- *Corresponding Author:
- Chong Shi
Department of Prenatal Diagnosis and Genetic Diagnosis Center
Tangshan Maternal and Child Health Care Hospital
P. R. China
E-mail: 18631530300@163.com
| Date of Received | 27 January 2020 |
| Date of Revision | 13 February 2021 |
| Date of Acceptance | 18 August 2021 |
| Indian J Pharm Sci 2021;83(4):823-829 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The therapeutic effect of combined drugs on cervical cancer has been confirmed. Whether antiprogrammed cell death protein 1 antibody combined with paclitaxel mediates the phosphoinositide 3-kinase-protein kinase B pathway to regulate cervical cancer still needs further research. 20 nude mice received subcutaneous administration of Henrietta Lacks cells to establish cervical cancer model which was then assigned into blank control group, control group A (programmed cell death protein 1 antibody (5 mg/ kg) administration), control group B (paclitaxel) and observation group (programmed cell death protein 1 antibody combined with paclitaxel) followed by analysis of cell proliferation, apoptosis, expression of phosphoinositide 3-kinase-protein kinase B signaling related proteins and messenger ribonucleic acids. Observation group had lowest tumor size, highest cell proliferation inhibition rate and cell apoptosis, which were all reversed in blank group with largest tumor size, lowest cell proliferation inhibition rate and cell apoptosis. There was no difference between control group A and control group B (p>0.05). The expressions of phosphoinositide 3-kinase, protein kinase B, tumor protein p53 and tumor protein p21 were lowest in observation group and highest in blank group. In addition, control group had no significant difference with control group B (p>0.05). Anti-programmed cell death protein 1 antibody combined with paclitaxel may inhibit the activity of phosphoinositide 3-kinase-protein kinase B signaling, thereby downregulating phosphoinositide 3-kinase, protein kinase B, tumor protein p53 and tumor protein p21, controlling cervical cancer cell division, promoting cell apoptosis and finally exerting anti-tumor effects.
Keywords
Cervical cancer, paclitaxel, programmed death protein-1, phosphoinositide 3-kinase-protein kinase B pathway
Cervical cancer is one of the common tumor diseases, with high mortality and incidence[1]. At present, comprehensive treatment methods are mainly used clinically, combining surgery with chemotherapy, etc., to a certain extent, can effectively prolong the survival time of patients[2]. Programmed death protein-1 (PD-1) receptors mainly work in conjunction with their programmed death-ligand 1 (PD-L1) to regulate the proliferation activity of T cells and the secretion of related cytokines, thereby controlling the body’s immune response. For tumor patients, the expression of PD-L1 increases, which promotes the apoptosis of T cells expressing PD-L1 molecules and finally causes the escape phenomenon of related tumor cells to play a regulatory role in the disease[3]. For the occurrence and development of cervical cancer, PD-1 antibody and PD-L1 ligand can participate in the immune response of cervical cancer and it is related to the prognosis of cervical cancer patients. PD-1 antibody is used as a target and can play an anti-tumor effect and at the same time, it can also regulate the immunotoxicity in the body, but whether it can be used as a target for the treatment of cervical cancer requires further research[4]. Paclitaxel has anti-tumor effects, mainly because paclitaxel can affect the balance of tubulin and dimers, thereby promoting the polymerization of tubulin, avoiding tubulin depolymerization and further promoting cell apoptosis. It is also used in breast cancer. In patients with cervical cancer, the mechanism of treatment for patients with cervical cancer is still unclear to be studied[5]. In the study of Che et al.[6], they stated that cervical cancer cells are mainly derived from cervical epithelial cells and that cervical cancer has a certain relationship with the phosphoinositide 3-kinase-protein kinase B (PI3K-Akt) pathway, which is generally manifested as the inhibition of cell apoptosis. Regarding the apoptosis process of cervical cancer cells, mediation and intervention are currently effective ways to control cervical cancer. However, whether anti-PD-1 antibody combined with paclitaxel has a therapeutic effect on cervical cancer through mediating the PI3KAkt pathway, needs to be further explored.
