- *Corresponding Author:
- Jing.Wu
Department of Ultrasound, Nantong Third People’s Hospital, Affiliated Nantong Hospital 3 of Nantong University, Nantong, Jiangsu 226006, China
E-mail: chinaaaachi@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “216-222” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the cardiac toxicity of anthracycline chemotherapy in patients with breast cancer and the effect of two-dimensional speckle tracking imaging technology. In this study, 83 patients with breast cancer were selected as the research objects and divided into the study group (n=43) and the control group (n=40) according to whether anthracycline was included in the chemotherapy regimen. The control group was treated with paclitaxel for 4 w, and the study group was treated with epirubicin (anthracycline) for 4 w and paclitaxel for 4 w. The treatment course was 4 cycles. The changes of peak strain dispersion, global longitudinal strain and left ventricular ejection fraction of patients before and after chemotherapy were examined by two-dimensional speckle tracking imaging and other techniques. The left atrial diameter, left ventricular end diastolic diameter, left ventricular end systolic diameter early to late mitral inflow velocity early diastolic mitral annular velocity (E), the ratio of early diastolic mitral inflow velocity to early diastolic annular velocity (E/e), the changes of myocardial enzymes and N-terminal pro-brain natriuretic peptide were observed. Before chemotherapy, there was no difference in the measured values of left atrial diameter, left ventricular end diastolic diameter, left ventricular end systolic diameter, E/A and E/E’ between the study group and the control group (p>0.05). After chemotherapy, there was still no statistically significant difference in these values (p>0.05). Before chemotherapy, the values of left ventricular ejection fraction, global longitudinal strain, global longitudinal strain-A4C, global longitudinal strain-A3C, global longitudinal strain-A2C, and peak strain dispersion between two groups were not statistically significant (p>0.05). After chemotherapy, the measured values of global longitudinal strain, peak strain dispersion, global longitudinal strain-A3C, global longitudinal strain-A2C in the study group were lower than those in the control group, with statistical significance (p<0.05), while the measured values of global longitudinal strain-A4C were not significantly different between two groups (p>0.05). Before and after chemotherapy, there was no significant difference in creatine kinase, lactate dehydrogenase and NT-proBNP between the study group and the control group (p>0.05). Breast cancer patients treated with anthracyclines accompanied by targeted drug chemotherapy have obvious toxicity to left ventricular function and two-dimensional speckle tracking imaging can detect these changes more sensitively than conventional cardiac ultrasound.
Keywords
Breast cancer, anthracyclines, chemotherapy, cardiotoxicity, two-dimensional speckle tracking imaging
Breast cancer is a common malignant tumor in women and is currently treated with a combination of surgery, chemotherapy, radiotherapy and endocrine therapy, which can prolong the survival time of patients[1]. Even though drugs with various chemotherapy regimens for breast cancer patients are available, anthracyclines belongs to cell cycle non-specific drugs with good antitumor effects[2]. Cardiac toxicity is the most common and dangerous side effect of anthracycline drugs, which can cause tachyarrhythmia, and even lead to a sudden cardiac death in severe cases. Beta (β)-receptor blockers aregenerally used for symptomatic treatment in clinic, while the overall efficacy is unsatisfactory[3,4].
As a new ultrasonic technology for evaluating myocardial function, Two-Dimensional Speckle Tracking Imaging (2D-STI) can evaluate the local and overall functions of myocardial tissue sensitively and accurately[5]. In the early stages of myocardial damage produced by anthracyclines, patients often have no changes in conventional echocardiographic parameters and obvious clinical symptoms and only have slight changes in left ventricular regional myocardial mechanics[6].
