- Corresponding Author:
- X. Feng
College of Medicine, Hunan Normal University, Changsha, Hunan 410006, P.R. China
E-mail: fengxing01@hotmail.com
| Date of Submission | 20 November 2013 |
| Date of Revision | 15 October 2014 |
| Date of Acceptance | 28 December 2014 |
| Indian J Pharm Sci 2015;77(1):103-107 |
Abstract
The aim of the study was to elucidate the therapeutic effects of Atracylodes rhizome polysaccharide on adenine-induced chronic renal failure in rats. Fifty male Sprague Dawley rats were selected and randomly divided in to 5 groups (n=10 rats per group): The normal control group, the chronic renal failure pathological control group, the dexamethasone treatment group and two Atracylodes rhizome polysaccharide treatment groups, treated with two different concentrations of the polysaccharide, the Atracylodes rhizome polysaccharide high group and the Atracylodes rhizome polysaccharide low group. All the rats, except those in the normal control group were fed adenine-enriched diets, containing 10 g adenine per kg food for 3 weeks. After being fed with adenine, the dexamethasone treatment group, Atracylodes rhizome polysaccharide high group and Atracylodes rhizome polysaccharide low group rats were administered the drug orally for 2 weeks. On day 35, the kidney coefficient of the rats and the serum levels of creatinine, blood urea nitrogen, total protein and hemalbumin were determined. Subsequent to experimentation on a model of chronic renal failure in rats, the preparation was proven to be able to reduce serum levels of creatinine, blood urea nitrogen and hemalbumin levels (P<0.05) and improve renal function. Atracylodes rhizome polysaccharide had reversed the majority of the indices of chronic renal failure in rats.
Keywords
Atracylodes rhizome polysaccharide, adenine, chronic renal failure, rats, beneficial effect
Traditional Chinese herbal medicine has been proven beneficial for certain diseases, including chronic renal failure (CRF). However, the essential compounds in the majority of formulae have not been identified, whereas the precise mechanisms of action of the formulae remain to be elucidated by using cellular and molecular approaches.
The Atractylodes rhizome polysaccharide is known as a tonic herb and vital energy nutrient, used for gastric and digestive disorders [1]. Pharmacological studies previously demonstrated that Atractylodes rhizome polysaccharide possesses immunostimulant, antioxidant, antihyperglycemic and antitumor properties [2,3]. CRF is characterized by progressive loss of nephrons caused by increased intraglomerular pressure and hyperfiltration. The loss of autoregulatory ability exposes the glomeruli to systemic blood pressure, leading to glomerular hypertrophy and sclerosis. Atractylodes rhizome polysaccharide has been shown to attenuate the progression of renal insufficiency in human and experimental animal models of CRF. To elucidate the molecular mechanisms underlying the action of Atractylodes rhizome polysaccharide, we investigated a CRF model in Sprague Dawley (SD) rats.
Chronic kidney disease is identified by a serum creatinine test. Higher levels of creatinine indicate a lower glomerular filtration rate and, as a result, a decreased ability of the kidneys to excrete waste products. Creatinine levels may be normal in the early stages of chronic kidney disease [4] and the condition may be suspected if urinalysis reveals the loss of protein or red blood cells in the urine. To investigate the underlying cause of kidney damage, various forms of medical imaging, blood tests and often, renal biopsy are employed to identify whether there is a reversible cause for renal malfunction. Recent professional guidelines have classified the severity of chronic kidney disease into five stages, with stage 1 being the mildest, usually causing a limited number of symptoms and stage 5 being severe illness with poor life expectancy if left untreated [5,6]. Stage 5 chronic kidney disease is also known as established chronic kidney disease and is synonymous with the now outdated terms end stage renal disease, chronic kidney failure or CRF.
The functions of Atractylodes rhizome polysaccharide include discharging poisonous substances by catharsis, invigorating the spleen, eliminating eczema and promoting blood circulation by relieving blood stasis. Indications include patients with azotemia or early stages of uraemia, which were diagnosed with hypospleen and hygrotoxin syndrome or hypospleen and blood stasis syndrome according to the traditional Chinese medicine. Atractylodes rhizome polysaccharide may decrease blood urea nitrogen and serum creatinine levels, protect the residual renal function and postpone the initiation of dialysis therapy. It may also be effective in improving the anaemia associated with renal disease and increasing blood calcium and decreasing blood phosphate levels.
To gain insight into the pharmacological effects of Atractylodes rhizome polysaccharide on CRF, a rat model to exhibit CRF-like characteristics was developed by feeding an adenine diet. Fifty male SD rats of 200-250 g body weight [7] were obtained from the Hunan Agricultural University (Changsha, China). The rats were allowed free access to water and feed composed of a standard powder diet containing 0.85% phosphorus, 1.12% calcium, 0.35% magnesium, 25.3% crude protein and 2.5 IU/g vitamin D3 [8].
