- *Corresponding Author:
- Zhongya Yan
Department of Cardiovascular Surgery, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province 230601, China
E-mail: yan20047@163.com
| Date of Received | 14 January 2023 |
| Date of Revision | 09 September 2023 |
| Date of Acceptance | 21 March 2024 |
| Indian J Pharm Sci 2024;86(2):537-545 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
At present, the effect of angiotensin II on the biological behavior of smooth muscle cells of thoracic aortic aneurysm is not clear. In this study, thoracic aortic aneurysm rat model (thoracic aortic aneurysm model group) was prepared by applying calcium chloride to blood vessels, and normal rats were taken as a sham operation group (sham group), with 10 rats in each group. The changes of angiotensin II content in plasma and left ventricular myocardial tissue were detected. Normal rat thoracic aortic smooth muscle cells were extracted and divided into the control group, angiotensin II group (1 μg/ml angiotensin II), and angiotensin II+angiotensin receptor blocker group (1×10-6 mol/l candesartan+1 μg/ml angiotensin II). The cholecystokinin cell counting kit-8, flow cytometry, Transwell chamber, scratch healing, and Western blot experiments were used to detect cell proliferation, apoptosis, invasion, migration, and the expression changes of Janus kinase/signal transducers and activators of transcription pathway-related proteins. The results showed that angiotensin II content in left ventricular myocardium of thoracic aortic aneurysm group was higher than that of Sham group (p<0.01). Compared with the control group, angiotensin II group showed increased cell proliferative activity, number of invaded cells, mobility, and expression levels of Ras-related C3 botulinum toxin substrate 1, phosphorylated-Janus kinase 2, phosphorylated-signal transducers and activators of transcription 1, and phosphorylated-signal transducers and activators of transcription 3 proteins, and decreased apoptosis rate (p<0.05). Compared with the angiotensin II group, the cell proliferative activity, the number of invaded cells, and mobility as well as the expression levels of Ras-related C3 botulinum toxin substrate 1, phosphorylated- Janus kinase 2, phosphorylated-signal transducers and activators of transcription 1, and phosphorylatedsignal transducers and activators of transcription 3 proteins in the angiotensin II+angiotensin receptor blocker group were decreased, and the apoptosis rate was increased (p<0.05). These results indicated that angiotensin II could induce the proliferation, invasion, and migration of thoracic aortic aneurysm smooth muscle cells, and promote apoptosis.
Keywords
Thoracic aortic smooth muscle cells, angiotensin II, Janus kinase, signal transducers, aneurysm
Aneurysm is the 2nd most common disease affecting the aorta. Approximately 26 000 out of 100 000 people die of aneurysm rupture every year[1]. The aorta progressively grows, and there are no direct therapeutic drugs. The main treatment methods include surgical resection or noninvasive drug treatment[2]. In recent years, the incidence of Thoracic Aortic Aneurysm (TAA) has risen yearly, and it is a disease of high-risk neoplastic expansion caused by structural impairment and weakened strength of the aortic wall[3]. Mutations in ACTA, which encodes filamentous protein A, and myosin light-chain kinase also lead to TAA [4-6]. Vascular smooth muscle cells are the main cellular component that constitutes the vascular wall structure and maintains vasotension. The structural or functional changes in vascular smooth muscle cells are the main pathological basis for the induction of hypertensive atherosclerosis, restenosis after angioplasty, and aneurysm[7,8]. In normal and mature vascular tissues, smooth muscle cells are in a quiescent state. Vascular injury causes the abnormal proliferation of smooth muscle cells[9]. The proliferation, apoptosis, migration, extracellular matrix synthesis, and phenotypic transformation of vascular smooth muscle cells play vital roles in vascular remodeling.
Angiotensin II (Ang II) is a factor that promotes vascular smooth muscle cell phenotypic transformation. It can effectively activate the proliferation, migration, and secretion of cells[10]. Ang II induces the proliferation, migration, and serious reactions of vascular smooth muscle cells and further induces pathological thickening. It was also verified that Ang II induces cell hypertrophy and programmed cell apoptosis. Furthermore, it is involved in atherosclerosis, vascular and cardiac remodeling[11]. Ang II receptors are divided into several subtypes, including Ang II Type 1 Receptor (AT1R), AT2R, AT3R, and AT4R. AT1R and AT2R play a dominant role[12]. Related research demonstrates that the pathophysiological role of Ang II is mediated by AT1R and that the activation of Ang II/AT1R engages in the proliferation and migration of cells[13]. Candesartan is an antagonist of AT1R that has an antagonistic effect on Ang II. The Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) signaling pathway is the common pathway for most cytokines and active substances to exert biological effects. It engages in the proliferation and anti-apoptosis processes of multiple cells[14].
To explore the role of Ang II in the biological behavior of thoracic aortic smooth muscle cells, the change in Ang II content in TAA was first analyzed. Then, the influence mechanism of Ang II and its antagonist (candesartan) on the proliferation, apoptosis, migration, and invasion of the cells was studied in vitro. This research was conducted to provide experimental materials for the regulation of Ang II- and candesartan-mediated JAK/STAT signaling pathways and the biological behavior of the cells.