Materials and Methods
Materials and instruments:
Henrietta Lacks (Hela) cell (Tongpai (Shanghai) Biotechnology), PD-1 antibody (Shanghai Saimai Biotechnology), paclitaxel (Shandong Lukangchenxin), hematoxylin reagent, eosin stain (Nanjing Dosif), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reagent (Xiamen) Xinchuang), cell lysate (Shanghai Liji), phosphate buffered saline (PBS) (Zhejiang Xinfu Pharmaceutical).
Inverted microscope (Bio-IEK), glutamine, dual luciferase kit, penicillin (Ribo biological company); transwell kit (Best Bio); LipofectamineTM 2000 (Sigma); 0.25 % trypsin, micro RNA (miRNA) 64 inhibitor, miRNA-64 mimics (Sigma); quantitative polymerase chain reaction (PCR) reagents (Sigma); fetal bovine serum, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Gibco); X-box binding protein 1 (XBP1), protein extraction kit (Bio-TEK Instruments).
Establishment of cervical cancer transplantation tumor model:
20 nude mice were selected and an appropriate amount of Hela cells were subcutaneously injected into the back of the rat to detect the size of the rat tumor tissue. The volume of tumor tissue=0.52×a×b2, where a represents the long diameter and b represents the short diameter[7].
Grouping and dosing schedule:
20 rats were randomly selected and divided into blank group, control group A, group B and observation group. Among them, the blank group was given only an appropriate amount of physiological saline solution, the control group A was given PD-1 antibody (53 mg/kg) via intraperitoneal injection and the control group B was also given the same dose by intraperitoneal injection of paclitaxel. The observation group was given the same dose of PD-1 antibody combined with paclitaxel.
Detection of tumor volume:
After 5 d, the size of tumor tissue was measured every 3 d and after 15 d, tumor tissue was taken for subsequent experiments.
HE staining tumor tissue:
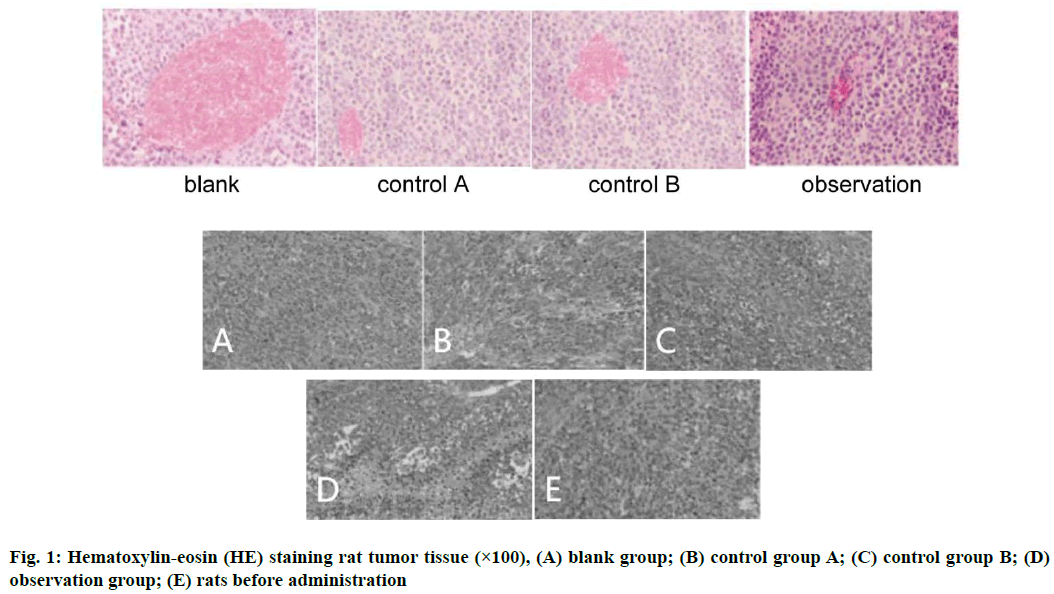
Take the above-mentioned tumor tissue, fix, PBS, hematoxylin dip, microscope observation, eosin staining and mount. Take the above-mentioned transplanted tumor tissue, give it an appropriate amount of formaldehyde solution for fixation and then place it in a liquid nitrogen tank for standby, take the nude mouse heart, lung, liver, spleen, kidney and other tissues and then apply an appropriate amount of formaldehyde solution for the fixation operation. Use tap water for cleaning and apply appropriate amount of alcohol solution for dehydration, namely 70 %, 80 %, 90 % and 100 %, xylene and appropriate amount of paraffin for embedding. HE staining: Put the above-mentioned tissues in the oven, after 90 min, apply appropriate amount of xylene I and II for dewaxing and then add alcohol I and