- *Corresponding Author:
- Ao Li
Department of Ophthalmology, The First Clinical Medical College, Nanjing Medical University, Nanjing, Jiangsu 211166, China
E-mail: strichelieu@163.com
| Date of Received | 24 December 2021 |
| Date of Revision | 14 October 2022 |
| Date of Acceptance | 19 July 2023 |
| Indian J Pharm Sci 2023;85(4):944-952 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To determine how butein affects the oxidative harm caused by high glucose levels on human retinal vascular endothelial cells, as well as its method of action in doing so (human retinal vascular endothelial cells). The high glucose group, high glucose+butein-low group, high glucose+butein-middle group, high glucose+buteinhigh group, control group, high glucose+microRNA-negative control group, high glucose+microRNA- 146a group, high glucose+butein+anti-microRNA-negative control group, and high glucose+butein+antimicroRNA- 146a group were each given their own group of human retinal vascular endothelial cells. We used flow cytometry, the dichloro-dihydro-fluorescein diacetate method and colorimetric methodology to assess the apoptotic rate, reactive oxygen species level, superoxide dismutase activity, and malondialdehyde content of human retinal vascular endothelial cells. To determine the expression of microRNA-146a, real time-qualitative polymerase chain reaction was employed. Superoxide dismutase activity and microRNA- 146a expression were both substantially lower (p<0.05) in the high glucose group than in the control group, although the apoptotic rate, reactive oxygen species level, and malondialdehyde content of human retinal vascular endothelial cells in the high glucose group were all considerably greater (p<0.05) than those in the control group. In contrast to the high glucose group, the high glucose+butein-low, high glucose+buteinmiddle, and high glucose+butein-high groups significantly reduced the apoptotic rate, reactive oxygen species level, and malondialdehyde content of human retinal vascular endothelial cells while significantly increasing superoxide dismutase activity and microRNA-146a expression (p<0.05). In comparison to the high glucose+microRNA-negative control group, the apoptotic rate, reactive oxygen species level and malondialdehyde content of human retinal vascular endothelial cells in the high glucose+microRNA-146a group were all substantially lower (p<0.05), and superoxide dismutase activity was also considerably greater (p<0.05). The apoptosis rate, reactive oxygen species level, and malondialdehyde content of human retinal vascular endothelial cells in the high glucose+butein+anti-microRNA-146a group were noticeably greater (p<0.05) than in the high glucose+butein+anti-microRNA-negative control group, whereas the superoxide dismutase activity was noticeably lower (p<0.05) in comparison. Butein may be able to lessen the oxidative harm than high glucose levels due to human retinal vascular endothelial cells by increasing the expression of microRNA-146a.
Keywords
Butein, retinal vascular endothelial cells, oxidative stress, apoptosis, microRNA-146a, diabetic retinopathy
One of the main causes of vision loss and blindness in the society is Diabetic Retinopathy (DR). While DR therapies including laser photocoagulation, antiangiogenic medications, and glucocorticoids have been established, there are still significant drawbacks. Human Retinal Vascular Endothelial Cells (HRECs) can undergo apoptosis and an oxidative stress response in response to hyperglycemia, which can lead to changes in the retinal micro vessels' function and integrity. As a result, inhibiting the high glucose-induced oxidative damage in HRECs is the primary research direction for managing DR[1]. Butein, a plant chalcone like compound, exhibits anticancer, anti-inflammatory, anti-lipogenic as well as antioxidant activities[2-4]. Studies have revealed that butein could reduce the inflammation and oxidative stress of neurons and exert neuroprotective agent function[5]. Moreover, butein could inhibit apoptosis, oxidative stress and inflammation by activating Silent Information Regulator 1 (SIRT1), thereby alleviating sepsis induced multi-organ damage[6]. Although these studies demonstrated that butein exhibited anti-apoptotic and anti-oxidative properties, its effect on DR progression remained unclear. MicroRNAs (miRNAs) are important members of the non-coding Ribonucleic Acid (RNA) family, which regulate gene expression by degrading messenger RNA (mRNAs) or translational repression, participating in multiple processes of apoptosis, angiogenesis, proliferation, inflammatory reaction, oxidative stress and other DR progression[7,8]. It has recently been shown that Traditional Chinese Medicine (TCM) compounds via miRNAs can affect DR progression[9,10]. In high hyperglycemia-induced HRECs, miR-146a expression was downregulated, and miR-146a overexpression prevented the apoptosis that high glucose causes in HRECs[11]. The pre-experiment demonstrated that butein administration corrected the high glucose-induced modification of miR-146a expression in HRECs. This work employed miR-146a as a starting point to investigate butein's protective effect and mechanism against high glucose-induced HRECs damage in the hope of establishing an experimental foundation for its usage as a DR therapy.