Materials and Methods
Materials:
The experimental materials included Ang II (Sigma-Aldrich, United States of America (USA)), male Sprague-Dawley (SD) rats (8~10 w old, 300~400 g, Beijing Huafukang Biotechnology Co., Ltd.,), pentobarbital sodium (Wuhan Jinnuo Chemical Co., Ltd.,); Ang II content detection kit (Beijing Furui Biotechnology Co., Ltd.,), candesartan (Dalian Meilun Biotechnology Co., Ltd.), Dulbecco's Modified Eagle Medium (DMEM)-F12 culture medium (Gibco, USA), fetal calf serum, Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) kit (Becton, Dickinson and Company, USA), Cell Counting Kit-8 (CCK-8) (Corning Incorporated, USA), Transwell cellule, Radio-Immunoprecipitation Assay (RIPA) lysis buffer (Shanghai Beyotime Biotechnology Co., Ltd.,), Polyvinylidene Fluoride (PVDF) membrane, Bicinchoninic Acid (BCA) concentration quantitative kit (BCA), Enhanced Chemiluminescence (ECL), anti-Rasrelated C3 botulinum toxin substrate 1 (Rac-1), anti-JAK2, anti-STAT1, anti-STAT3, and anti- Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (Beijing Annoron Biotechnology Co., Ltd.,). All experiments in this study were conducted in strict accordance with the operating rules for experimental animals and were approved by the Ethics Committee of Shanghai 10th people’s hospital (No: 20211425).
Preparation of TAA animal models:
Twenty SD rats were randomly enrolled into the sham and TAA groups (10 in each). Then, 0.4 % 40 mg/kg pentobarbital sodium (China Pharmaceutical Group Shanghai Chemical Reagent Company) was injected into the abdominal cavity for anesthesia. After tracheotomy, endotracheal intubation and fixation were performed on the rats in the TAA group. Next, the Serine Palmitoyltransferase (SPT)-ALC-V8S small animal ventilator (Nanjing Weimeite Scientific Instrument Co., Ltd., China) was connected. Respiratory frequency, the ratio of inspiration/expiration, and pressure support were set as 100 times/min, 1:1.5, and 0.01 MPa, respectively. After that, thoracotomy was performed at the 5th intercostal space of the left margin of the sternum, and the thoracic aorta was fully exposed and dissociated for 1 cm. Next, the tunica adventitia was covered with cotton gauze soaked with 0.5 mol/l calcium chloride for 20 min. After the blood vessel changed and apparently dilated by 1.5 times, the cotton gauze was removed, and then the thoracic cavity was rinsed with physiological saline. Next, the ventilator was adjusted to promote left lung recruitment. Moreover, the thoracic cavity drainage tube was indwelled, venting was performed, and pleural effusion was extracted. Next, the ventilator was removed, the discharge in the respiratory tract was cleared, and the respiratory tract and skin were closed successively. After treatment, 10 000 IU penicillin was administered for anti-infection for 3 consecutive days. After tracheotomy, the skin of rats in the sham group was sutured, and antiinfection was carried out.
Detection of Ang II in plasma and left ventricular myocardial tissue:
Then, 0.4 % 40 mg/kg pentobarbital sodium was injected into the abdominal cavity for anesthesia. Then, 3 ml arterial blood was extracted from the abdominal cavity, and anticoagulation was performed. After that, it was centrifuged at 4000 rpm/min for 20 min for serum acquisition. After blood collection, pulpotomy was performed to sacrifice the rats. Then, 500 mg of left ventricular myocardial tissue was extracted, minced, and homogenized. Next, it was centrifuged at low temperature and 10 000 rpm/min for 20 min, after that supernatant was removed. A radioimmunoassay was adopted to detect Ang II content in serum and left ventricular myocardial tissue using an Ang II content detection kit.
Culture and grouping of thoracic aortic smooth muscle cells:
SD rats (4 w old) were injected with 0.4 % 40 mg/ kg pentobarbital sodium through the abdominal cavity for anesthesia. Then, pulpotomy was performed to sacrifice them. After thoracotomy, the thoracic aorta was dissociated along the aortic arch and slit pores of the aorta. After that, it was completely cut and placed in precooled phosphate buffer. Next, blood vessels were cut into small cubes of 1 mm2 and then placed into a culture flask. DMEM-F12 culture medium containing 10 % fetal calf serum and 1 % streptomycin was added. Next, they were incubated for 4 h in a D180 incubator containing 5 % Carbon dioxide (CO2) at a constant temperature and humidity of 37° (China, Shenzhen RWD Life Technology Co., Ltd.,). The culture flask was turned over, and the culture was continued for 7 d after the tissue was covered with the medium. After the flask bottom was filled with cells, passage was carried out. The 3rd to 8th generations of thoracic aortic smooth muscle cells were selected for subsequent tests. The cells were counted, and the density was adjusted to 3×105/ml. Next, the cells were seeded into a 12-well plate, and the culture was continued for 14 d. The culture solution was changed every 3 d.
Thoracic aortic smooth muscle cells were seeded into a 96-well plate (3×103/well) and divided into 3 groups (3 repeated wells in each). The cells in the control group were routinely cultured in DMEM-F12 culture medium containing 10 % fetal calf serum and 1 % streptomycin. Based on the above culture method, the cells in the Ang II group were treated with 1 μg/ml Ang II, while those in the Ang II+Angiotensin Receptor Blocker (ARB) groups were first treated with 1×10-6 mol/l candesartan and cultured for 24 h. After that, 1 μg/ ml Ang II was added.
