNF, IL-6, and IL-1 content comparing the positive control group with the aescin high-dose group as shown in Table 3.
| Group | IL-1β (μg/l) | IL-6 (μg/l) | TNF-α (μg/l) |
|---|---|---|---|
| Blank control | 2.32±0.39 | 107.33±24.52 | 125.93±14.43 |
| Model control | 10.37±0.93a | 249.58±22.76a | 273.29±24.39a |
| Aescin low-dose | 6.85±0.71ab | 203.89±22.37ab | 209.93±19.27ab |
| Aescin medium-dose | 5.61±0.52abc | 181.29±20.13abc | 182.99±12.74abc |
| Aescin high-dose | 4.41±0.43abcd | 159.16±18.15abcd | 163.85±16.32abcd |
| Positive control | 4.32±0.41abcd | 159.38±18.21abcd | 162.79±16.28abcd |
| F | 319.924 | 77.013 | 123.709 |
| p | 0.000 | 0.000 | 0.00 |
Note: Compared with the blank control group, ap<0.05; compared with the model control group, bp<0.05; compared with the aescin low-dose group, cp<0.05 and compared with the aescin medium-dose group, dp<0.05
Table 3: Serum Levels of TNF-α, IL-6 and IL-1 In the Various Rat Groups are Compared (x?±s, n=15)
In contrast, model control rats had more HMGB1 and RAGE proteins in their sciatic nervous tissues than the blank control group (p<0.05). There was a dosage effect (p<0.05) and reduced levels of HMGB1 and RAGE proteins in baseline sciatic nerves tissues of rats treated with each dose of aescin compared in the model control group as shown in fig. 2 and Table 4.
| Group | HMGB1 | RAGE |
|---|---|---|
| Blank control | 0.58±0.09 | 0.62±0.10 |
| Model control | 1.47±0.12a | 1.59±0.14a |
| Aescin low-dose | 1.17±0.07ab | 1.22±0.11ab |
| Aescin medium-dose | 1.06±0.05abc | 1.03±0.09abc |
| Aescin high-dose | 0.73±0.06abcd | 0.89±0.09abcd |
| Positive control | 0.78±0.09abcd | 0.92±0.08abcd |
| F | 235.276 | 153.448 |
| p | 0.000 | 0.000 |
Note: Compared with the blank control group, ap<0.05; compared with the model control group, bp<0.05; compared with the aescin low-dose group, cp<0.05 and compared with the aescin medium-dose group, dp<0.05
Table 4: HMGB1 and RAGE Protein Expression in Sciatic Nerve Tissue of Rats from Each Group (x?±s, n=15)
The main factors contributing to the pathogenesis of diabetes are inflammatory response, oxidative stress as well as abnormal polyol metabolism in the body[17,18]. DPN is its complication, which mostly occurs in the lower extremities, injures autonomic and bilateral peripheral nerves and, in severe cases, leads to amputation of the patients[19]. Modern medicine has not completely identified the pathophysiology of DPN, and several investigations have discovered higher levels of inflammatory markers including IL-6 and TNF-α in the serum of diabetic patients[20,21], congruent with our findings, pointing to a crucial role for inflammation in the development of illness. The pathogenesis of DPN is still unknown, and it is generally believed to have a close relationship with the body's glucose metabolism, oxidative stress, as well as inflammatory response[22]. Therefore, in the treatment of DPN, with the body inflammatory response as the therapeutic target, there may be an important clinical application value.
HMGB1 is widely found in lymphoid tissues, liver, brain, kidney and other tissues, and is a non-histone chromosomal structural protein. Stress causes the production of HMGB1, an inflammatory component and damaged signaling factor that controls inflammatory responses, triggers the secretion of additional inflammatory agents and supports the upregulation of RAGE transcription. Chronic problems of diabetes include HMGB1, which binds with great attraction to RAGE, the primary receptor in the classical communication cascade of HMGB1[23]. Numerous studies have documented that Advanced Glycation End Products (AGEs)/RAGE/Nuclear Transcription Factors-Kappa B (NF-κB) signaling pathways are involved in oxidative stress and excessive inflammatory response in diabetes treatment[24-26]. Some scholars have confirmed that the inflammatory signaling pathway axis of HMGB1-Toll-Like Receptor 4 (TLR4)/NF-κB is capable of mounting an inflammatory response that leads to demyelinating injury of the nerve[27]. We found that the amount of protein generated of HMGB1 and RAGE was significantly higher all through the sciatic nerve and other tissues regarding model control rats in comparison with creatures in the blank control group, suggesting that wherever DPN occurs, the degree of gene expression for HMGB1 and RAGE increases, leading to injury to the body. This may be related to HMGB1 and RAGE activation which in turn leads to oxidative stress and inflammatory response, but whether this is the case needs to be confirmed by further studies.