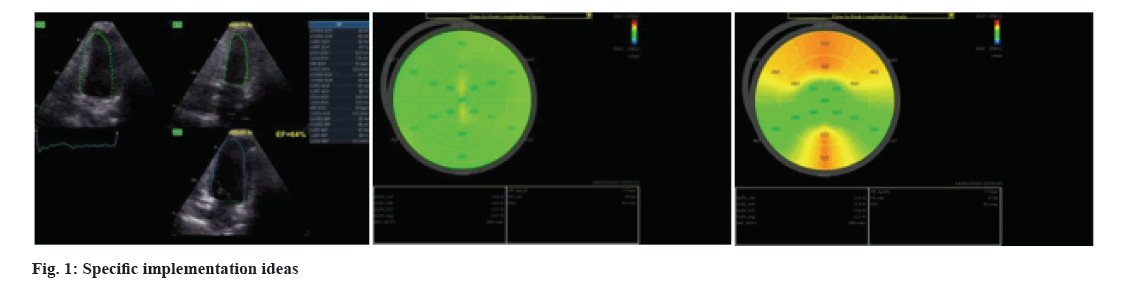
In this study, we explored the cardiotoxicity of anthracycline chemotherapy in patients with breast cancer, and applied 2D-STI technology in the early stage to evaluate the cardiac function, so as to detect subclinical cardiac injury. Anthracycline combined with targeted drug chemotherapy is a common treatment for breast cancer patients, but it can produce significant toxicity to the left ventricular function of patients. Conventional echocardiography does not sensitively reflect early myocardial injury. In the research, 2D-STI was used for evaluating myocardial injury in breast cancer patients after anthracycline combined with targeted drug chemotherapy. It was found that 2D-STI was more sensitive to detect these changes than conventional cardiac ultrasound (fig. 1)
Materials and Methods
General information:
A retrospective study was conducted and 83 people with breast cancer admitted to our hospital from December 2019 to December 2021 were selected by randomized number table method.
Inclusion criteria:
Patients between 19 y to 59 y old; patients who are diagnosed with breast cancer in accordance with the criteria in the “guidelines and norms for the diagnosis and treatment of breast cancer of the Chinese AntiCancer Association” (2019 edition)[7]; the condition of patients were confirmed by breast palpation, X-ray mammography and pathological examination; patients received anthracycline chemotherapy in our oncology department for 4 cycles; the survival time of patients were expected to be >6 mo and the Karnofsky Performance Scale (KPS) score of patients before chemotherapy was not <70 points; the study meets the basic requirements of human experimentation and medical ethics and the informed consent was signed with the patients along with their family members are included in the study.
Exclusion criteria:
Patients with metastatic breast cancer; patients with malignant tumors; patients with a history of chemo radiotherapy; patients with hypertensive heart disease, severe heart, liver and kidney underlying diseases; patients received <2 courses of chemotherapy due to intolerance of chemotherapy drugs and patients who transferred hospital for treatment are excluded from the study.
Chemotherapy methods:
The control group was treated with paclitaxel chemotherapy with the dose of 175 mg/m2, intravenous drip more than 3 times, once every 3 w. Intravenous dexamethasone 20 mg, intramuscular diphenhydramine 50 mg and intravenous ranitidine 50 mg were given 30-60 min before treatment. The study group was treated with epirubicin (anthracycline) and paclitaxel sequential chemotherapy. Intravenous drip of epirubicin 100-120 mg/m2, once every 3 w and the usage of paclitaxel was the same as the control group. All patients underwent 4 courses of chemotherapy.
Indicator detection methods:
The Left Atrial Diameter (LAD), Left Ventricular End Diastolic Diameter (LVEDD), Left Ventricular End Systolic Diameter (LVESD), early to late mitral inflow velocity (E/A), early mitral filling velocity (E) and early diastolic mitral annular velocity (E') were observed. 2D-STI was used to examine the Left Ventricular Ejection Fraction (LVEF), Global Longitudinal Strain (GLS), GLS-Apical 4-Chamber (GLS-A4C), GLS-Apical 3-Chamber (GLS-A3C), GLS-Apical 2-Chamber (GLS-A2C), Peak Strain Dispersion (PSD). The changes of myocardial enzymes, including Creatine Kinase (CK), Lactate Dehydrogenase (LDH) and N-Terminal Pro-B-type Natriuretic Peptide (NT-proBNP) were detected and analyzed.
All patients underwent conventional echocardiography and 2D-STI to evaluate cardiac function. Echocardiography was performed before and after 4 cycles of chemotherapy. Patients were asked to breathe calmly to reduce respiratory disturbance in the left lateral position. By using conventional echocardiography, the parameters were evaluated which includes LVEDD, LVESD, E/A ratio, E', and E/E'. LVEF and GLS, GLS-A4C, GLS-A3C, GLS-A2C, PSD was detected by 2D-STI technology to obtain the heart sections of A4C, A3C and A2C. All images were obtained at frame rate >50 frames/s. Three consecutive cardiac cycle images were acquired and analyzed in real time, and three sets of each were acquired to get the average value.