Detection of proliferative activity by CCK-8:
Thoracic aortic smooth muscle cells were seeded into a 96-well plate (3×103/well) and divided into 3 groups (3 repeated wells in each). After that, a CCK-8 kit was employed to detect proliferative activity. Next, they were incubated at 37° for 0 h, 12 h, 24 h, 36 h, and 48 h. Then, 20 μl CCK-8 reagent was added to each well and cultured in the incubator for 4 h. Finally, a multifunctional enzyme marker was utilized to measure the absorbance (Optical Density (OD)) of each well at 490 nm.
Detection of cell apoptosis by flow cytometry:
Thoracic aortic smooth muscle cells were enrolled into different groups and then resuspended in phosphate buffer. Then, 5 μl Annexin V-FITC was added and mixed. Next, 5 μl PI was added, mixed, and incubated at room temperature for 15 min. After 400 μl binding buffer was added to the mixture, the mixture was immediately subjected to the test machine, and apoptosis and necrosis were detected using Attune CytPix flow cytometry (Thermo Fisher Scientific, US). Finally, FlowJo 7.6.1 (Becton, Dickinson & Company, USA) was used to analyze and calculate the cell apoptosis rate.
Detection of cell invasion by Transwell cellule assay:
During passage, 100 μl of cell suspension was added to the Transwell cellule, and thoracic aortic smooth muscle cells were enrolled into different groups. The Transwell cellules in the control, Ang II, and Ang II+AT1R groups were added to routine culture medium, medium containing Ang II, and medium containing Ang II and AT1R. Then, they were routinely cultured for 6 h. Next, Transwell cellules were extracted, and the original medium was eliminated. After that, the cells were rinsed with aseptic phosphate buffer and then fixed with polyformaldehyde solution for 10 min. After air drying, the cells were stained with crystal violet, and the upper nonmigrating cells were slightly swabbed with a sterile cotton swab. After they were rinsed with phosphate buffer, they were observed under an E100 light microscope (Nikon, Japan) and then photographed for counting.
Detection of cell migration by scratch healing:
Thoracic aortic smooth muscle cells were seeded into a 6-well plate. Once confluence reached 90 %, a 100 μl sterilization probe was employed to line the bottom of the petri dish. Then, the cells were rinsed with phosphate buffer and grouped. Scratch healing was observed at 0 h and 24 h during the culture, and the cell migration rate was calculated based on equation.
Cell migration rate=(scratch width at 0 h-scratch width at 24 h)/scratch width at 0 h×100 %
Detection of protein levels by Western blot:
Thoracic aortic smooth muscle cells were grouped and collected after 24 h. Then, RIPA buffer was added for the extraction of total protein. The BCA method was adopted to detect the concentration of extracted total protein. In addition, an appropriate amount of separation and spacer gel was prepared, and then Sodium Dodecyl Sulphate (SDS) polyacrylamide gel electrophoresis was performed to separate target proteins. Next, protein bands were cut and transferred to a PVDF membrane. After that, they were placed in the confining liquid containing 5 % skimmed milk powder and sealed at room temperature for 2 h. Then, Rac- 1, p-JAK2, p-STAT1, p-STAT3, and GAPDH (working concentration 1:300) primary antibodies were added and incubated at 4° overnight. After that, horseradish peroxidase-conjugated Immunoglobulin G (IgG) secondary antibody (working concentration 1:2000) was added and incubated at room temperature for 2 h. Next, ECL reagent was utilized for color rendering of protein bands, and they were developed and photographed in a Bio-5000 Plus gel imaging system (Bio-Rad, USA). Finally, the relative expression of target proteins was analyzed with ImageJ (National Institutes of Health, USA).
Statistical processing:
Statistical Package for the Social Sciences (SPSS) 19.0 (IBM company USA) was employed for data analysis and processing. All experimental data were denoted by the mean±standard deviation (x̄ ±s). One-factor analysis of variance was carried out for the differences among several groups, and pairwise comparison was performed on the data that conformed to homogeneity of variance using the Least Significant Difference (LSD) test. p<0.05 and p<0.01 revealed suggested significant and extremely significant statistical significance.
Results and Discussion
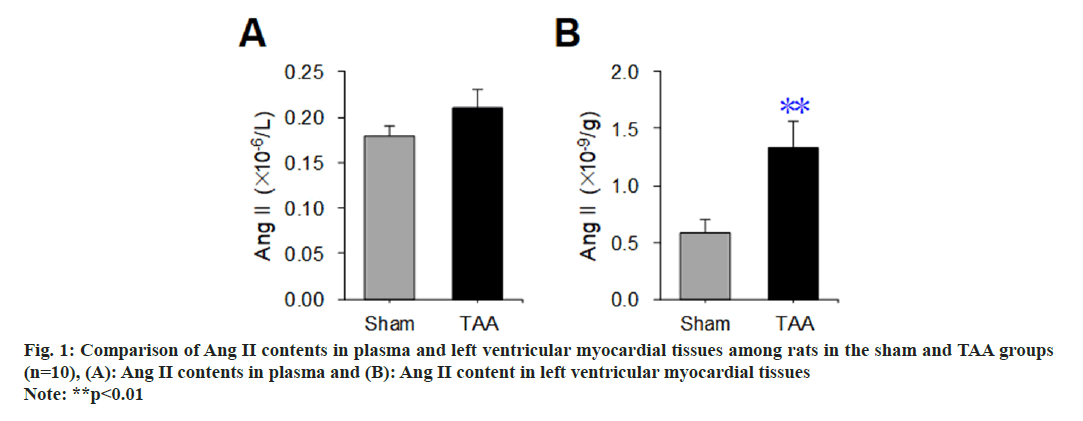
A radioimmunoassay was used to detect the difference in Ang II content in plasma and left ventricular myocardial tissue of rats between the sham operation group and the TAA model, and the results are shown in fig. 1. Compared with the sham operation group, there was no significant difference in the plasma Ang II content of rats with TAA (p>0.05) (fig. 1A). Compared with the sham operation group, the Ang II content in the left ventricular myocardial tissue of rats with TAA was increased significantly (p<0.01) (fig. 1B).