In recent years, TCM has attracted much attention in the treatment of diabetes and its complications. Aescin is a natural plant medicine with potent anti-inflammatory and anti-stress effects, and the main component is sodium aescin. Aescin is able to eliminate edema, maintain cell membrane stability, and the body microcirculation is improved, and it also inhibits the progression of inflammation. Clinical trials have shown that sodium aescin can inhibit inflammatory factor expression in rat alveolar lavage fluid and attenuate pulmonary fibrosis in rats with acute lung injury[28]. In addition, aescin can protect brain nervous tissue to some extent and reduce the risk of apoptosis and inflammatory response caused by ischemia-reperfusion[29,30]. In this study, we found that different doses of aescin downregulated the protein expression of HMGB1 and RAGE in rat sciatic nerve tissue to different degrees compared with the model control group, indicating that aescin can inhibit the activation of HMGB1 and RAGE in DPN rats. Further studies revealed that different doses of aescin all significantly reduced the contents of inflammatory factor TNF-α, IL-6 and IL-1β in serum, effectively alleviated the lesions of sciatic nerve tissue, and the effect of high-dose aescin was consistent with that of the positive control drug, indicating that aescin can achieve the effect of alleviating DPN by alleviating the inflammatory reaction mediated by HMGB1-RAGE, with a dose effect. However, whether oxidative stress is involved in this process still needs to be deeply investigated. In addition, in this study, we found that high doses of aescin significantly increased MNCV, whereas low and medium-doses of aescin did not significantly improve MNCV, indicating that high doses of aescin can improve DPN in rats by increasing MNCV. Our findings show that aescin, particularly at high dosages, may reduce DPN by blocking inflammatory reactions controlled by HMGB1 and RAGE.
In conclusion, aescin effectively ameliorates inflammatory response and further alleviates DPN in vivo by regulating HMGB1-RAGE levels, which lays the foundation for the clinical treatment of DPN.
Conflict of interests:
The authors declared no conflict of interests.
References
- Jin YP, Su XF, Li HQ, Wu JD, Ding B, Sun R, et al. The therapeutic effect of pancreatic kininogenase on treatment of diabetic peripheral neuropathy in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2016;124(10):618-21.
[Crossref] [Google Scholar] [PubMed]
- Vinik AI. Diabetic sensory and motor neuropathy. New Engl J Med 2016;374(15):1455-64.
- Zhou Y, Liu SX, Zhou YN, Wang J, Ji R. Research on the relationship between RAGE and its ligand HMGB1, and prognosis and pathogenesis of gastric cancer with diabetes mellitus. Eur Rev Med Pharmacol Sci 2021;25(3):1339-50.
[Crossref] [Google Scholar] [PubMed]
- Han R, Liu Z, Sun N, Liu S, Li L, Shen Y, et al. BDNF alleviates neuroinflammation in the hippocampus of type 1 diabetic mice via blocking the aberrant HMGB1/RAGE/NF-κB pathway. Aging Dis 2019;10(3):611-25.
[Crossref] [Google Scholar] [PubMed]
- Fukumoto J, Cox Jr R, Fukumoto I, Cho Y, Parthasarathy PT, Galam L, et al. Deletion of ASK1 protects against hyperoxia-induced acute lung injury. PloS One 2016;11(1):e0147652.
[Crossref] [Google Scholar] [PubMed]
- Yan H, Zhu L, Zhang Z, Li H, Li P, Wang Y, et al. HMGB1-RAGE signaling pathway in pPROM. Taiwan J Obstet Gynecol 2018;57(2):211-6.
[Crossref] [Google Scholar] [PubMed]
- Dias-Freitas F, Metelo-Coimbra C, Roncon-Albuquerque Jr R. Molecular mechanisms underlying hyperoxia acute lung injury. Respir Med 2016;119:23-8.
[Crossref] [Google Scholar] [PubMed]
- Chen Z, Yu S. Protective effect and mechanism of aescin against blood-retinal barrier damage in diabetic rats. Zhejiang J Integr Tradit Chin Med Western Med 2019;29(7):530-3.
- Gupta SC, Kunnumakkara AB, Aggarwal S, Aggarwal BB. Inflammation, a double-edge sword for cancer and other age-related diseases. Front Immunol 2018;9(2):2160.
[Crossref] [Google Scholar] [PubMed]
- Cheng CL, Chao WT, Li YH, Ou YC, Wang SS, Chiu KY, et al. Escin induces apoptosis in human bladder cancer cells: An in vitro and in vivo study. Eur J Pharmacol 2018;840:79-88.