Following an acclimatization period of 7 d, the rats were randomly divided into five groups (n=10 rats per group). The first group continued to receive the same diet without treatment until the end of the study (normal control group). The second group was switched to a powder diet containing adenine, obtained from the Chinese Hui Xing Biochemical Reagents Co. Ltd. (0.75% w/w in the feed) for 21 d (pathological control group). The third group was administered adenine in the feed (as in pathological control group) plus dexamethasone (0.1 mg/kg/d) for 21 d (dexamethasone group). The fourth and fifth groups were administered adenine in the feed (as in pathological control group), plus a low Atracylodes rhizome polysaccharide dose (400 mg/kg/d) or a high Atracylodes rhizome polysaccharide dose (800 mg/kg/d), respectively, for 21 d. Atracylodes rhizome polysaccharide was provided by Professor Xi-min Qiu, Hunan Normal University, Department of Pharmacy. The local ethics committee approved this study protocol and the experiments were conducted according to the National research council guidance’s for the care and use of laboratory animals.
During the course of the treatment the rats were weighed weekly and placed individually in metabolic cages to collect the urine voided in 24 h. Twenty-four hours after the completion of the treatment, the rats were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (5 mg/kg), and blood (3 ml) was collected from the anterior vena cava into heparinized tubes. The two kidneys were excised, blotted on a filter paper and weighed. Kidney coefficient was calculated as double kidney weight×100/rat weight.
The rats were placed in metabolic cages on day 21 and urine samples were collected over a period of 24 h. Blood was collected from the inferior vena cava in plain plastic tubes with and without anticoagulant (sodium citrate), left to stand at 4° for 1 h and centrifuged at 900×g at 5° for 15 min to separate serum and plasma. The rats were sacrificed with euthanasia. The kidneys were removed from the rats and washed with ice-cold saline. A small piece from the left kidney was fixed in 10% buffered formalin. The medullary portion of the kidney was homogenized in ice-cold saline to produce 10% (w/v) tissue homogenate.
Serum levels of creatinine, the most common surrogate of glomerular filtration rate, were estimated. Blood urea nitrogen was measured to evaluate renal function. Abnormalities in blood urea nitrogen levels served as an indicator of impaired glomerular function. Renal tissue protein content was estimated according to the method of Lowry et al. [9], using bovine serum albumin as the standard. The absorbance was determined spectrophotometrically at 750 nm. Albumin, the most important body protein is synthesized in the liver and albumin plasma concentrations decrease in renal failure.
All results were expressed as mean±standard error of the mean (SEM). Biochemical estimation data were statistically analyzed using the one way analysis of variance (ANOVA) followed by Tukey’s multiple range test employing SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
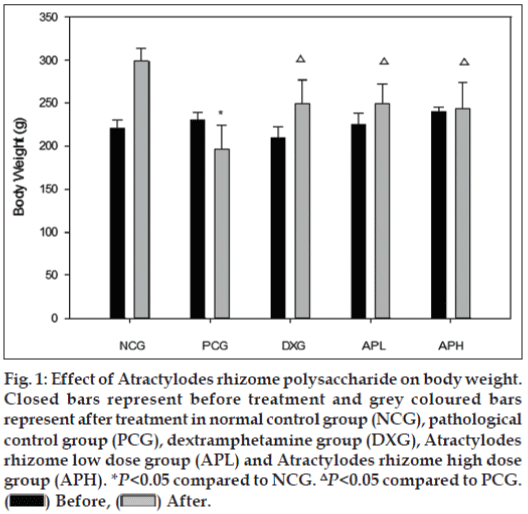
Results obtained in the present investigation demonstrated that the pathological control group rats exhibited a marked decrease in total body weight compared to the normal control group rats. However, pretreatment with Atracylodes rhizome polysaccharide and dexamethasone attenuated this change compared to pathological control group (fig. 1).
Figure 1: Effect of Atractylodes rhizome polysaccharide on body weight.
Closed bars represent before treatment and grey coloured bars
represent after treatment in normal control group (NCG), pathological
control group (PCG), dextramphetamine group (DXG), Atractylodes
rhizome low dose group (APL) and Atractylodes rhizome high dose
group (APH). *P<0.05 compared to NCG. ΔP<0.05 compared to PCG.  Before,
Before,  After.
After.