II, wash with double distilled water, stain with hematoxylin, wash with tap water and eosin dyeing, washing with tap water, adding 70 %, 80 %, 95 % alcohol in the order, respectively, for 5 min, then respectively giving 95 % alcohol II, 100 % alcohol I and 100 % alcohol II for 10 min and then giving appropriate amounts of xylene I, Ⅱ, 10 min in all, again using gum for mounting operation, using an optical microscope to observe the morphology of each tissue.
Cell counting kit-8 (CCK-8) assay:
Under normal environment, cervical cancer cells were cultured and after 72 h, 100 μl 10 % CCK-8 was added for 2 h followed by measuring the absorption value. Growth inhibition rate (%)=(Optical density (OD) control group−OD experimental group)/OD control group×100 %.
TUNEL staining:
Take the cells, PBS, 3 times, 5 min/time, add Triton X-100, 3 min, PBS, 3 times, 5 min/time, culture in a dark environment and then wash with an appropriate amount of PBS solution, 3 times, 5 min/time, give proper amount of glycerin for sealing operation.
Western blot:
Cells were inoculated. After inoculation, cells were collected when reaching a confluence of 90 % and lysed to extract cell protein which was then quantified and separated on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) for western blot analysis using primary antibodies (PI3K, Akt, tumor protein p53 (p53) and tumor protein p21 (p21), dilution ratios are all 1:500).
Reverse transcription polymerase chain reaction (RT-PCR):
RNA was extracted followed by reverse transcription and PCR amplification with conditions: 95° 10 min, 1 cycles; 95° 15 s, 60° 60 s, 40 cycles. Gene was analyzed using 2-ΔΔCt method and β-actin was the reference. The primer sequences were shown in Table 1.
| Gene | Sequences | Length (base pair (bp)) | |
|---|---|---|---|
| β-actin | Forward | 5’-ctgtattccgtctccttggttc-3’ | 105 |
| Reverse | 5’-ctgtattccgtctccttggttc-3’ | ||
| PI3K | Forward | 5’-ctgtattcgtcttcctcggttc-3’ | 126 |
| Reverse | 5’-ctgtatcgtctcttgctcgttc-3’ | ||
| Akt | Forward | 5’-ctgtagtctccgccttttgttc-3’ | 78 |
| Reverse | 5’-ctgtattccgtgctccttgttc-3’ | ||
| p-Akt | Forward | 5’-ctgtattcccggtctcttgttc-3’ | 133 |
| Reverse | 5’-ctgtttgctcattctccggttc-3’ | ||
| p53 | Forward | 5’-ctgtatcggttccttgttctcc-3’ | 111 |
| Reverse | 5’-ctgtactctggttgttcccttc-3’ | ||
| p21 | Forward | 5’-ctgtacttgtttctggcccttc-3’ | 103 |
| Reverse | 5’-ctggttgttactctgtcccttc-3’ | ||
| p-PI3K | Forward | 5’-ctgctgtccgttgttactcttc-3’ | 121 |
| Reverse | 5’-ctggtctgtctgttactccttc-3’ | ||
Table 1: Primer Sequence
Observation indicators:
Observe the cell proliferation activity, number and apoptosis number of each group, observe the expression of PI3K-Akt pathway related proteins and mRNA in each group.
