- *Corresponding Author:
- J. Liu
Department of Cardiology, People’s Hospital of Chongqing Hechuan, Chongqing 401520, China
E-mail: liujun800528@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “210-215” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the efficacy and safety of atorvastatin combined with Shakubactrivalsartan in patients with coronary heart disease complicated with cardiac insufficiency. A total of 188 patients with coronary heart disease combined with cardiac dysfunction were included in this study, of which 73 patients received atorvastatin and 115 patients received atorvastatin combined with sakubactrivalsartan. According to the efficacy criteria, the efficacy of Atorvastatin group and Atorvastatin+sakubactrivalsartan group was determined. The changes of left ventricular mass index, left ventricular ejection fraction, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, left ventricular end-diastolic volume and N-terminal pro-brain natriuretic peptide before and after treatment were detected in the two groups. The inflammatory response of patients was evaluated by comparing the changes of interleukin-6 and high-sensitivity C-reactive protein before and after treatment. Adverse reactions occurred during treatment were collected to determine the safety of treatment. Before treatment, there was clearly no significant difference between the two groups. After treatment, most of the patients in the Atorvastatin and Atorvastatin+sakubactrivalsartan groups showed significant efficacy, while the efficacy in the Atorvastatin+sakubactrivalsartan group was better than that in the Atorvastatin group. After 6 mo of treatment, left ventricular mass index, left ventricular ejection fraction, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, left ventricular end-diastolic volume and N-terminal pro-brain natriuretic peptide in Atorvastatin+sakubactrivalsartan group were superior to those in Atorvastatin group. At the same time, the levels of inflammatory cytokines in the Atorvastatin+sakubactrivalsartan group were obviously lower than those in the Atorvastatin group after 6 mo of treatment. The adverse events that occurred during treatment in the Atorvastatin and Atorvastatin+sakubactrivalsartan groups mainly included hypotension, hyperkalemia, fecal occult blood, hematuria, and arrhythmia. There was no significant difference in the incidence of adverse reactions in the Atorvastatin+sakubactrivalsartan group compared with the atorvastatin group. The efficacy of atorvastatin plus sakubactrivalsartan group was higher than that of atorvastatin group. Atorvastatin combined with sakubatravalsartan has a significant effect on patients with coronary heart disease and cardiac insufficiency.
Keywords
Atorvastatin, sacubitril-valsartan, cardiac insufficiency, interleukin-6
Coronary heart disease is one of the common cardiovascular diseases. Coronary heart disease is based on atherosclerosis, caused by vascular cavity stenosis or obstruction, resulting in myocardial ischemia and hypoxia or necrosis[1]. The course of coronary heart disease is prolonged, the progress of the disease can make the structure and function of the heart change, myocardial systolic function will be affected and decreased, promote the heart forward reduced blood and then cause systemic circulation or pulmonary circulation congestion, i.e., left cardiac insufficiency. In patients with coronary heart disease, patients with left ventricular insufficiency have a high proportion[2]. This kind of patients are mainly manifested by precardiac pain, night sweats, rapid heart rate, etc., and also have a high risk of shock and sudden death[3].
Statins have good lipid-lowering effects and are currently the first-line and second-line drugs for prevention and treatment of cardiovascular and cerebrovascular diseases. Many studies have shown that statins can stabilize or alleviate the progress of atherosclerosis, and have good effects on coronary heart disease. Foreign studies have shown that statins can also improve vascular endothelial function and alleviate the inflammatory reaction of the body. Sacubitril-valsartan can not only inhibit angiotensin, but also enhance the vasodilation of endogenous natriuretic titanium and inhibit ventricular remodeling[4,5].
At present, there are no studies on the efficacy of atorvastatin in combination with sacubitril-valsartan in the treatment of coronary heart disease with cardiac insufficiency. Therefore, this study mainly discussed the efficacy of atorvastatin combined with sacubitril-valsartan in the treatment of patients with coronary heart disease and cardiac insufficiency[6-10].