Instrument:
Vivid E95 Doppler ultrasound diagnostic instrument from General Electric (GE), United States of America (USA), equipped with an M5Sc probe at 1.7-3.3 MHz. The consistency of 2D-STI technology in evaluating left ventricular systolic synchronization was evaluated, and the images were collected and analyzed by two experienced doctors.
Before and after 4 cycles of chemotherapy, 3 ml fasting peripheral venous blood samples were collected and placed in vacuum. An hour later, the blood was centrifuged with the speed of 3000 r/min for 10 min. The serum was analyzed by Electrochemiluminescence for CK and LDH, and NT-proBNP was detected by enzyme-linked immunosorbent assay.
Statistical analysis:
The GLS, GLS-A4C, GLS-A3C, GLS-A2C, PSD values collected in this study were tested and expressed as (x̄±s), which were in accordance with the approximate normal distribution or normal distribution, and t test was used to made comparisons between them. Chi-square (χ2) test was used to made comparisona between groups of non-grade counting data and professional Statistical Package for Social Sciences (SPSS) 21.0 software was used for data processing with test level Alpha (α)=0.05.
Results and Discussion
The basic information of the two groups was compared and shown in Table 1. There was no statistically significant difference in age, Body Mass Index (BMI), blood pressure, heart rate, lesion diameter and Tumor, Node, Metastasis (TNM) stage between the two groups (p>0.05).
| General information | Study (n=43) | Control (n=40) | t/χ2 | p |
|---|---|---|---|---|
| Age (years) | 45.38±7.70 | 47.02±7.84 | -0.961 | 0.339 |
| BMI (kg/m2) | 22.48±1.20 | 22.26±1.13 | 0.858 | 0.393 |
| Systolic pressure (mmHg) | 120.6±6.4 | 118.5±7.0 | 1.428 | 0.157 |
| Diastolic pressure (mmHg) | 74.3±7.0 | 75.5±6.8 | -0.791 | 0.431 |
| Heart rate (time/min) | 76.5±6.9 | 74.8±7.3 | 1.091 | 0.279 |
| Lesion diameter (mm) | 24.71±5.72 | 26.20±6.14 | -1.145 | 0.256 |
| TNM staging (%) | 2.069 | 0.355 | ||
| α | 12 (27.91) | 14 (37.84) | ||
| β | 25 (58.14) | 24 (60.00) | ||
| χ | 6 (13.95) | 2 (5.41) |
Table 1: Comparison of Baseline Data between the two Groups of Patients
Before and after chemotherapy, there was no statistically significant difference in the measured values of LAD, LVEDD, LVESD, E/A and E/E' between the two groups (p>0.05) (Table 2).
| Group | n | LAD (cm) | LVEDD (cm) | LVESD (cm) | E/A | E/E’ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | ||
| Study | 43 | 31.30±3.47 | 32.66±3.81 | 45.92±4.76 | 47.30±5.51 | 29.57±4.10 | 31.13±4.73 | 1.34±0.31 | 1.22±0.28 | 8.49±2.15 | 9.42±2.27 |
| Control | 40 | 31.03±3.51 | 32.14±3.94 | 45.44±4.63 | 46.73±5.11 | 29.10±3.95 | 30.68±4.98 | -0.434 | -1.278 | 8.25±1.94 | 8.87±2.43 |
| t | 0.352 | 0.611 | 0.465 | 0.488 | 0.531 | 0.422 | 0.666 | 0.205 | 0.533 | 1.066 | |
| p | 0.726 | 0.543 | 0.643 | 0.627 | 0.597 | 0.674 | -0.434 | -1.278 | 0.596 | 0.290 | |
Table 2: Comparison Of Cardiac Ultrasound Indices before and after Treatment between the two Groups (x̄±s)
Before chemotherapy, there was no statistically significant difference in the values of GLS, GLS-A4C, GLS-A3C, GLS-A2C, PSD, measurements between the two groups (p>0.05). But after chemotherapy, the measured values of GLS, GLS-A3C, GLS-A2C and PSD in the study group were lower than those in the control group, and the differences were statistically significant (p<0.05), while there was no statistically significant difference in the measured values of LVEF, GLS-A4C (p>0.05) as shown in Table 3.