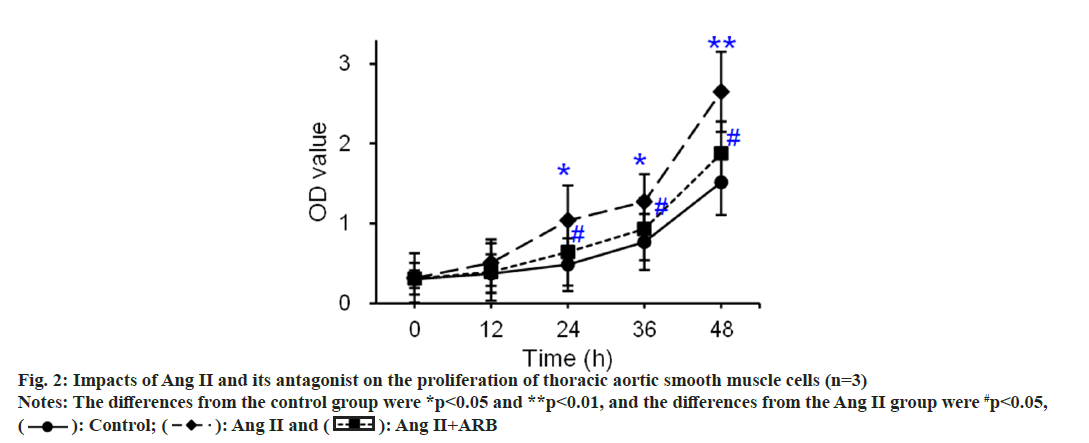
The thoracic aorta was cultured with tissue mass method, and primary thoracic aortic vascular smooth muscle cells were identified by α2-actin. The results showed that α2 actin was positive in thoracic aortic vascular smooth muscle cells. Under the inverted microscope, the passaged thoracic aortic vascular smooth muscle cells showed typical “peak-and-valley” like growth. The CCK-8 method was used to detect and compare the effects of Ang II and its receptor antagonists on the proliferation of thoracic aortic vascular smooth muscle cells, and the results are shown in fig. 2. The OD values of the control, Ang II, and Ang II+ARB groups gradually increased as the culture progressed. Compared with that in the control group, the OD value apparently rose at 24 h and 36 h (p<0.05) and dramatically rose at 48 h (p<0.01) in the Ang II group. Compared with that in the Ang II group, the OD value notably increased at 24 h, 36 h, and 48 h in the Ang II+ARB group (p<0.05).
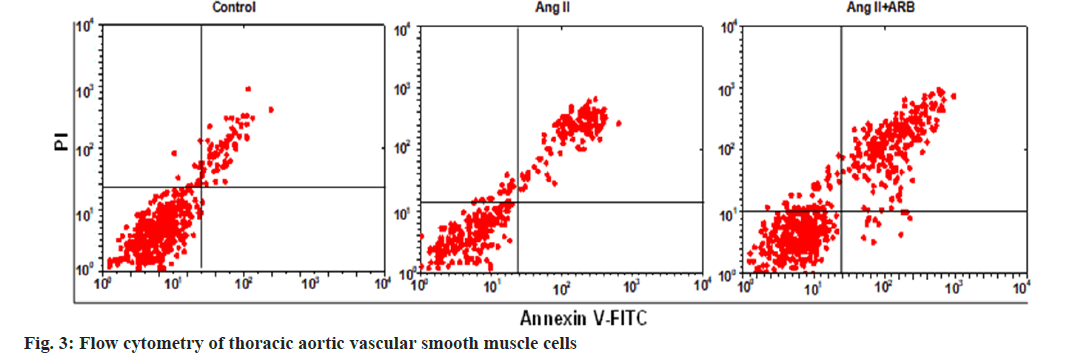
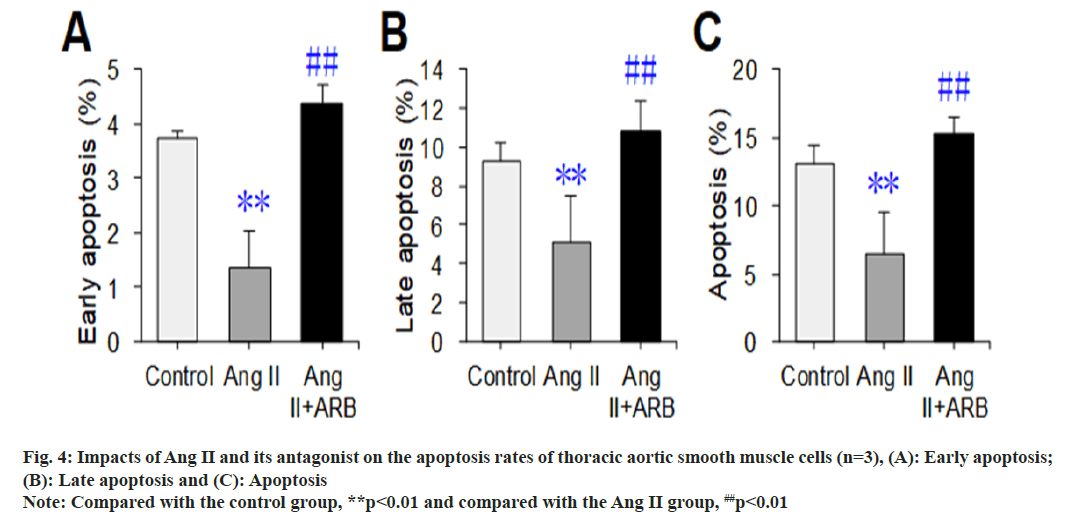
After apoptosis was detected by flow cytometry, the flow cytometry image was divided into four phenomena, namely, LL, UL, UR, and LR, where LL represented normal cells, UL represented necrotic cells, UR represented late apoptotic cells, and LR represented early apoptotic cells. In this study, early apoptosis, late apoptosis and total apoptosis (early apoptosis+late apoptosis) were selected to compare the apoptosis rates, and the results are shown in fig. 3 and fig. 4. The early, late, and total apoptosis rates of the cells in the Ang II groups were dramatically lower than those in the control group (p<0.01). Compared with those in the Ang II group, the early, late, and total apoptosis rates of the cells dramatically increased in the Ang II+ARB group (p<0.01).