Statistical methods:
Statistical package for the social sciences (SPSS) 26.0 software analyzed the data which were described as mean±standard deviation and assessed by F test, p<0.05 indicates a significance.
Results and Discussion
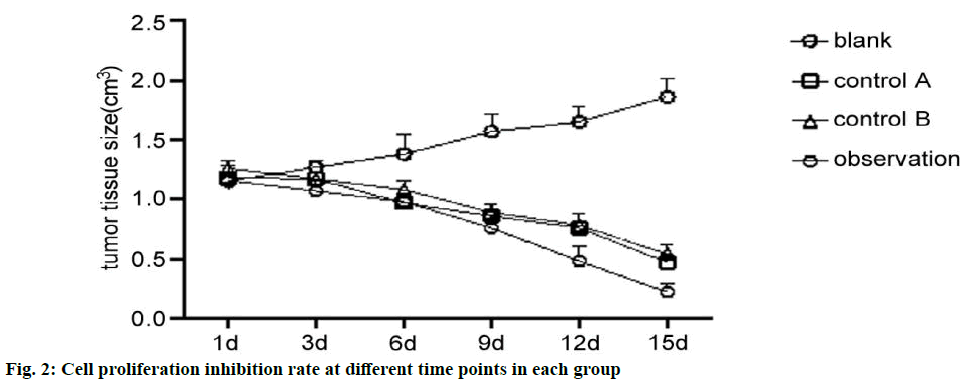
Anti-PD-1 antibody combined with paclitaxel drugs decrease tumor tissue size. The tumor volume test results showed that after administration, tumor tissue size from the 1st to the 15th d of each group was compared and there were significant differences. Among them, observation group had smallest tumor tissue size, the blank group had the largest size without difference between control group A and control group B (p>0.05) (Table 2 and fig. 1).
| Group | Sample size | 1 d | 3 d | 6 d | 9 d | 12 d | 15 d |
|---|---|---|---|---|---|---|---|
| Blank | 5 | 1.15±0.07 | 1.27±0.06 | 1.38±0.16 | 1.57±0.14 | 1.65±0.13 | 1.86±0.16 |
| Control A | 5 | 1.18±0.05 | 1.17±0.05 | 0.97±0.08 | 0.86±0.05 | 0.76±0.12 | 0.47±0.07 |
| Control B | 5 | 1.26±0.07 | 1.17±0.06 | 1.08±0.08 | 0.89±0.07 | 0.78±0.05 | 0.54±0.08 |
| Observation | 5 | 1.16±0.13 | 1.07±0.08 | 0.98±0.06 | 0.76±0.11 | 0.48±0.12 | 0.22±0.07 |
| F | - | 3.793 | 12.342 | 19.307 | 43.380 | 139.000 | 186.013 |
| p | - | 0.031 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Blank vs. control A | p | 0.197 | 0.002 | 0.029 | 0.000 | 0.000 | 0.000 |
| Blank vs. control B | p | 0.005 | 0.002 | 0.057 | 0.000 | 0.000 | 0.001 |
| Blank vs. observation | p | 0.453 | 0.000 | 0.024 | 0.000 | 0.000 | 0.000 |
| Control A vs. control B | p | 0.078 | 0.840 | 0.451 | 0.564 | 0.782 | 0.067 |
| Control A vs. observation | p | 0.572 | 0.028 | 0.994 | 0.289 | 0.000 | 0.004 |
| Control B vs. observation | p | 0.026 | 0.042 | 0.270 | 0.111 | 0.000 | 0.000 |
Table 2: Changes in Tumor Tissue Size of Rats in Each Group (cm3)
Anti-PD-1 antibody combined with paclitaxel drugs inhibit the proliferation of cervical cancer cells and promote apoptosis. The CCK-8 test results showed that the cell inhibition rate of observation group was highest at different time points. In addition, comparison between the control group A and the control group B showed no difference (p>0.05) (Table 3 and fig. 2).