Materials and Methods
Patients:
A total of 188 patients with coronary heart disease combined with cardiac dysfunction who were treated from October 2021 to October 2022 were included in this study. All patients were examined by Electrocardiogram (ECG), echocardiography, hematology and coronary artery Computerized Tomography (CT) after admission, and met the World Health Organization (WHO) diagnostic criteria for coronary heart disease. The patients were divided into two groups according to the treatment plan they received. 73 patients treated with atorvastatin were included in the atorvastatin group. 115 patients who were treated with atorvastatin combined with sacubitril-valsartan were included in the atorvastatin+sacubitril-valsartan group.
Inclusion criteria:
Patients diagnosed as coronary heart disease by ECG and other examinations, the Left Ventricular Ejection Fraction (LVEF) index of patients is lower than 35 %. The duration of patients with stable angina pectoris was more than 30 d. The patient has a history of previous myocardial infarction[11-14].
Exclusion criteria:
Patients with the following conditions were excluded; those who have received cardiac surgery or interventional therapy for coronary heart disease, left atrial flutter or fibrillation, left ventricular aneurysm, severe hypertension, severe liver and renal insufficiency, drug allergy, severe valve disease and mental disease.
Therapeutic methods:
Both groups of patients maintained their normal living habits before treatment and received routine symptomatic treatment. Patients in the atorvastatin group received oral atorvastatin calcium capsules in addition to conventional symptomatic treatment. Atorvastatin+sacubitril-valsartan patients were given atorvastatin calcium capsules and sacubitril- valsartan in addition to conventional symptomatic treatment. Atorvastatin calcium capsules are taken orally at a dose of 10 mg once daily. The oral dose of sacubitril-valsartan was 2 tablets each time, 3 times a day.
Criteria for determining efficacy:
After treatment, the patient's clinical symptoms such as precordial pain, night sweats, fatigue, and heart rate acceleration basically disappeared, the use of nitroglycerin was reduced by 80 % or more, and the ECG was clearly improved, which was an obvious effect of treatment; the clinical symptoms of the patients were clearly reduced, the use of nitroglycerin was reduced by 50 % to 79 %, and the ECG was improved, which was effective for treatment; if the clinical symptoms, nitroglycerin dosage and ECG of the patient do not change clearly or worsen, the treatment is invalid. Total effective rate of treatment=(number of markedly effective cases+number of effective cases)/total number of cases+100 %.
Cardiac function index detection:
Compare the changes of cardiac function indexes Left Ventricular Mass Index (LVMI), LVEF, Left Ventricular End-Diastolic Diameter (LVEDD), Left Ventricular End-Systolic Diameter (LVESD), Left Ventricular End-Diastolic Volume (LVEDV), N-Terminal Pro-Brain Natriuretic Peptide (NT- proBNP) before and after treatment in the atorvastatin group and atorvastatin+sacubitril-valsartan group. Collect adverse reactions of patients during treatment.
Enzyme-Linked Immunosorbent Assay (ELISA):
Before and after treatment, 2.5 ml of fasting venous blood was taken from the patients, and the serum was separated by high-speed centrifugation and sent to the laboratory. Interleukin (IL)-6 detection kit was selected to detect the level of IL-6 by ELISA. The high-sensitivity C-Reactive Protein (hsCRP) detection kit was selected to detect the level of hs-CRP by ELISA.
Statistical analysis:
All data in this study were statistically analyzed by Statistical Package for the Social Sciences (SPSS) 26.0 software (SPSS, Chicago, Illinois, United States of America (USA)). p<0.05 means the difference is statistically obvious.