As shown in Table 1, compared to normal control group, the kidney coefficients were significantly increased (P<0.05), with the kidneys in the chronic renal failure group exhibiting significant changes, as previously described [10]. Compared to the chronic renal failure group, the kidney coefficients of the low Atracylodes rhizome polysaccharide group, the high Atracylodes rhizome polysaccharide group and the dexamethasone group were significantly reduced, which confirms that Atracylodes rhizome polysaccharide exerted a protective effect against adenine-induced renal failure.
| Group | No. | Body weight(g) | Double Kidney weight (g) | Kidney coefficient (g. 100g−1) |
|---|---|---|---|---|
| NCG | 10 | 298.50±14.92 | 1.99±0.09 | 0.67±0.03 |
| PCG | 10 | 248.80±27.31 | 7.98±1.95 | 3.11±0.53* |
| DXG | 10 | 196.50±27.33 | 6.49±1.55 | 3.02±0.47 |
| APLG | 10 | 249.27±23.03 | 5.82±1.41 | 2.34±0.57▲ |
| APHG | 10 | 243.42±30.04 | 5.93±0.86 | 2.47±0.44▲ |
Table 1: Effects of ap on kidney coefficient induced by adenine in crf rats
Adenine led to a significant elevation of the serum creatinine levels in rats. Compared to the normal control group, the serum levels of creatinine and serum blood urea nitrogen were significantly elevated in pathological control group rats (P<0.05), indicating that this was a successful experimental model, as previously described [11,12] (Table 2).
| Group | SCR (μmol/l) | BUN (mmol/l) | TP (g/l) | ALB (g/l) |
|---|---|---|---|---|
| NCG | 34.00±3.66 | 8.34±1.16 | 81.89±5.58 | 42.10±3.97 |
| PCG | 95.80±18.20* | 29.12±3.82* | 73.84±5.39* | 34.58±6.16* |
| DXG | 93.63±14.89 | 25.22±10.56 | 76.96±2.36 | 35.00±3.21 |
| APLG | 67.27±11.55▲ | 19.90±4.02▲ | 81.40±5.30▲ | 41.91±4.96▲ |
| APHG | 53.00±16.21▲ | 18.77±4.59▲ | 80.36±3.40▲ | 40.28±3.24▲ |
Table 2: Effects of ap on scr, bun, tp and alb changes induced by adenine in crf rats
These results demonstrated that pathological control group rats exhibited a marked increase in blood urea nitrogen levels, compared to normal control group rats. However, treatment with low and high doses of Atracylodes rhizome polysaccharide led to significant improvement in the serum and urinary biomarker changes, which were comparable to normal control group (Table 2).
The results obtained in the current investigation also demonstrated that pathological control group rats had a decreased tissue total protein. Treatment with low and high doses of Atracylodes rhizome polysaccharide significantly ameliorated adenine-induced renal tissue biomarker alterations, which were comparable to normal control group (Table 2).
Compared to normal control group, albumin levels in pathological control group rats were significantly reduced, indicating that the experimental model was successful, as previously described [13]. Following treatment by low and high doses of Atracylodes rhizome polysaccharide, the rat albumin levels were significantly elevated, confirming the protective effect of Atracylodes rhizome polysaccharide against adenine-induced renal failure in rats (Table 2).
Excessive adenine feeding establishes a model of moderate to severe CRF [14,15], which also induces uremia related vascular calcification [16]. Dietary administration of 0.75% adenine for 4 weeks resulted in stable renal function impairment 2-10 weeks after the initiation of adenine administration, with serum levels of creatinine values comparable to those previously reported for this model. Although there is considerable biological variation in the development of vascular calcifications, the adenine model exhibits distinct advantages over the remnant kidney model, with significantly milder renal function impairment, resulting only after long follow-up periods in mild and mostly intra organ calcifications [17,18].
In this investigation, Atracylodes rhizome polysaccharide was shown to be beneficial in pre end stage renal failure. The properties of this compound have led to its prescription use in the management of humans and experimental animals with renal failure. Therefore, we used adenine-induced chronic renal failure rats in order to analyze kidney histology and investigate the effects of Atracylodes rhizome polysaccharide on adenine-induced renal injury.
In our study, the adenine-induced renal failure rat model resembled chronic renal failure in humans, biochemically and morphologically. The histological characteristic of our model was a scattered distribution of damaged tubules. In addition, there was development of massive albuminuria and an increase in the serum concentrations of creatinine and blood urea nitrogen, resembling the clinical course of progressive human kidney disease. We considered this model as being particularly suitable for testing novel treatment methods, due to the rapid course of the disease and the fact that there is little variation between individual rats. Thus, any beneficial effects of treatment may be detected using a minimum number of animals.
To investigate the effect of Atracylodes rhizome polysaccharide, we conducted this study in order to validate the protective action of this compound. In this study, the administration of adenine was associated with malnutrition, essentially due to a reduction of food intake. We were able to demonstrate a significant recovery of food intake and progressive decrease of proteinuria, serum levels of creatinine and blood urea nitrogen levels following Atracylodes rhizome polysaccharide administration in adenine fed rats. During the 4 weeks of administration, we also observed that Atracylodes rhizome polysaccharide attenuated the histological severity of kidney injury.