| Group | Sample size | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Blank | 5 | 0 | 0 | 0 |
| Control A | 5 | 10.84±1.26 | 20.43±3.26 | 36.19±3.44 |
| Control B | 5 | 11.42±1.19 | 22.43±3.19 | 35.21±3.37 |
| Observation | 5 | 34.28±3.78 | 48.28±6.29 | 50.91±5.87 |
| F | - | 138.101 | 116.478 | 223.850 |
| p | - | 0.000 | 0.000 | 0.000 |
| Blank vs. control A | p | 0.000 | 0.000 | 0.000 |
| Blank vs. control B | p | 0.000 | 0.001 | 0.000 |
| Blank vs. observation | p | 0.001 | 0.001 | 0.000 |
| Control A vs. control B | p | 0.943 | 0.998 | 0.237 |
| Control A vs. observation | p | 0.003 | 0.006 | 0.000 |
| Control B vs. observation | p | 0.003 | 0.003 | 0.000 |
Table 3: Comparison of Cervical Cancer Cell Proliferation Inhibition Rate (%)
The TUNEL test results showed that observation group had highest number of apoptosis, blank group had lowest number without difference between control group A and control group B (p>0.05) (Table 4).
| Group | Sample size | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Blank | 5 | 1.08±0.02 | 1.04±0.01 | 1.07±0.03 |
| Control A | 5 | 10.43±1.14 | 20.23±3.34 | 36.25±3.23 |
| Control B | 5 | 11.24±1.23 | 22.51±3.27 | 35.32±3.43 |
| Observation | 5 | 33.45±2.36 | 48.37±6.32 | 50.62±5.63 |
| F | - | 511.797 | 356.504 | 518.522 |
| p | - | 0.000 | 0.000 | 0.000 |
| Blank vs. control A | p | 0.002 | 0.002 | 0.000 |
| Blank vs. control B | p | 0.000 | 0.000 | 0.000 |
| Blank vs. observation | p | 0.000 | 0.000 | 0.000 |
| Control A vs. control B | p | 0.978 | 0.710 | 1.000 |
| Control A vs. observation | p | 0.000 | 0.000 | 0.000 |
| Control B vs. observation | p | 0.000 | 0.000 | 0.000 |
Table 4: Comparison of the Number of Apoptosis in Each Group (%)
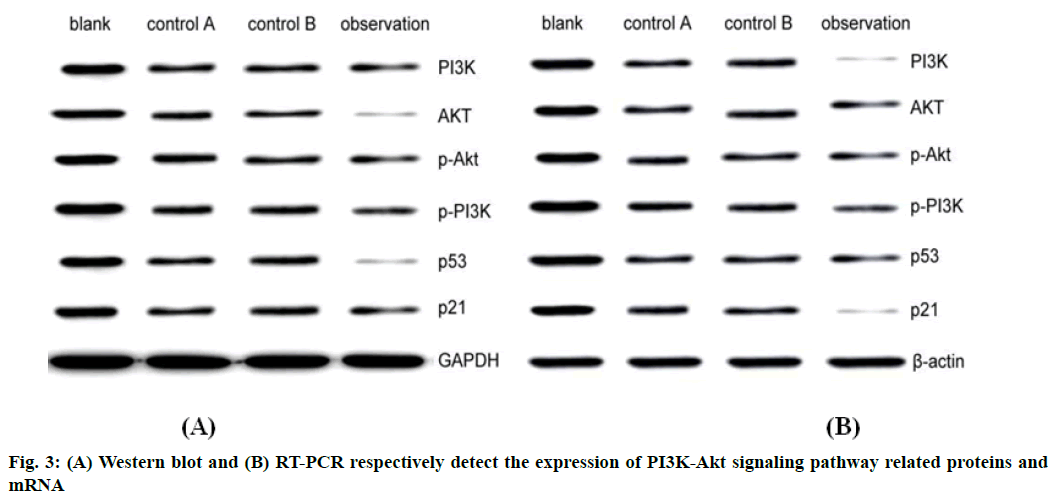
Anti-PD-1 antibody combined with paclitaxel regulate PI3K-Akt signaling pathway, thereby exerting a therapeutic effect on cervical cancer cells. Western blot and RT-PCR results showed that the expression of PI3K-Akt signaling pathway related proteins was lowest in observation group and highest in blank group. In addition, compared with control group B, control group A had no significant difference (p>0.05) (Table 5 and fig. 3).