Results and Discussion
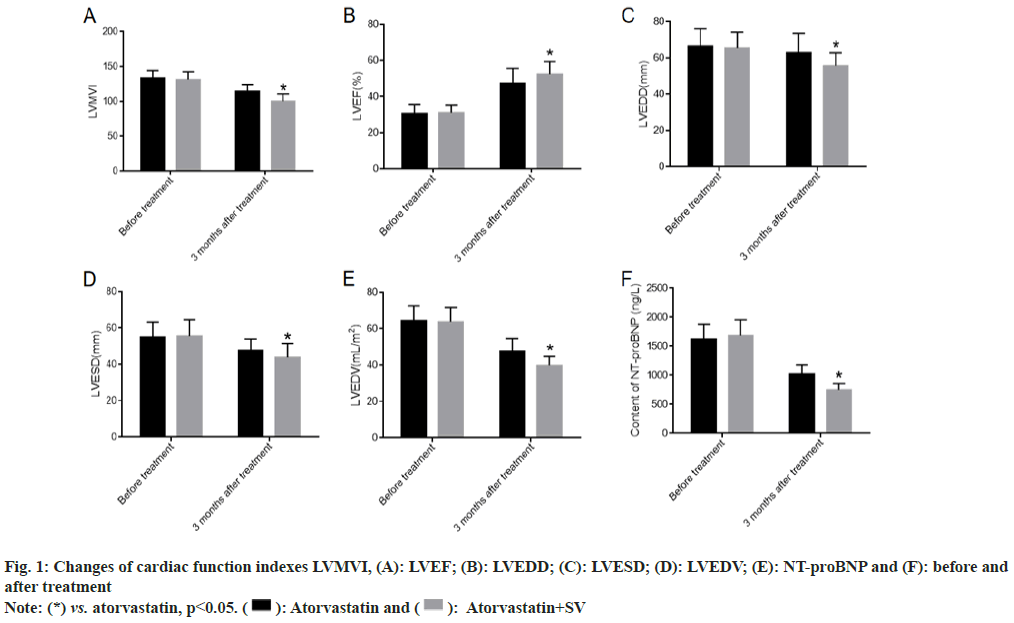
We collected the changes of LVMVI, LVEF, LVEDD, LVESD, LVEDV and NT-proBNP before treatment and 6 mo after treatment. There was no obvious difference between the atorvastatin group and atorvastatin+sacubitril-valsartan group before treatment. After 6 mo of treatment, all patients showed significant improvements in cardiac function, with atorvastatin combined with sacubitril-valsartan showing even greater improvements as shown in fig. 1.
After 6 mo of treatment, cardiac function grades were evaluated in both groups to determine changes in impaired cardiac function. The results showed that patients treated with atorvastatin calcium capsules combined with sacubitril-valsartan had significantly better cardiac function classification than those treated with atorvastatin calcium capsules alone as shown in Table 1.
| Index | SV (n=73) | Atorvastatin+SV (n=115) | t/χ2 | p |
|---|---|---|---|---|
| Gender (male) | 46 | 63 | 1.242 | 1.114 |
| Age | 62.53±10.64 | 64.71±12.05 | 1.264 | 0.208 |
| Course of disease | 5.94±2.16 | 6.07±2.84 | 0.334 | 0.739 |
| Cardiac function classification | 9.912 | 0.019 | ||
| I | 19 | 24 | ||
| II | 37 | 49 | ||
| III | 22 | 11 | ||
| IV | 14 | 31 |
Table 1: Comparison of General Data of Patients
We further explored the changes in the inflammation level of patients before and after treatment to evaluate the effect of medication on the clearance of inflammation in the body[15-20]. There was no obvious difference in IL-6 and hs-CRP before treatment between the two groups. After 6 mo of treatment, the levels of IL-6 and hs-CRP decreased clearly in both groups and were more pronounced in patients treated with atorvastatin and sacubitril-valsartan as shown in fig. 2.