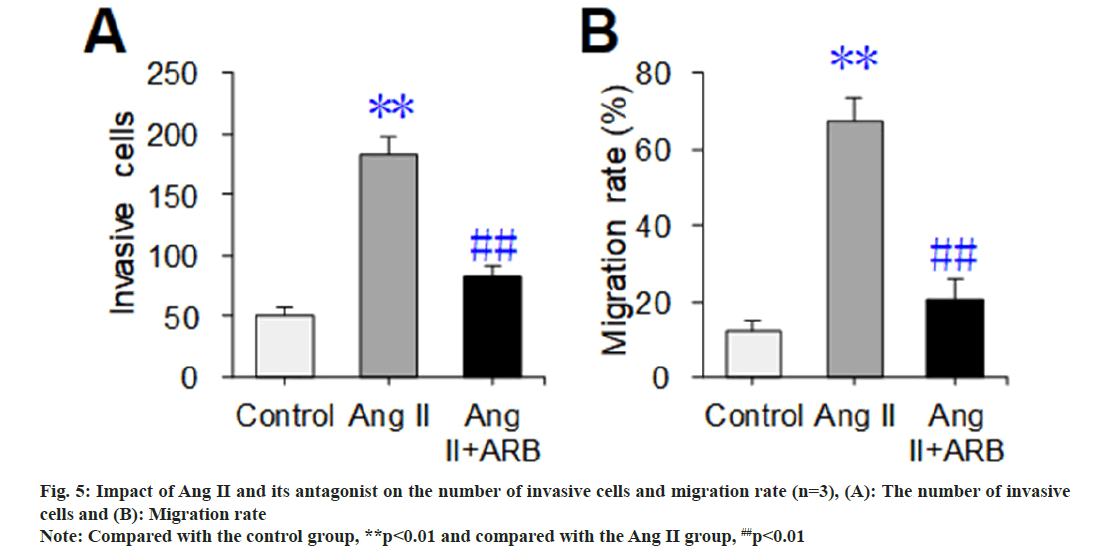
The Transwell chamber test was used to detect and compare the effects of Ang II and its receptor antagonists on the invasion and mobility of thoracic aortic vascular smooth muscle cells, and the results are shown in fig. 5. The number of invasive cells and migration rate dramatically increased in the Ang II group compared with the control group (p<0.01). Compared with those in the Ang II group, the number of invasive cells and migration rate were dramatically lower in the Ang II+ARB group (p<0.01).
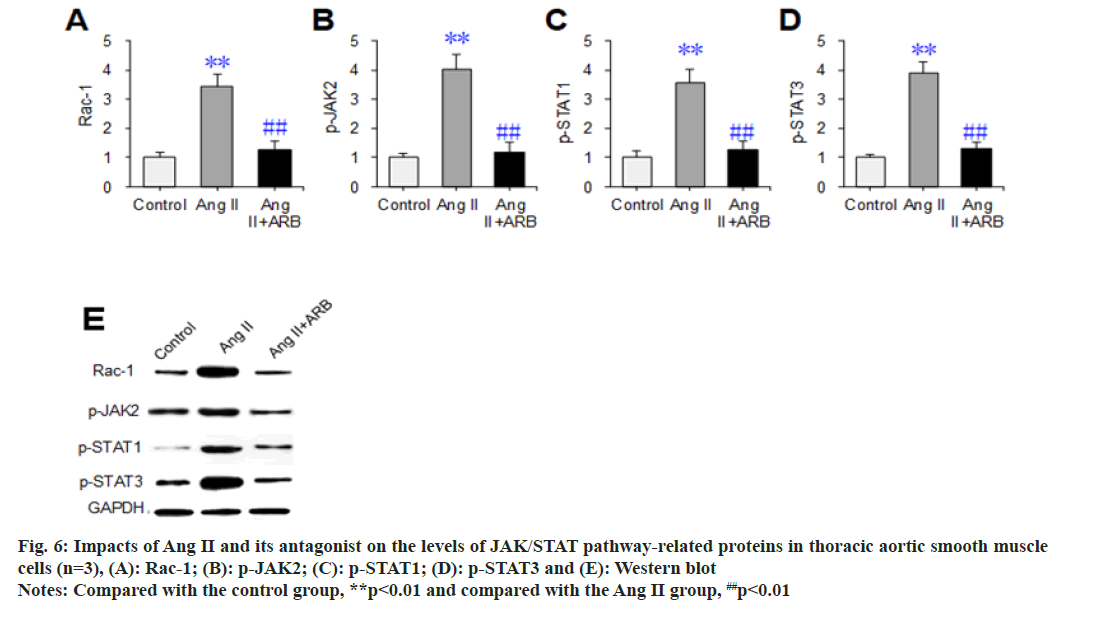
Western blot assays were used to detect and compare the effects of Ang II and its receptor antagonists on the expression levels of the JAK/ STAT signaling pathway-related proteins Rac- 1, p-JAK2, p-STAT1, and p-STAT3 in thoracic aortic vascular smooth muscle cells. The results are shown in fig. 6. The relative levels of Rac- 1, p-JAK2, p-STAT1, and p-STAT3 dramatically increased in the Ang II group compared with the control group (p<0.05). Compared with those in the Ang II group, the relative levels of the above proteins were dramatically lower in the Ang II+ARB group (p<0.01).