| Group | Sample size | PI3K | AKT | p-Akt | p-PI3K | p53 | p21 |
|---|---|---|---|---|---|---|---|
| Blank | 5 | 1.58±0.12 | 1.54±0.11 | 1.37±0.13 | 1.64±0.04 | 1.75±0.05 | 1.67±0.06 |
| Control A | 5 | 1.03±0.04 | 1.23±0.04 | 1.25±0.03 | 1.06±0.03 | 1.05±0.04 | 1.08±0.02 |
| Control B | 5 | 1.04±0.03 | 1.01±0.07 | 1.02±0.03 | 1.04±0.04 | 1.06±0.01 | 1.04±0.08 |
| Observation | 5 | 0.45±0.06 | 0.37±0.02 | 0.62±0.03 | 0.38±0.04 | 0.41±0.06 | 0.42±0.05 |
| F | - | 492.526 | 555629.170 | 128.444 | 2.308 | 517556.105 | 506.379 |
| p | - | 0.000 | 0.000 | 0.000 | 0.115 | 0.000 | 0.000 |
| Blank vs. control A | p | 0.000 | 0.000 | 0.720 | 0.104 | 0.000 | 0.000 |
| Blank vs. control B | p | 0.000 | 0.000 | 0.000 | 0.551 | 0.000 | 0.000 |
| Blank vs. observation | p | 0.000 | 0.000 | 0.000 | 0.241 | 0.000 | 0.000 |
| Control A vs. control B | p | 1.000 | 0.017 | 0.000 | 0.033 | 0.634 | 0.368 |
| Control A vs. observation | p | 0.000 | 0.000 | 0.000 | 0.619 | 0.000 | 0.000 |
| Control B vs. observation | p | 0.000 | 0.000 | 0.000 | 0.087 | 0.000 | 0.000 |
Table 5: Changes in the Expression of PI3K-AKT Signaling Pathway Related Proteins (%)
In this study, in order to explore the effect of anti-PD-1 antibody combined with paclitaxel on cervical cancer rats by regulating the PI3K-Akt pathway, the rats were first induced to cervical cancer using Hela cells. After drugs were given, the rats were further tested. Tumor tissue size, the results showed that after administration, the tumor tissue size of each group was compared from the 1st to the 15th d and there were significant differences. Among them, the observation group had the smallest tumor tissue size, the blank group was the largest and the control group A and the control group B, showed no difference, indicating that anti-PD-1 antibody combined with paclitaxel can significantly inhibit the growth of tumor tissue and the combined effect is significantly better than the treatment effect of single drug and it also indicates that the cervical cancer model is successfully made and can be used for follow-up experiments.
This study further explored the effect of anti-PD-1 antibody combined with paclitaxel on cervical cancer cells. The results showed that the inhibition rate of cell proliferation and cell apoptosis was the highest in observation group at different time points and lowest in blank group. The results of this study further indicate that the anti-PD-1 antibody combined with paclitaxel can significantly inhibit the proliferation of cervical cancer cells, while effectively promoting apoptosis of cervical cancer cells and has an effect on the proliferation of cervical cancer cells. Paclitaxel can control the process of cell division, inhibit cell growth and promote cell apoptosis by promoting the polymerization of microtubules. In addition, anti-PD-1 antibodies can affect cervical cancer cells. PD-1 antibodies and PD-L1 ligand can stimulate T cell activity, so that a large number of cells are blocked in resting phase/growth 1 phase (G0/G1) phase, inhibit cell growth and promote their apoptosis. In addition, anti-PD-1 antibody combined with paclitaxel can inhibit extracellular regulated kinase (ERK) and PI3K-Akt signaling pathway and further inhibit the expression of proteins and genes related to the mitotic process, thereby affecting cell cycle, further inhibiting the proliferation of cervical cancer cells and promoting cell apoptosis[8-17]. In the study of Li et al.[18], the effect of paclitaxel chemotherapy on nude mice with cervical cancer transplantation tumors was assessed. After paclitaxel treatment, the volume of transplanted tumors in nude mice decreased significantly. Anti-PD-1 antibody combined with paclitaxel has a better effect on inhibiting tumor tissue size than paclitaxel alone.