[Crossref] [Google Scholar] [PubMed]
- Yan B, Liu S, Zhu M. Clinical effects of Urinary Kallindinogenase combined with sodium aescin in diabetes mellitus with acute cerebral infarction. Med J Chin PeoplE Health 2020;32(2):2-4.
- Wang L, Zhao X. Effect of alprostadil combined with sodium aescin in the treatment of peripheral neuropathy in type 2 diabetes mellitus. J Chin Prescrip Drug 2019;17(10):97-8.
- Wang H, Wang Y, Ou W. Pain model in a rat model of diabetic peripheral neuropathy. Chin J Pain Med 2007;13(1):43-5.
- Wu X, Yuan D, Chen K. Effect of aescin on the pharmacokinetics of lovastatin in a hyperlipidemic rat model in vivo. Pract Pharm Clin Remedies 2020;23(3):5.
- Fang Y, Wang Y, Zhou W. Effects of Huangqi Guizhi Wuwu decoction on AGEs/RAGE/NF-κB signaling pathway in a rat model of diabetic peripheral neuropathy. Chin J Exp Tradit Med Formula 2020;26(13):52-8.
- Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary P, Waberski B. Subclinical neuropathy among diabetes control and complications trial participants without diagnosable neuropathy at trial completion: Possible predictors of incident neuropathy? Diabetes Care 2007;30(10):2613-8.
[Crossref] [Google Scholar] [PubMed]
- Gnudi L, Coward RJ, Long DA. Diabetic nephropathy: Perspective on novel molecular mechanisms. Trends Endocrinol Metab 2016;27(11):820-30.
[Crossref] [Google Scholar] [PubMed]
- Gao F. Effect of emodin on blood glucose and peripheral neuropathy in diabetic rats. Chin J Clin Res 2019;11(7):30-2.
- Hu J, Chen Z. Surgical management of diabetic peripheral neuropathy. Fudan Univ J Med Sci 2016;43(5):615-9.
- Park BK, Lee EA, Kim HY, Lee JC, Kim KS, Jeong WH, et al. Fatty liver and insulin resistance in the liver-specific knockout mice of mitogen inducible gene-6. J Diabetes Res 2016;2016:1632061.
[Crossref] [Google Scholar] [PubMed]
- Bruchfeld A, Wendt M, Miller EJ. Macrophage migration inhibitory factor in clinical kidney disease. Front Immunol 2016;7:8.
[Crossref] [Google Scholar] [PubMed]
- Liu M, Liang J. Treatment of acupuncture drug combination in rheumatics syndrome of diabetes. Nei Mongol J Tradit Chin Med 2018;37(8):41.
- Mei YW, Huang TL, Chen X, Yu SX, Li J, Zhang Z, et al. HMGB1-RAGE pathway contributes to the abnormal migration of endogenous Subventricular zone neural progenitors in an experimental model of focal Microgyria. J Mol Neurosci 2022;72(1):56-68.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Jiao N, Jiang M, Liu L, Zhu Y, Wu H, et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J Cell Mol Med 2020;24(11):6083-95.
[Crossref] [Google Scholar] [PubMed]
- Han L, Yuan X, Yang Z. Regulation of HMGB1/RAGE/NF-κB pathway by Pueraria mirifica on ameliorating cognitive impairment in diabetic rats. Pharmacol Clin Chin Materia Med 2020;36(1):7.
- Gao X, Shen Y. Relationship of expression of key proteins in HMGB1-RAGE/TLRs-NF-κB signaling pathway and diabetic nephropathy. Transl Med J 2020;51(6):15-23.
- Cheng S, Zhou X, Li X. Exploration of the mechanisms underlying the protective effects of Danggui Sini decoction on peripheral neuropathy in diabetic rats based on NF-κB signaling pathway. J Changchun Univ Chin Med 2019;35(1):128-31.
- Huang S, Lin T, Meng L. Sodium aescin blocks TGF-β mediated signaling pathways on pulmonary fibrosis and inflammatory factors in rats with acute lung injury. Guiding J Tradit Chin Med Pharmacol 2022;28(6):40-4373.
- Zhang L, Fei M, Wang H, Zhu Y. Sodium aescinate provides neuroprotection in experimental traumatic brain injury via the Nrf2-ARE pathway. Brain Res Bull 2020;157:26-36.
[Crossref] [Google Scholar] [PubMed]
- Guo Q, Huo K, Song Y. Protective mechanism of β-aescin sodium against focal cerebral ischemia-reperfusion injury in rats. Genom Appl Biol 2020;39(7):3312-7.