Materials and Methods
Cells and reagents:
HRECs were bought from American Type Culture Collection (ATCC) in the United States; miR-146a mimics, miR-NC, miR-146a and anti-miR-NC were bought from Sangon Biotech Co., Ltd.; LipofectamineTM 2000 (Cat No. 11668-027) was purchased from Invitrogen, United States of America (USA); butein (purity 98 %, Cat No. XY-BZP-3047) was purchased from Shanghai Xuanya Biotechnology Co., Ltd.; Reactive Oxygen Species (ROS) level assay kit (Cat No. BB-47051), Fluorescein Isothiocyanate (FITC)-(Cat No. BB-4101) was purchased from Shanghai Bestbio Co., Ltd.; Glyceraldehyde Phosphate Dehydrogenase (GAPDH) RabMab (AF1186), Superoxide Dismutase (SOD) activity assay kit (Cat No. S0101S), goat anti rabbit secondary antibody (Cat No. A0208), Malondialdehyde (MDA) content assay kit (Cat No. S0131S), cleaved-caspase-3 RabMab (Cat No. AC033) was purchased from Beyotime Biotech Inc., Shanghai; miRNA reverse transcription (Cat No. MT0006) and fluorescent quantitative Polymerase Chain Reaction (PCR) kit (Cat No. ALH190) was purchased from Beijing Baiao Laibo Biotechnology Co., Ltd.; Cleaved caspase-9 rabbit polyclonal antibody (Cat No.AF5244) was purchased from Affinity, USA.
Proposed methods:
Cell culture and modeling: The HRECs were grown in Dulbecco's Modified Eagle Medium (DMEM) with the addition of 10 % fetal bovine serum and 1.0 % double antibody. The cells were preserved at a temperature of 37° in a Carbon dioxide (CO2) incubator. For the experiments, cells from passages three to five were utilized. To generate a cellular model for DR, HRECs were treated with a culture medium that had a high concentration of glucose (30 mmol/l) for 48 h. In simpler terms, HRECs were grown in a specific type of medium with added nutrients and were kept in a controlled environment for optimal growth. For the experiments, cells from a specific passage were chosen. To create a model for DR, the cells were exposed to a culture medium that had a high level of glucose for 2 d[12].
Cell transfection: Prior to transfection, HRECs were grown in 24-well plates. Once the cells reached a confluence of 60 %, they were ready for transfection. The transfection was performed using a substance called Lipo2000, which is used to deliver nucleic acids to cells. Four different types of nucleic acids were used for the transfection; anti-miR-146a, miR-146a mimic, anti-miR-Negative Control (NC), and miR-NC. Each nucleic acid was administered at a dose of 40 nM. After transfection, the cells were allowed to incubate for 48 h, during which time they were allowed to grow and proliferate. At the verge of the completion of the incubation period, the cells were collected for further experimentation. This involve analysis of their gene expression, protein levels, or other characteristics to determine the effects of the nucleic acid transfection on the cells. Overall, the experiment aimed to examine the role of miR-146a in HRECs and how modulating its expression may impact cellular function.
Experimental grouping: The motive of the study is to visualize the impact of high glucose levels on HRECs under various experimental conditions. To accomplish this, different groups of HRECs were subjected to distinct treatments. One group was treated with a nutrient solution that contained 5.5 mmol/l glucose, which served as the control group. Another group was treated with a high glucose based concentration of 30 mmol/l to induce high glucose stress. In addition, several other groups of cells were treated with 30 mmol/l glucose and varying concentrations of butein (0.5 μmol/l, 1 μmol/l, or 2 μmol/l), which were labeled as high glucose+butein-low, high glucose+butein-middle and high glucose+butein-high groups, respectively. These groups were created to examine the effects of butein, a plant-derived flavonoid, on high glucose-induced stress in HRECs. Another set of cells were transfected with miR-146a mimics or miR-NC, which served as the control, and then incubated with 30 mmol/l glucose. These groups were labeled as high glucose+miR-146a and high glucose+miR-NC groups, respectively. The purpose of this experiment was to investigate the role of miR-146a, a type of miRNA, in high glucose-induced stress in HRECs. Lastly, cells were transfected with anti-miR-146a and incubated with 30 mmol/l glucose and 2 μmol/l butein. These groups were labeled as high glucose+butein+anti-miR-146a and high glucose+butein+anti-miR-NC groups. The goal of this experiment was to examine the interaction between butein and miR-146a in high glucose-induced stress in HRECs. All groups of HRECs were then incubated for 48 h to observe any changes in the cells caused by the different experimental conditions. Overall, this study aimed to gain insights into the effects of high glucose levels on HRECs and to identify potential therapeutic targets for DR.
Apoptosis of HRECs by flow cytometry detection: Cells from diverse experimental groups have been cleaned with Phosphate Buffered Saline (PBS), which is a solution used to maintain cell integrity and function during laboratory procedures. After the washing step, the cells were resuspended in a solution to create a homogeneous cell suspension with a concentration of 1 million cells per milliliter. To examine the level of cell apoptosis, 5 ml of FITC-Annexin V and 5 ml of propidium iodide were added to the cell suspension. FITC-Annexin V is a protein that binds to the cell membrane of apoptotic cells, while propidium iodide is a Deoxyribonucleic Acid (DNA) binding dye that labels dead cells. The cell suspension was then incubated in the dark at 25° for 15 min, allowing the FITC-Annexin V and propidium iodide to penetrate the cell membrane and bind to their respective targets. Flow cytometry, a technique used to analyze the physical and chemical properties of cells, was then employed to examine the level of cell apoptosis. This method involves passing the cell suspension through a flow cytometer, which uses lasers and detectors to analyze the properties of individual cells in the sample. By measuring the fluorescence emitted by FITC-Annexin V and propidium iodide, the percentage of apoptotic and dead cells in each sample can be quantified.