Fig. 6: Impacts of Ang II and its antagonist on the levels of JAK/STAT pathway-related proteins in thoracic aortic smooth muscle cells (n=3), (A): Rac-1; (B): p-JAK2; (C): p-STAT1; (D): p-STAT3 and (E): Western blot Note: Compared with the control group, **p<0.01 and compared with the Ang II group, ##p<0.01
The diameter of the thoracic aorta with TAA is 1.5 times as long as that of the normal thoracic aorta[15]. TAA is a high-risk disease that is pathologically based on local dilatation of thoracic arterial vessels due to dysplasia. TAA results from hypertension, atherosclerosis, or viral infection[16,17]. At present, the methods for constructing aneurysm models mainly include trypsin intraluminal injection, thinning of the vascular middle layer, balloon dilatation, surgical insertion of blood vessels, and vascular patch method. However, these operations are complex, and abdominal aortic aneurysm is the main aneurysm[18,19]. In recent years, some researchers have pointed out that calcium chloride external application to blood vessels is effective for TAA animal models, and it has a good effect in simulating the formation of human aneurysms[20]. Therefore, in this study, the rats were used as experimental animals, and the TAA animal model was prepared by applying calcium chloride to the blood vessels to investigate the role of Ang II in the biological behavior of aortic smooth muscle cells of TAA. The proliferation and migration of arterial smooth muscle cells are the initiating factors for angioproliferative diseases, and smooth muscle cells have become key target cells for the prevention and treatment of cardiovascular diseases. Myocardial endogenous Ang II is an essential effector that induces myocardial hypertrophy and cardiac fibrosis[21]. According to related research, Ang II regulates the apoptosis, proliferation, and migration of cells and leads to the deposition of extracellular collagen interstitium at different periods[22,23]. Moreover, Ang II plays a vital role in maintaining normal functions and structural integrity of the vessel wall. The research findings suggested that Ang II content was apparently higher in left ventricular myocardial tissue among TAA rats than among normal rats. The results suggested that TAA caused the increase of Ang II content in the left ventricular myocardial tissue of rats, indicating that there might be a correlation between Ang II content and TAA. Ang II is the key vasoactive substance in the renin-Ang system, and it maintains the stability of cardiovascular functions[24]. Ang IImediated vascular inflammatory reactions could link the interaction between hypertension and other cardiovascular diseases and are involved in the progression of cardiovascular diseases through multiple mechanisms.
Thoracic arterial smooth muscle cells are involved in TAA progression, and their excessive proliferation causes dysplasia of arterial vessels and induces TAA[25]. It was found that 1 μg/ml Ang I induced the proliferative activity of thoracic arterial smooth muscle cells and promoted cell invasion and migration, which was consistent with the findings of Zhang et al.[26]. They showed that Ang II could induce the proliferation, migration, and invasion of the cells. Ang II exerts its physiological effect mainly by combining AT1R with AT2R. Moreover, it can activate AT1R and enable it to promote tumor growth and metastasis[27]. AT2R activation leads to vasodilatation and programmed cell death and suppresses cell proliferation[28]. AT1R antagonist suppressed angiogenesis and vascular metastasis in tumors. According to the research findings, the proliferative activity, invasion, and migration of Ang II-induced thoracic arterial smooth muscle cells were apparently inhibited after candesartan was added. It was demonstrated that Ang II engaged in the proliferation, invasion, and migration of the cells by regulating AT1R levels.