| Group | n | GLS (%) | GLS-A4C (%) | GLS- A3C (%) | GLS- A2C (%) | PSD | LVEF (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | After | Pre | After | Pre | After | Pre | After | Pre | After | Pre | After | ||
| Study | 43 | -22.04±1.17 | -17.66±1.32 | -22.94±1.46 | -17.43±1.20 | -22.57±1.27 | -17.52±1.32 | -22.53±1.64 | -17.48±1.43 | 29.64±2.77 | 30.82±2.80 | 67.83±5.20 | 65.14±5.74 |
| Control | 40 | -21.78±1.30 | -19.40±1.66 | -22.67±1.60 | -18.56±1.43 | -22.28±1.43 | -18.67±1.48 | -22.80±1.55 | -18.34±1.48 | 29.11±2.56 | 30.45±2.66 | -0.565 | -1.418 |
| t | -0.959 | 5.304 | -0.804 | 3.91 | -0.978 | 3.741 | 0.769 | 2.692 | 0.903 | 0.616 | 0.574 | 0.160 | |
| p | 0.34 | 0.0001 | 0.424 | 0.0001 | 0.331 | 0.0001 | 0.444 | 0.009 | 0.369 | 0.540 | -0.565 | -1.418 | |
Table 3: Comparison of 2D-STI Indices between the two Groups (x̄±s)
Before and after chemotherapy, there was no statistically significant difference in CK, LDH and NT-proBNP between the study group and the control group (p>0.05) as shown in Table 4.
| Group | n | CK (U/l) | LDH (U/l) | NT-proBNP (pg/ml) | |||
|---|---|---|---|---|---|---|---|
| Pre | After | Pre | After | Pre | After | ||
| Study | 43 | 46.43±8.40 | 48.40±11.27 | 176.4±38.2 | 183.1±36.8 | 243.1±54.0 | 251.6±49.5 |
| Control | 40 | 50.02±9.14 | 52.15±10.67 | 181.6±40.5 | 188.4±32.5 | 235.8±59.5 | 242.1±55.3 |
| t | -1.83 | -1.521 | -0.59 | -0.678 | 0.575 | 0.811 | |
| p | 0.071 | 0.132 | 0.557 | 0.5 | 0.567 | 0.42 | |
Table 4: Comparison Of CK, LDH, and NT-proBNP Values between the two Patient Groups (x̄±s)
Anthracene chemotherapeutic drugs have significant effect on treating breast cancer. After treatment, the 5 y and 10 y survival rate of patients have increased to 73 % and 61 %, respectively[8]. However, anthracyclines can cause type I cardiotoxicity, which can cause irreversible myocardial damage. The mechanism is complex, including free reactive oxygen species generation, oxidative stress, Deoxyribonucleic Acid (DNA) damage, etc.,[9,10]. Type I cardiac toxicity was dose-dependent, with a high incidence (48 %) of cardiac injury, when cumulative doses of anthracyclines were up to 700 mg/m2[11,12]. As a humanized monoclonal antibody against Human Epidermal growth factor Receptor-2 (HER2), trastuzumab has a good anti-tumor effect on HER2 positive breast cancer patients, but it can cause type II cardiotoxicity, which can lead to myocardial cell dysfunction and hinder cell repair by inhibiting the cardiac HER2 neuromodulator protein system and disrupting endogenous oxidative stress regulation[13,14]. Type II cardiotoxicity is reversible and dose-independent[15,16]. A survey found that the incidence of trastuzumab-related cardiotoxicity was 7 %-27 %[17]. Cardio toxic injury can be increased when trastuzumab and anthracycline chemotherapeutic agents are combined, while the exact mechanism remains unclear[18-20].
As commonly used clinical indicators of myocardial enzyme profile, the increased serum levels of CK and LDH can sensitively reflect the degree of myocardial injury[21,22]. NT-proBNP is an inactive fragment released equally with BNP, which has good stability and long half-life. It is often used in clinical evaluation of cardiac function, because its serum level is less affected by drugs[23,24]. It has been suggested that serum NT-proBNP levels were related to the degree of heart failure[25]. This study found that the measured values of CK, LDH and NT-proBNP in the two groups were similar at 4 w, suggesting that the changes of CK, LDH and NT-proBNP were not obvious at the early stage of cardiac injury.