The adverse reactions of patients in the atorvastatin group and atorvastatin+sacubitril-valsartan group during treatment mainly include hypotension, hyperkalemia, stool occult blood, hematuria and arrhythmia. Compared with the atorvastatin group, there was no obvious difference in the incidence of adverse reactions in the atorvastatin+sacubitril- valsartan group as shown in Table 2. The total effective rate of atorvastatin group was 89.07 %, and that of atorvastatin+sacubitril-valsartan group was 98.26 %. The effectiveness of atorvastatin+sacubitril- valsartan group was higher than that of atorvastatin group as shown in Table 3.
| n | Hypotension | Hyperkalemia | FOBT | Hematuresis | Arrhythmia | |
|---|---|---|---|---|---|---|
| SV | 73 | 0 (0.00) | 1 (1.37) | 0 (0.00) | 1 (1.37) | 1 (1.37) |
| Atorvastatin+SV | 115 | 1 (0.87) | 0 (0.00) | 2 (1.74) | 1 (0.87) | 0 (0.00) |
| χ2 | 0.050 | |||||
| p | 0.824 | |||||
Table 2: Statistical Analysis of Adverse Reactions of Patients in Each Group [n (%)]
| n | Significant effect | Effective | Inefficiency | Total efficiency | |
|---|---|---|---|---|---|
| SV | 73 | 40 (54.79) | 25 (34.25) | 8 (10.96) | 65 (89.04) |
| Atorvastatin+SV | 115 | 69 (60.00) | 44 (38.26) | 2 (1.74) | 113 (98.26) |
| χ2 | 7.541 | ||||
| p | 0.023 | ||||
Table 3: Analysis of Treatment Effectiveness of Patients in Each Group [n (%)]
Coronary heart disease combined with cardiac insufficiency is a common disease in cardiology. The incidence rate is increasing year by year, and the mortality and disability rates are high. Some studies have shown that patients with coronary artery stenosis, myocardial ischemia and hypoxia, lead to impaired glucose uptake capacity of cells, a large amount of lactic acid accumulation of cells, and then damage myocardial cells, causing cardiovascular events, with a high mortality[21,22].
Atorvastatin can inhibit the synthesis of valproic acid in the body, reduce the cholesterol content, make the liver produce low density lipoprotein cholesterol to supplement the cholesterol content of the body, so as to achieve the goal of reducing the content of triacylglycerol, dredge the heart and blood vessels, and reduce the incidence of heart disease[23]. In addition, atorvastatin has the function of resisting inflammation and protecting vascular endothelium, reducing the production of angiotensin and promoting the production of human insulin, thus resisting human inflammation, improving vascular sclerosis, increasing the blood supply function of the heart, avoiding myocardial hypoxia and regulating the state of smooth muscle, making the heart gangrene partially regenerate, expanding the coronary vessels of the heart, and dredging the blood accumulated in the pulmonary circulation, make blood circulation more smooth, so as to improve left heart function. Sacubitril-valsartan is a new type of angiotensin-enkephalin inhibitor[24,25]. Patients can degrade the natriuretic peptide after taking it orally, so as to improve the level of natriuretic peptide in the body, and then play the role of water excretion, vasodilation and inhibition of sympathetic system[26]. However, it is rarely used in coronary heart disease with cardiac insufficiency at present. Some studies have shown that sacubitril-valsartan include two components, sacubitril and valsartan. Sacubitril is an enkephalin inhibitor, which can decompose natriuretic peptide and maintain the stability of the neuroendocrine system by inhibiting the secretion of aldosterone. Valsartan is an angiotensin receptor antagonist, which can inhibit vasoconstriction and improve ventricular remodeling by blocking angiotensin receptor I[27].
In this study, Atorvastatin alone and combined with sacubitril-valsartan significantly improved cardiac function indicators such as LVEF, LVMVI, LVEDD, LVESD, LVEDV, and NT-proBNP in patients with coronary heart disease complicated with cardiac dysfunction, repaired cardiac function injury, and reduced the level of inflammatory factors[28-30]. But atorvastatin combined with sacubitril-valsartan was more effective. This may be due to the fact that sacubitril-valsartan can more effectively promote the binding of troponin and calcium ions, and directly improve the lesion. At the same time, the combination of troponin and calcium ions can activate the potassium channel of the patient's body, expand the peripheral veins of the patient, reduce the load of the patient's heart, and thus improve the function of the patient's heart. In addition, atorvastatin combined with sacubitril-valsartan has a better effect on inflammation, which may further improve the cardiac environment and thereby improve cardiac function[31,32].