The JAK/STAT pathway is mainly composed of the tyrosine kinase-associated receptor, tyrosine kinase JAK, and its production effector transcription factor STAT, which is highly conserved in the process of cell proliferation and blood production. The pathway is regulated by a large number of internal and environmental stimuli, which can increase the plasticity of cell or tissue responses. The JAK/STAT pathway mediates almost all immune responses and is also capable of promoting tumor cell survival, immune escape, and persistent inflammatory responses[29]. The cytokine effect mediated by the JAK/STAT pathway cannot only mediate the interferon effect of the antiviral/antiproliferative agent but also mediate the effects of erythropoietin for the treatment of anemia, thrombopoietin for the treatment of thrombocytopenia, and Granulocyte Colony-Stimulating Factor (G-CSF) for the treatment of neutropenia[30]. At present, it has been confirmed that the JAK/STAT pathway is closely related to hematological diseases such as polycythemia, thrombocytosis, and leukemia, as well as autoimmune diseases such as rheumatoid arthritis, lupus erythematosus, and mandatory spondylitis[31]. Rabkin et al.[32] indicated that Matrix Metalloproteinases (MMPs) were related to the pathogenesis of aortic aneurysm, while MMPs could activate JAK/STAT signaling pathways and thus reduce the related components in the aortic wall, resulting in the ineffective maintenance of aortic size, vascular dilation and aneurysm formation. The research findings demonstrated that Ang II inhibited the apoptosis of thoracic arterial smooth muscle cells and promoted Rac- 1, p-JAK2, p-STAT1, and p-STAT3, which was consistent with the conclusion drawn by Ruwhof et al.[33]. They showed that the phosphorylation level of JAK/STAT rose in myocardial cells after Ang II stimulation. The above research findings revealed that Ang II induced smooth muscle cells by regulating JAK/STAT signal transduction. In addition, it was found that the levels of Rac-1, p-JAK2, p-STAT1, and p-STAT3 were lower after candesartan was added. Hence, AT1R antagonists could activate downstream signaling pathways by inhibiting Ang II for the effective treatment of cell proliferation, hypertrophy, and cardiovascular remodeling.
Based on TAA animal model, the role of Ang II in the biological behavior of aortic smooth muscle cells of TAA was explored, and it was found that Ang II levels abnormally increase in TAA, and Ang II can induce the proliferation, invasion, and migration of thoracic arterial smooth muscle cells and suppress their apoptosis. Candesartan, known as an AT1R antagonist, could inhibit the signal transduction of JAK/STAT pathways. Furthermore, it inhibited the proliferation, invasion, and migration of the cells and promoted apoptosis. In subsequent research, TAA rat models should be prepared to analyze the mechanism of AT1R and AT2R antagonists in the progression of TAA. The research findings provided references for the search for therapeutic targets and the formulation of treatment plans for TAA.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang W, Guo Z, Xie D, Lin Z, Lin R. Relationship between MMP-9 gene polymorphism and intracranial aneurysm. Cell Mol Biol 2022;68(1):14751.
[Crossref] [Google Scholar] [PubMed]
- Tawk RG, Hasan TF, D’Souza CE, Peel JB, Freeman WD. Diagnosis and treatment of unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage. Mayo Clin Proc 2021;96(7):1970-2000.
[Crossref] [Google Scholar] [PubMed]
- Jian D, Tianxing N. Prognostic biomarkers and molecular mechanisms for thoracic aortic aneurysms. J Biol Regul Homeost Agents 2022;36(3):599-609.
- Morisaki H, Akutsu K, Ogino H, Kondo N, Yamanaka I, Tsutsumi Y, et al. Mutation of ACTA2 gene as an important cause of familial and nonfamilial nonsyndromatic thoracic aortic aneurysm and/or dissection (TAAD). Hum Mutat 2009;30(10):1406-11.
[Crossref] [Google Scholar] [PubMed]
- Siddiqui ST, Fisher SD. Heritable FLNA gene mutation in a patient with thoracic aortic aneurysm. Case Rep 2022;4(2):87-90.
[Crossref] [Google Scholar] [PubMed]
- Shalata A, Mahroom M, Milewicz DM, Limin G, Kassum F, Badarna K, et al. Fatal thoracic aortic aneurysm and dissection in a large family with a novel MYLK gene mutation: Delineation of the clinical phenotype. Orphanet J Rare Dis 2018;13(1):41.
[Crossref] [Google Scholar] [PubMed]
- Grootaert MO, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res 2018;114(4):622-34.
[Crossref] [Google Scholar] [PubMed]
- Petsophonsakul P, Furmanik M, Forsythe R, Dweck M, Schurink GW, Natour E, et al. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation: Involvement of vitamin K-dependent processes. Arterioscler Thromb Vasc Biol 2019;39(7):1351-68.
[Crossref] [Google Scholar] [PubMed]
- Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc Res 2018;114(4):590-600.
[Crossref] [Google Scholar] [PubMed]
- Zhuo X, Gao W, Li Y, Liu Y, Lun X, Cui Y. Promoting effect of the interaction between interleukin-10 and AT1-R above hypothalamic paraventricular nucleus on heart failure. Acta Med Mediter 2022;38(4):2427-34.
- Annoni F, Moro F, Caruso E, Zoerle T, Taccone FS, Zanier ER. Angiotensin-(1-7) as a potential therapeutic strategy for delayed cerebral ischemia in subarachnoid hemorrhage. Front Immunol 2022;13:841692.
- Hayashi C, Matsumoto K, Shinya K, Nakajima T, Nakao T, Komatsu A, et al. The expression of angiotensin II receptors mRNA in granulosa-lutein cells in endometriosis patients who underwent ovarian surgery before in vitro fertilization. Clin Exp Obstetr Gynecol 2019;46(6):910-3.
- Mondaca-Ruff D, Riquelme JA, Quiroga C, Norambuena-Soto I, Sanhueza-Olivares F, Villar-Fincheira P, et al. Angiotensin II-regulated autophagy is required for vascular smooth muscle cell hypertrophy. Front Pharmacol 2019;9:1553.