Echocardiography is a widely used clinical auxiliary examination method that can noninvasively evaluate myocardial systolic and diastolic function with the advantages of being easy to perform, no trauma, no radiation exposure and repeatability[26,27]. However, conventional echocardiography cannot reflect early cardiac injury[28-30]. The study found that after chemotherapy, there was no significant difference in LAD, LVEDD, LVESD, LVEF, E/A, E/E' and other conventional echocardiographic indices between the two groups, suggesting that conventional echocardiography could not sensitively detect early changes in cardiac toxic injury, which is generally consistent with the existing research.
2D-STI technology is a new ultrasonic technology, which uses the unique gray value mode in myocardial tissue ultrasound images, namely “spots”, to track spots frame by frame in the cardiac cycle to evaluate myocardial deformation. It has been applied in the evaluation of subclinical left ventricular dysfunction[31,32]. GLS can evaluate the overall and segmental ventricular function, which has good detection value for subclinical myocardial dysfunction[33-35]. In this study, it was applied for the evaluation of cardiac toxicity induced by chemotherapy. After chemotherapy, it was found that the measured values of PSD, GLS, GLS-A3C and GLS-A2C in the patients treated with targeted drugs combined with anthracycline drugs were lower than those in the patients treated with targeted drugs only.
PSD reflects the standard deviation of the time to peak strain of the 17 segments. PSD is a reliable tool for accurate evaluation of the coordination and synchronization of myocardial mechanical movement in the early stage of disease, the smaller the PSD then the better the synchrony of myocardial motion. The result suggests that anthracycline combined with targeted drug chemotherapy in patients with breast cancer has obvious toxicity to the left ventricular function of patients, and 2D-STI is more sensitive to these changes than conventional echocardiography.
In conclusion, breast cancer patients treated with anthracycline accompanied by targeted drug chemotherapy have obvious toxicity to the left ventricular function of patients, and 2D-STI is more sensitive to these changes than conventional echocardiography.
However, this study also has certain limitations. Although patients with hypertensive heart disease and severe heart, liver and kidney basic diseases are excluded from the study, those with stable disease in the early stage of coronary heart disease are still included, it may have a certain bias on the results. In the future clinical work, patients with cardiac disease should be eliminated as much as possible to improve the accuracy of the results.
Funding:
This work was supported by Nantong Municipal Health Commission (No: MB2021055).
Conflict of interests:
The authors declared no conflict of interests.
References
- Benmalek R, Krikez I, Maaroufi A, Azzouzi L, Habbal R. Do excess weight and obesity increase the risk of cardiotoxicity in breast cancer Moroccan patients treated with Anthracyclines and Trastuzumab? Arch Cardiovasc Dis Suppl 2020;12(1):194.
- Jin Z, Chenghao Y, Cheng P. Anticancer effect of tanshinones on female breast cancer and gynecological cancer. Front Pharmacol 2022;12:1-9.
[Crossref] [Google Scholar] [PubMed]
- van der Voort A, Van Ramshorst MS, Van Werkhoven ED, Mandjes IA, Kemper I, Vulink AJ, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual ERBB2 blockade in patients with ERBB2-positive breast cancer: A secondary analysis of the TRAIN-2 randomized, phase 3 trial. JAMA Oncol 2021;7(7):978-84.
[Crossref] [Google Scholar] [PubMed]
- Bergler-Klein J. Myocardial damage in anthracyclines and breast cancer: Take a look at the bull’s eye. Eur Heart J Cardiovasc Imaging 2021;22(4):416-7.
[Crossref] [Google Scholar] [PubMed]
- Halima MB, Boudiche S, Mechri M, Jebali H, Ghabi H, Rais L, et al. Early detection of cardiac involvement in hemodialysis patients. Arch Cardiovasc Dis Suppl 2020;12(1):70-1.
- Kim J, Cho SG, Kang SR, Yoo SW, Kwon SY, Min JJ, et al. Association between FDG uptake in the right ventricular myocardium and cancer therapy-induced cardiotoxicity. J Nucl Cardiol 2020;27(6):2154-63.
[Crossref] [Google Scholar] [PubMed]
- Breast Cancer Professional Committee of China Anti-Cancer Association. Guidelines for Diagnosis and Treatment of Breast Cancer (2019 Edition). Chin J Cancer 2019;29(8):609-79.