The results of this study also showed that atorvastatin combined with sacubitril-valsartan was more effective in the treatment of patients with coronary heart disease combined with cardiac insufficiency, and there was no difference in the occurrence of adverse reactions compared with atorvastatin monotherapy. This suggests that atorvastatin combined with sacubitril-valsartan has good efficacy and high safety in the treatment of patients with coronary heart disease complicated with cardiac insufficiency[33-35].
In conclusion, atorvastatin combined with sacubitril- valsartan has good efficacy in patients with coronary heart disease complicated with cardiac insufficiency, and can effectively reduce inflammatory response with good safety.
Conflict of interests:
The authors declared no conflict of interests.
References
- Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, et al. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2021;6(10):1130-43.
[Crossref] [Google Scholar] [PubMed]
- Nichols S, McGregor G, Breckon J, Ingle L. Current insights into exercise-based cardiac rehabilitation in patients with coronary heart disease and chronic heart failure. Int J Sports Med 2021;42(01):19-26.
[Crossref] [Google Scholar] [PubMed]
- Wang N, Sun Y, Zhang H, Wang B, Chen C, Wang Y, et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur Heart J 2021;42(40):4180-8.
[Crossref] [Google Scholar] [PubMed]
- Trøseid M, Andersen GO, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020;52:102649.
[Crossref] [Google Scholar] [PubMed]
- McMurray JJV, Wheeler DC, Stefansson BV, Jongs N, Postmus D, Correa-Rotter R, et al. DAPA-CKD trial committees and investigators. Effects of dapagliflozin in patients with kidney disease, with and without heart failure. JACC Heart Fail 2021;9(11):807-20.
- Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest 2020;50(5):e13230.
[Crossref] [Google Scholar] [PubMed]
- Saldarriaga C, Atar D, Stebbins A, Lewis BS, Abidin IZ, Blaustein RO, et al. Vericiguat in patients with coronary artery disease and heart failure with reduced ejection fraction. Eur J Heart Failure 2022;24(5):782-90.
[Crossref] [Google Scholar] [PubMed]
- Li J, Li Y, Gong F, Huang R, Zhang Q, Liu Z, et al. Effect of cardiac rehabilitation training on patients with coronary heart disease: A systematic review and meta-analysis. Ann Palliat Med 2021;10(11):119011909.
[Crossref] [Google Scholar] [PubMed]
- Peng H, Wang S, Wang M, Ye Y, Xue E, Chen X, et al. Nonalcoholic fatty liver disease and cardiovascular diseases: A Mendelian randomization study. Metabolism 2022;133:155220.
[Crossref] [Google Scholar] [PubMed]
- Parikh PB, Bhatt DL, Bhasin V, Anker SD, Skopicki HA, Claessen BE, et al. Impact of percutaneous coronary intervention on outcomes in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 2021;77(19):2432-47.
[Crossref] [Google Scholar] [PubMed]
- Zhang P, Guo D, Xu B, Huang C, Yang S, Wang W, et al. Association of serum 25-hydroxyvitamin D with cardiovascular outcomes and all-cause mortality in individuals with prediabetes and diabetes: Results from the UK biobank prospective cohort study. Diabetes Care 2022;45(5):1219-29.
[Crossref] [Google Scholar] [PubMed]
- Beyene SS, Yacob O, Melaku GD, Hideo-Kajita A, Kuku KO, Brathwaite E, et al. Comparison of patterns of coronary artery disease in patients with heart failure by cardiac amyloidosis status. Cardiovasc Revasc Med 2021;27:31-5.