[Crossref] [Google Scholar] [PubMed]
- Ohno T, Aoki H, Ohno S, Nishihara M, Furusho A, Hiromatsu S, et al. Cytokine profile of human abdominal aortic aneurysm: Involvement of JAK/STAT pathway. Ann Vasc Dis 2018;11(1):84-90.
[Crossref] [Google Scholar] [PubMed]
- Wheeler AP, Yang Z, Cordes TM, Markham LW, Landis BJ. Characterization of the rate of aortic dilation in young patients with thoracic aortic aneurysm. Pediatr Cardiol 2021;42(1):148-57.
[Google Scholar] [PubMed]
- Mendez P, Lineros R, Ospina D, Ringa M, Eyheremendy E. Endovascular management of iatrogenic renal vascular injuries: Case series and systematic review. Arch Esp Urol 2022;75(6):524-31.
[Crossref] [Google Scholar] [PubMed]
- Rodrigues Bento J, Meester J, Luyckx I, Peeters S, Verstraeten A, Loeys B. The genetics and typical traits of thoracic aortic aneurysm and dissection. Ann Rev Genom Hum Genet 2022;23:223-53.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Mi X, Wang Z, Zhang M, Hou J, Jiang S, et al. Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis 2020;11(6):454.
- Faraji A, Sahebi M, Salavati DS. Numerical investigation of different viscosity models on pulsatile blood flow of thoracic aortic aneurysm (TAA) in a patient-specific model. Comput Methods Biomech Biomed Eng 2023;26(8):986-98.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Zhang X, Yang W, Li M, Wang G, Luo Q. Ferrostatin-1 ameliorates liver dysfunction via reducing iron in thioacetamide-induced acute liver injury in mice. Front Pharmacol 2022;13:869794.
[Crossref] [Google Scholar] [PubMed]
- Lv SL, Zeng ZF, Gan WQ, Wang WQ, Li TG, Hou YF, et al. Lp-PLA2 inhibition prevents Ang II-induced cardiac inflammation and fibrosis by blocking macrophage NLRP3 inflammasome activation. Acta Pharmacol Sin 2021;42(12):2016-32.
[Crossref] [Google Scholar] [PubMed]
- Gan L, Liu D, Liu J, Chen E, Chen C, Liu L, et al. CD38 deficiency alleviates Ang II-induced vascular remodeling by inhibiting small extracellular vesicle-mediated vascular smooth muscle cell senescence in mice. Signal Transduct Target Ther 2021;6(1):223.
- Liu L, Yang X, Liao Y, Wang C, Wang Y. Resveratrol alleviates Ang II-induced vascular smooth muscle cell senescence by upregulating E2F1/SOD2 axis. Toxicol Res 2022;11(5):831-40.
[Crossref] [Google Scholar] [PubMed]
- Abuiessa SA, Helmy MM, El-Gowelli HM, El-Gowilly SM, El-Mas MM. Dysregulated ACE/Ang II/Ang1-7 signaling provokes cardiovascular and inflammatory sequelae of endotoxemia in weaning preeclamptic rats. Eur J Pharmacol 2022;936:175344.
[Crossref] [Google Scholar] [PubMed]
- Pedroza AJ, Tashima Y, Shad R, Cheng P, Wirka R, Churovich S, et al. Single-cell transcriptomic profiling of vascular smooth muscle cell phenotype modulation in Marfan syndrome aortic aneurysm. Arterioscler Thromb Vasc Biol 2020;40(9):2195-211.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Sun Y. Chromodomain Helicase DNA Binding Protein 1-like, a negative regulator of Forkhead box O3a, promotes the proliferation and migration of Angiotensin II-induced vascular smooth muscle cells. Bioengineered 2022;13(2):2597-609.
[Crossref] [Google Scholar] [PubMed]
- Simpson NJ, Ferguson AV. Tumor necrosis factor-α potentiates the effects of angiotensin II on subfornical organ neurons. Am J Physiol Regul Integr Comp Physiol 2018;315(3):R425-33.
[Crossref] [Google Scholar] [PubMed]
- Fatima N, Patel S, Hussain T. Angiotensin AT2 receptor is anti-inflammatory and reno-protective in lipopolysaccharide mice model: Role of IL-10. Front Pharmacol 2021;12:600163.
[Crossref] [Google Scholar] [PubMed]
- Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci 2018;27(12):1984-2009.
[Crossref] [Google Scholar] [PubMed]
- Yan Z, Gibson SA, Buckley JA, Qin H, Benveniste EN. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin Immunol 2018;189:4-13.
[Crossref] [Google Scholar] [PubMed]
- Merli P, Quintarelli C, Strocchio L, Locatelli F. The role of interferon-gamma and its signaling pathway in pediatric hematological disorders. Pediatr Blood Cancer 2021;68(4):e28900.
[Crossref] [Google Scholar] [PubMed]
- Rabkin SW. The role matrix metalloproteinases in the production of aortic aneurysm. Prog Mol Biol Transl Sci 2017;147:239-65.
[Crossref] [Google Scholar] [PubMed]
- Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: Mechanisms and signal transduction pathways. Cardiovasc Res 2000;47(1):23-37.
[Crossref] [Google Scholar] [PubMed]
 ): Control; (
): Control; ( ): Ang II and (
): Ang II and ( ): Ang II+ARB
): Ang II+ARB