- Pricope DL, Mitu F. The importance of multidisciplinary cardiooncology team in the breast cancer treatment with anthracyclines. Int Med 2021;18(4):67-75.
- Park JM, Reed GD, Liticker J, Putnam WC, Chandra A, Yaros K, et al. Effect of doxorubicin on myocardial bicarbonate production from pyruvate dehydrogenase in women with breast cancer. Circul Res 2020;127(12):1568-70.
[Crossref] [Google Scholar] [PubMed]
- Battisti NM, Andres MS, Lee KA, Ramalingam S, Nash T, Mappouridou S, et al. Incidence of cardiotoxicity and validation of the heart failure association-international cardio-oncology society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res Treat 2021;188(1):149-63.
[Crossref] [Google Scholar] [PubMed]
- Tesch ME, Chia SK, Simmons CE, LeVasseur N. Impact of sequence order of anthracyclines and taxanes in neoadjuvant chemotherapy on pathologic complete response rate in HER2-negative breast cancer patients. Breast Cancer Res Treat 2021;187(1):167-76.
[Crossref] [Google Scholar] [PubMed]
- Nigdelis MP, Karamouzis MV, Kontos M, Alexandrou A, Goulis DG, Lambrinoudaki I. Updates on the treatment of invasive breast cancer: Quo Vadimus? Maturitas 2021;145:64-72.
[Crossref] [Google Scholar] [PubMed]
- Vaitiekus D, Muckiene G, Vaitiekiene A, Maciuliene D, Vaiciuliene D, Ambrazeviciute G, et al. Impact of arterial hypertension on doxorubicin-based chemotherapy-induced subclinical cardiac damage in breast cancer patients. Cardiovasc Toxicol 2020;20(3):321-7.
[Crossref] [Google Scholar] [PubMed]
- Inoue K, Nagai SE, Saito T, Sakurai T, Kimizuka K, Yamada H, et al. TS-1 add-on therapy in Japanese patients with triple-negative breast cancer after neoadjuvant or adjuvant chemotherapy: A feasibility study. Invest New Drugs 2020;38(1):140-7.
[Crossref] [Google Scholar] [PubMed]
- Lothstein L, Soberman J, Parke D, Gandhi J, Sweatman T, Seagroves T. Pivarubicin is more effective than doxorubicin against triple-negative breast cancer in vivo. Oncol Res 2020;28(5):451-65.
[Crossref] [Google Scholar] [PubMed]
- Yamashita K, Tanaka H, Hatazawa K, Tanaka Y, Sumimoto K, Shono A, et al. Association between clinical risk factors and left ventricular function in patients with breast cancer following chemotherapy. Int J Cardiovasc Imaging 2021;37(1):197-205.
[Crossref] [Google Scholar] [PubMed]
- Barac A, Isaacs C, M Shara N, Lynce F, Desale S, Haynes K, et al. Trends in the use of cardiac imaging for women with newly diagnosed breast cancer. J Cardiovasc Transl Res 2020;13(3):478-89.
[Crossref] [Google Scholar] [PubMed]
- Lee J, Hur H, Lee JW, Youn HJ, Han K, Kim NW, et al. Long‐term risk of congestive heart failure in younger breast cancer survivors: A nationwide study by the SMARTSHIP group. Cancer 2020;126(1):181-8.
[Crossref] [Google Scholar] [PubMed]
- Jacobse JN, Steggink LC, Sonke GS, Schaapveld M, Hummel YM, Steenbruggen TG, et al. Myocardial dysfunction in long‐term breast cancer survivors treated at ages 40-50 years. Eur J Heart Fail 2020;22(2):338-46.
[Crossref] [Google Scholar] [PubMed]
- Nisha Y, Dubashi B, Bobby Z, Sahoo JP, Kayal S. Effect of cytotoxic chemotherapy on bone health among breast cancer patients. Does it require intervention? Support Care Cancer 2021;29(11):6957-72.
[Crossref] [Google Scholar] [PubMed]
- Wiethan GÁ, Sari MH, Ferreira LM. Scalp cooling impact in alopecia of patients under treatment for breast cancer-literature review. SN Compr Clin Med 2020;2(12):2825-33.
- Beaudry RI, Kirkham AA, Thompson RB, Grenier JG, Mackey JR, Haykowsky MJ. Exercise intolerance in anthracycline‐treated breast cancer survivors: The role of skeletal muscle bioenergetics, oxygenation, and composition. Oncologist 2020;25(5):e852-60.