[Crossref] [Google Scholar] [PubMed]
- Minhas AM, Jain V, Maqsood MH, Pandey A, Khan SS, Fudim M, et al. Non-alcoholic fatty liver disease, heart failure, and long-term mortality: Insights from the national health and nutrition examination survey. Curr Probl Cardiol 2022;47(12):101333.
[Crossref] [Google Scholar] [PubMed]
- Simon TG, Roelstraete B, Hagström H, Sundstrom J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut 2022;71(9):1867-75.
[Crossref] [Google Scholar] [PubMed]
- Li J, Liu FH, Guo J, Yu YF, Li CQ. Retrospective analysis of renal prognosis in elderly coronary artery disease patients complicated with renal insufficiency. Aging (Albany NY) 2021;13(19):22856.
[Crossref] [Google Scholar] [PubMed]
- Wang K, Tian J, Zheng C, Yang H, Ren J, Liu Y, et al. Interpretable prediction of 3-year all-cause mortality in patients with heart failure caused by coronary heart disease based on machine learning and SHAP. Comput Biol Med 2021;137:104813.
- Shashu BA, Baru A. Factors associated with the extent of coronary artery disease and the attained outcome of percutaneous coronary intervention at Gesund cardiac and medical center, Addis Ababa, Ethiopia. Ethiop J Health Sci 2022;32(3):539-48.
[Google Scholar] [PubMed]
- Naito R, Kasai T. Obstructive coronary artery disease, a common and curable but critical comorbidity in acute decompensated heart failure. Eur J Heart Fail 2022;24(11):2150-1.
[Crossref] [Google Scholar] [PubMed]
- Tona F, Montisci R, Iop L, Civieri G. Role of coronary microvascular dysfunction in heart failure with preserved ejection fraction. Rev Cardiovasc Med 2021;22(1):97-104.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Xing J, Zhao B, Wang Y, Zhang L, Wang Y, et al. The effects of high-intensity interval training on exercise capacity and prognosis in heart failure and coronary artery disease: A systematic review and meta-analysis. Cardiovasc Ther 2022;2022:4273809.
[Crossref] [Google Scholar] [PubMed]
- Seno A, Antiochos P, Lichtenfeld H, Rickers E, Qamar I, Ge Y, et al. Prognostic value of T1 mapping and feature tracking by cardiac magnetic resonance in patients with signs and symptoms suspecting heart failure and no clinical evidence of coronary artery disease. J Am Heart Assoc 2022;11(2):e020981.
[Crossref] [Google Scholar] [PubMed]
- Ly HQ, Noly PE, Nosair M, Lamarche Y. When the complex meets the high-risk: Mechanical cardiac support devices and percutaneous coronary interventions in severe coronary artery disease. Can J Cardiol 2020;36(2):270-9.
[Crossref] [Google Scholar] [PubMed]
- Bjarnason-Wehrens B, Schwaab B, Reiss N, Schmidt T. Resistance training in patients with coronary artery disease, heart failure, and valvular heart disease: A review with special emphasis on old age, frailty, and physical limitations. J Cardiopulm Rehabil Prev 2022;42(5):304-15.
[Crossref] [Google Scholar] [PubMed]
- Kato S, Kitamura H, Hayakawa K, Fukui K, Tabata E, Otoshi R, et al. Coronary artery disease and heart failure in patients with idiopathic pulmonary fibrosis. Heart and Vessels. 2021;36(8):1151-8.
[Crossref] [Google Scholar] [PubMed]
- Wang Y. The efficacy and safety of bisoprolol in the treatment of myocardial infarction with cardiac insufficiency. Comput Math Methods Med 2022;2022:3098726.
[Crossref] [Google Scholar] [PubMed]
- Zheng J, Heidenreich PA, Kohsaka S, Fearon WF, Sandhu AT. Variability in coronary artery disease testing for patients with new-onset heart failure. J Am Coll Cardiol 2022;79(9):849-60.