In the process of cell mitosis, there are multiple signaling pathways involved. This study mainly explored whether the PI3K-Akt signaling pathway mediates anti-PD-1 antibody combined with paclitaxel to exert anti-tumor effects on cervical cancer. The results show that in the observation group, the expressions of PI3K, Akt, p53 and p21 proteins were the lowest and the blank group was the highest. In addition, compared with the control group B, the difference was not significant, suggesting that anti- PD-1 antibody combined with paclitaxel may inhibit PI3K-Akt pathway activity, thereby inhibiting PI3K, Akt, p53, p21 protein expression, play an anti-tumor effect. Analyze the reason. Paclitaxel mainly promotes the polymerization of microtubules, participates in the process of cell division, phosphorylates PI3K and Akt and transmits inhibitory signals. At the same time, anti-PD-1 antibody binds to PD-L1 to inhibit PI3KAkt signal. The expression of PI3K, Akt, p53 and p21 proteins in the pathway produces immune resistance, thereby inhibiting T cell apoptosis, further preventing cervical cancer cells from immune escape and inhibiting the progression of cervical cancer[19-21].
In summary, anti-PD-1 antibody combined with paclitaxel may inhibit the activity of PI3K-Akt pathway, thereby inhibiting the expression of PI3K, Akt, p53 and p21 proteins, controlling the division process of cervical cancer cells, promoting apoptosis and finally exerting anti-tumor effect. This study has certain limitations. For example, this study mainly explores that anti-PD-1 antibody combined with paclitaxel can mediate the PI3K-Akt pathway and participate in the division and apoptosis of cervical cancer cells, but whether the PI3K-Akt pathway is involved in the cervical cancer cell proliferation and apoptosis need to be further explored. In addition, this study only explored the possible involvement of p53, p21, PI3K and other proteins in the PI3K-Akt pathway in anti-tumor effects. Whether there are other pathway factors involved in the above process remains to be studied; Anti-PD-1 antibody combined with paclitaxel can inhibit the expression of proteins and genes related to cell mitosis, but the specific proteins and genes need to be further studied.
Conflicts of interest:
The authors declared no conflict of interest.
References
- Dong P, Hao F, Dai S, Tian L. Combination therapy Eve and Pac to induce apoptosis in cervical cancer cells by targeting PI3K/AKT/mTOR pathways. J Recept Signal Transduct Res 2018;38(1):83-8.
- Li S, Ma YM, Zheng PS, Zhang P. GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp Clin Cancer Res 2018;37(1):1-4.
- Tamura R, Yoshihara K, Saito T, Ishimura R, Martinez-Ledesma JE, Xin H, et al. Novel therapeutic strategy for cervical cancer harboring FGFR3-TACC3 fusions. Oncogenesis 2018;7(1):1-2.
- Wang N, Hou MS, Zhan Y, Shen XB, Xue HY. MALAT1 promotes cisplatin resistance in cervical cancer by activating the PI3K/AKT pathway. Eur Rev Med Pharmacol Sci 2018;22(22):7653-9.
- Dong P, Xiong Y, Yu J, Chen L, Tao T, Yi S, et al. Control of PD-L1 expression by miR-140/142/340/383 and oncogenic activation of the OCT4–miR-18a pathway in cervical cancer. Oncogene 2018;37(39):5257-68.