In the present study, several physiological, biochemical and histopathological parameters were used to determine whether Atracylodes rhizome polysaccharide consumption is able to mitigate CRF, as reported by certain traditional medicinal practices [19]. Our results suggested that Atracylodes rhizome polysaccharide, at the doses tested and for the duration it was used, was in fact effective in significantly, although incompletely, reversing the majority of the indices of CRF.
The mechanism(s) by which Atracylodes rhizome polysaccharide improved renal function in CRF has not been elucidated. First, Atracylodes rhizome polysaccharide may inhibit the progression of renal failure by preventing dihydroxyadenine deposition in the kidneys, particularly since dihydroxyadenine deposition led to CRF in a model of renal failure similar to that used in the present study [20]. Second, it has been hypothesized that Atracylodes rhizome polysaccharide increases the amount of energy available to the colonies of bacteria that ferment dietary fibers and absorb nitrogen as they grow.
In conclusion, the mechanism(s) underlying the nephroprotective actions of Atracylodes rhizome polysaccharide have not been fully elucidated, although it is hypothesized that antioxidant and/or antiinflammatory actions are involved. This study provided experimental evidence that Atracylodes rhizome polysaccharide attenuated adenine-induced renal dysfunction, suggesting a potential role for Atracylodes rhizome polysaccharide in the protection against renal failure progression. There are ongoing investigations on the effects of Atracylodes rhizome polysaccharide on the consequences of adenine-induced CRF.
Acknowledgements
This research was supported in part by China Scholarship Council (CSC, No. 201306725014) grants from the construct Program of the Key discipline of Basic medicine in Hunan Province, Provincial Science and Technology Plan of Hunan, China (No2014SK3238), Traditional Chinese Medicine of Hunan Province, China (No 201204).
References
- Pharmacopoeia of People’s Republic of China. Vol. 1. Guangzhou Guangdong: Sciences and Technology Press; 1995. p. 84-5.
- Cao G, Zhang XY, Cong XD. The research progress of polysaccharides from AtractylodesmacrocephalaKoidz. J Beijing Union Univ (Nat Sci) 2009;23:14-8.
- ONeill WC, Lomashvili KA, Malluche HH, Faugere MC, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int 2011;79:512-7.
- Sayegh MH, Carpenter CB. Transplantation 50 years later -progress, challenges, and promises. N Engl J Med 2004;351:2761-6.
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004;4:378-83.
- Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 2004;4:1289-95.
- Neugarten J, Golestaneh L. Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis 2013;20:390-5.
- Da Costa e Silva A, Todorov JC, D’Arrochela Lobo O. Effect of experimentally induced chronic renal failure upon the behavior of rats. Nephron 1979;24:78-80.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J BiolChem 1951;193:265-75.
- Wang TS, Long ZJ, Huang JL, Wang L, Wang W, Zhang L. Preventive and therapeutic effects of Shenlixin granules on chronic renal failure rats caused by adenine. Trad Chin Drug Res ClinPharmacol 2005;16:399-401.
- Liu Y, Yu B, Ma W. Effect of Shenshuai I on blood NO and NIS of rats with chronic renal failure induced by adenine. Chin J Trad Med Sci Technol 2000;7:361-2.
- Wang WM. Experimental study on chronic renal failure rats induced by adenine prevented with Shenshuai I decoction. Chin J Tradit Med Sci Technol 2001;8:79-80.
- Jin W, Wang NS. Advances in nutrition in the treatment of acute renal failure. Chin J IntegrTrad West Nephrol 2008;9:927-9.
- Okada H, Kaneko Y, Yawata T, Uyama H, Ozono S, Motomiya Y, et al. Reversibility of adenine-induced renal failure in rats. ClinExpImmunol 1999;3:82-8.
- Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron 1986;44:230-4.
- Katsumata K, Kusano K, Hirata M, Tsunemi K, Nagano N, Burke SK, et al. Sevelamer hydrochloride prevents ectopic calcification and renalosteodystrophy in chronic renal failure rats. Kidney Int 2003;64:441-50.
- Cozzolino M, Dusso AS, Liapis H, Finch J, Lu Y, Burke SK, et al. The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J Am SocNephrol 2002;13:2299-308.
- Cozzolino M, Staniforth ME, Liapis H, Finch J, Burke SK, Dusso AS, et al. Sevelamer hydrochloride attenuates kidney and cardiovascularcalcifications in long-term experimental uremia. Kidney Int 2003;64:1653-61.
- Ali BH, Alqarawi AA, Ahmed IH. Does treatment with gum Arabic affect experimental chronic renal failure in rats? FundamClinPharmacol 2004;18:327-9.
- Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron 1986;44:230-4.