[Crossref] [Google Scholar] [PubMed]
- Bollano E, Redfors B, Rawshani A, Venetsanos D, Volz S, Angeras O, et al. Temporal trends in characteristics and outcome of heart failure patients with and without significant coronary artery disease. ESC Heart Fail 2022;9(3):1812-22.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Xu G, Xu L, Zhang Y, Huang Y. Perioperative cardiac complications in patients over 80 years of age with coronary artery disease undergoing noncardiac surgery: The incidence and risk factors. Clin Interv Aging 2020;15:1181-91.
[Crossref] [Google Scholar] [PubMed]
- Tsampasian V, Vassiliou VS. Sex-related risk of heart failure in suspected or known coronary artery disease: Adding a piece to the puzzle. Eur J Prev Cardiol 2021;28(15):1720-1.
[Crossref] [Google Scholar] [PubMed]
- Qi RX, Shao J, Jiang JS, Ruan XW, Huang S, Zhang Q, et al. Myocardial extracellular volume fraction quantitation using cardiac dual-energy CT with late iodine enhancement in patients with heart failure without coronary artery disease: A single-center prospective study. Eur J Radiol 2021;140:109743.
[Crossref] [Google Scholar] [PubMed]
- Guo R, Wu T, Zheng N, Wan Y, Wang J. Clinical features of acute coronary syndrome in patients with coronary heart disease and its correlation with tumour necrosis factor in cardiology. Comput Math Methods Med 2022;2022:3439768.
[Crossref] [Google Scholar] [PubMed]
- Janus SE, Hajjari J, Chami T, Karnib M, Al-Kindi SG, Rashid I. Myeloperoxidase is independently associated with incident heart failure in patients with coronary artery disease and kidney disease. Curr Probl Cardiol 2022;47(11):101080.
[Crossref] [Google Scholar] [PubMed]
- Yamamoto K, Matsumura-Nakano Y, Shiomi H, Natsuaki M, Morimoto T, Kadota K, et al. Effect of heart failure on long-term clinical outcomes after percutaneous coronary intervention vs. coronary artery bypass grafting in patients with severe coronary artery disease. J Am Heart Assoc 2021;10(15):e021257.
[Crossref] [Google Scholar] [PubMed]
- D’Andrea A, Ciampi Q, Russo A, Forni A, Mangia C, Picano E. The effects of lockdown-induced air quality changes on the results of cardiac functional stress testing in coronary artery disease and heart failure patients. Environ Sci Pollut Res 2021;28(30):41423-30.
[Crossref] [Google Scholar] [PubMed]
- Baig M, Imran HM, Gaw A, Stabile L, Wu WC. Cardiac rehabilitation in women; comparison of enrollment, adherence and outcomes between heart failure and coronary artery disease. Heart Lung 2021;50(2):223-9.
[Crossref] [Google Scholar] [PubMed]
 Atorvastatin+SV
Atorvastatin+SV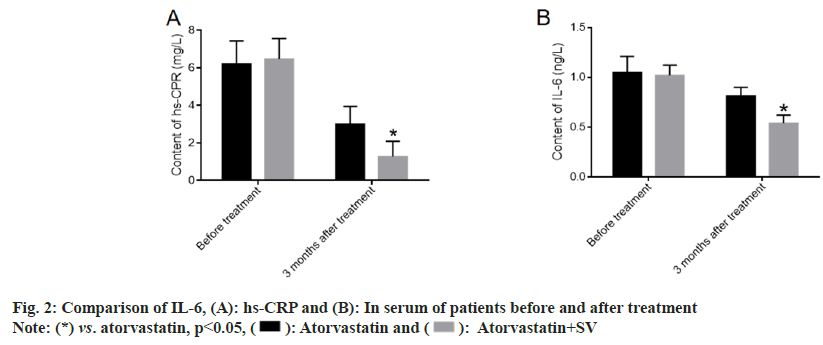
 Atorvastatin+SV
Atorvastatin+SV