- Che Y, Li Y, Zheng F, Zou K, Li Z, Chen M, et al. TRIP4 promotes tumor growth and metastasis and regulates radiosensitivity of cervical cancer by activating MAPK, PI3K/AKT and hTERT signaling. Cancer Lett 2019;452:1-3.
- Xia C, Liu C, He Z, Cai Y, Chen J. Metformin inhibits cervical cancer cell proliferation by modulating PI3K/Akt-induced major histocompatibility complex class I-related chain A gene expression. J Exp Clin Cancer Res 2020;39(1):1-2.
- Rader JS, Pan A, Corbin B, Iden M, Lu Y, Vellano CP, et al. Identification and validation of a prognostic proteomic signature for cervical cancer. Gynecol Oncol 2019;155(2):324-30.
- Chen X, Xiong D, Ye L, Yang H, Mei S, Wu J, et al. SPP1 inhibition improves the cisplatin chemo-sensitivity of cervical cancer cell lines. Cancer Chemother Pharmacol 2019;83(4):603-13.
- Nair VB, Manasa VG, Sinto MS, Jayasree K, James FV, Kannan S. Differential expression of microRNAs in uterine cervical cancer and its implications in carcinogenesis; an integrative approach. Int J Gynecol Cancer 2018;28(3); 553-62.
- Munoz JP, Carrillo-Beltran D, Aedo-Aguilera V, Calaf GM, Leon O, Maldonado E, et al. Tobacco exposure enhances human papillomavirus 16 oncogene expression via EGFR/PI3K/Akt/c-Jun signaling pathway in cervical cancer cells. Front Microbiol 2018;9:3022.
- Hu R, Wang MQ, Niu WB, Wang YJ, Liu YY, Liu LY, et al. SKA3 promotes cell proliferation and migration in cervical cancer by activating the PI3K/Akt signaling pathway. Cancer Cell Int 2018;18(1):1-6.
- Tian Q, Wang L, Sun X, Zeng F, Pan Q, Xue M. Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion and PI3K/AKT signalling pathway. J BUON 2019;24(3):997-1002.
- Pappa KI, Christou P, Xholi A, Mermelekas G, Kontostathi G, Lygirou V, et al. Membrane proteomics of cervical cancer cell lines reveal insights on the process of cervical carcinogenesis. Int J Oncol 2018;53(5):2111-22.
- Zong S, Liu X, Zhou N, Yue Y. E2F7, EREG, miR-451a and miR-106b-5p are associated with the cervical cancer development. Arch Gynecol Obstet 2019;299(4):1089-98.
- Dai B, Yu R, Fan M, Yang T, Wang B, Zhang Y. HMQ-T-F2 suppresses migration of the human cervical cancer HeLa cells by reversing EMT via the PI3K/Akt signaling pathway. Oncol Rep 2019;42(4):1451-8.
- Yu X, Yang Y, Li Y, Cao Y, Tang L, Chen F, et al. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3 K/Akt pathway. Int J Biochem Cell Biol 2018;94:107-18.
- Li J, Qi C, Liu X, Li C, Chen J, Shi M. Fibulin-3 knockdown inhibits cervical cancer cell growth and metastasis in vitro and in vivo. Sci Rep 2018;8(1):1-8.
- Xu J, Zhu W, Chen L, Liu L. MicroRNA-433 inhibits cell growth and induces apoptosis in human cervical cancer through PI3K/AKT signaling by targeting FAK. Oncol Rep 2018;40(6):3469-78.
- Li C, Zheng X, Li W, Bai F, Lyu J, Meng QH. Serum miR-486-5p as a diagnostic marker in cervical cancer: with investigation of potential mechanisms. BMC Cancer 2018;18(1):1-10.
- Su J, Zhang F, Li X, Liu Z. Osthole promotes the suppressive effects of cisplatin on NRF2 expression to prevent drug-resistant cervical cancer progression. Biochem Biophys Res Commun 2019;514(2):510-7.