[Crossref] [Google Scholar] [PubMed]
- Malta CE, Carlos AC, de Alencar MC, Alves e Silva EF, Nogueira VB, Alves AP, et al. Photobiomodulation therapy prevents dysgeusia chemotherapy induced in breast cancer women treated with doxorubicin plus cyclophosphamide: A triple-blinded, randomized, placebo-controlled clinical trial. Support Care Cancer 2022;30(3):2569-80.
[Crossref] [Google Scholar] [PubMed]
- Bisoc A, Ciurescu D, Radoi M, Tântu MM, Rogozea L, Sweidan AJ, et al. Elevations in high-sensitive cardiac Troponin T and N-terminal prohormone brain natriuretic peptide levels in the serum can predict the development of anthracycline-induced cardiomyopathy. Am J Ther 2020;27(2):e142-50.
[Crossref] [Google Scholar] [PubMed]
- Dempsey N, Rosenthal A, Dabas N, Kropotova Y, Lippman M, Bishopric NH. Trastuzumab-induced cardiotoxicity: A review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res Treat 2021;188(1):21-36.
[Crossref] [Google Scholar] [PubMed]
- Benmalek R, Krikez I, Maaroufi A, Azzouzi L, Habbal R. Left ventricular echocardiographic parameters as predictors of cardiotoxicity in breast cancer patients with a left ventricular ejection fraction of 50 %-60 % treated with anthracyclines. Arch Cardiovas Dis Suppl 2020;12(1):66.
- Papanikolaou V, Chrysovergis A, Mastronikolis N, Tsiambas E, Ragos V, Peschos D, et al. Topoisomerase IIa protein expression patterns in laryngeal squamous cell carcinoma. Anticancer Res 2020;40(2):807-11.
[Crossref] [Google Scholar] [PubMed]
- Saleh Y, Abdelkarim O, Herzallah K, Abela GS. Anthracycline-induced cardiotoxicity: Mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail Rev 2021;26(5):1159-73.
[Crossref] [Google Scholar] [PubMed]
- Bines J, Small IA, Sarmento R, Kestelman F, Silva S, Rodrigues FR, et al. Does the sequence of anthracycline and taxane matter? The NeoSAMBA Trial. Oncologist 2020;25(9):758-64.
[Crossref] [Google Scholar] [PubMed]
- Bartsch R, Singer CF, Pfeiler G, Hubalek M, Stoeger H, Pichler A, et al. Conventional vs. reverse sequence of neoadjuvant epirubicin/cyclophosphamide and docetaxel: Sequencing results from ABCSG-34. Br J Cancer 2021;124(11):1795-802.
[Crossref] [Google Scholar] [PubMed]
- Fourati N, Charfeddine S, Chaffai I, Dhouib F, Farhat L, Boukhris M, et al. Subclinical left ventricle impairment following breast cancer radiotherapy: Is there an association between segmental doses and segmental strain dysfunction? Int J Cardiol 2021;345:130-6.
[Crossref] [Google Scholar] [PubMed]
- Narezkina A, Narayan HK, Zemljic-Harpf AE. Molecular mechanisms of anthracycline cardiovascular toxicity. Clin Sci 2021;135(10):1311-32.
[Crossref] [Google Scholar] [PubMed]
- Grakova EV, Shilov SN, Kopeva KV, Berezikova EN, Popova AA, Neupokoeva MN, et al. Anthracycline-induced cardiotoxicity: The role of endothelial dysfunction. Cardiol 2021;146(3):315-23.
[Crossref] [Google Scholar] [PubMed]
- Vaitiekus D, Muckiene G, Vaitiekiene A, Sereikaite L, Inciuraite R, Insodaite R, et al. HFE gene variants' impact on anthracycline-based chemotherapy-induced subclinical cardiotoxicity. Cardiovasc Toxicol 2021;21(1):59-66.
[Crossref] [Google Scholar] [PubMed]
- Alizadehasl A, Ghadimi N, Kaveh S, Maleki M, Ghavamzadeh A, Noohi F, et al. Prevention of anthracycline-induced cardiotoxicity: A systematic review and network meta-analysis. Int J Clin Pharm 2021;16(2):477-86.
[Crossref] [Google Scholar] [PubMed]