Detection of ROS level in HRECs by Dichloro-Dihydro-Fluorescein Diacetate (DCFH-DA) assay: To measure the levels of ROS in HRECs, DCFH-DA was used. DCFH-DA is a non-fluorescent probe that can be converted into fluorescent DCF by ROS, making it an effective tool for detecting ROS levels. To prepare the DCFH-DA solution, it was mixed with serum-free medium and then diluted 1:1000. The HRECs were then mixed with this solution and incubated at a concentration of 1×106 cells per milliliter for 20 min at 37°. During the incubation, the mixture was gently mixed every 5 min to ensure that the probe and cells were in contact. After the incubation, the cells were cleaned with serum-free medium to eliminate any excess DCFH-DA. The levels of ROS were then measured using flow cytometry, which is a technique that can detect and analyze multiple physical and chemical characteristics of individual cells as they pass through a laser beam. In this case, the flow cytometry detected the fluorescence of DCF, which allowed for the quantification of ROS levels in the HRECs.
Colorimetric detection of SOD activity and MDA content in HRECs: After the incubation period, the cell culture solution was eliminated from the cells, and they were washed once with chilled PBS. Then, approximately one million cells were mixed with 100 μl of lysate and lysed by pipetting. The resulting mixture was subjected to centrifugation at 8000 revolutions per minute for 5 min at 4°, and the liquid above the pellet was collected as the sample for analysis. The activity of intracellular SOD, which is an enzyme that protects against oxidative stress, and the content of MDA, which is a marker for lipid peroxidation and oxidative stress, were measured using specific assay kits. These assays provided a quantitative measure of the amount of SOD activity and MDA content in the cells, which can be used to assess the effects of different treatments or conditions on oxidative stress and cellular damage.
RT-qPCR detection of miR-146a expression in HRECs: In this experiment, Trizol reagent was added to the different groups of HRECs to extract total RNA. The extracted RNA was then used to synthesize cDNA by a miRNA reverse transcription kit. After synthesizing cDNA, a miRNA fluorescence quantitative PCR kit was used to amplify the cDNA and determine the expression level of miR-146a. The relative expression of miR-146a to U6 was determined using the 2−ΔΔCt method, which is a widely used technique to measure gene expression. The primer sequences utilized for the PCR are demonstrated as; miR-146a upstream primer 5'- CAA CAC CAG TCG ATG GGC TGT-3’, reverse primer 5'-CCC ATG GAA TTC AGT TCT cat T-3'; U6 upstream primer and reverse primer. These primers are specific to the miR-146a and U6 genes, respectively. The experiment aimed to determine the expression level of miR-146a in HRECs under different conditions.
Western blotting for cleaved caspase-3 and cleaved caspase-9 protein levels: To obtain all the proteins from HRECs, Radioimmunoprecipitation Assay Buffer (RIPA) buffer was used to disrupt the cells. After extraction, 40 μl of the protein samples have been segregated by Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), a technique used to separate proteins by size. The proteins were then transported from the gel to a membrane using wet electroblotting. The membrane was then blocked to prevent nonspecific binding of antibodies and treated with diluted primary and secondary antibodies for 2 h at room temperature. The primary and secondary antibodies bind to specific proteins of interest. After the incubation, the immunoblots were identified using an Odyssey system of imaging, and the relative densitometry values of cleaved caspase-3 and cleaved caspase-9 bands were determined using Image J software. This analysis allows for the quantification of protein expression levels.
Statistical methods:
The data obtained from the experiments was analyzed using statistical methods, and the results were presented as mean±standard deviation (x͞ ±s). The Statistical Package for the Social Sciences (SPSS) 20.0 version was utilized for the statistical interpretations. In order to make comparison of two groups, an independent sample based t-test was performed. Meanwhile, for comparing differences among multiple groups, one-way Analysis of Variance (ANOVA) and Least Significant Difference (LSD) test, t-test were used. p<0.05 was taken as benchmark for all analysis. This means that if the p value was lower than 0.05, the difference between the groups was considered relevant statistically, and the probability that the observed modification arisen by chance was <5 %. Using the statistical analysis methods mentioned above helps to ensure that the data obtained is reliable and can be used to draw valid conclusions. It also helps in determining whether the observed differences in the data are due to the treatment or intervention being tested, or if they occurred by chance.
Results and Discussion
The study found that levels of ROS and MDA were remarkably higher, while SOD activity was pointedly lower, in HRECs of the high glucose group associated to the control group (p<0.05). However, in HRECs of the high glucose+butein-low, high glucose+butein-middle, and high glucose+butein-high groups, ROS and MDA levels were remarkably lower. SOD activity was considerably higher as compared to the high glucose group (p<0.05). These findings are summarized in Table 1.
| Group | ROS level (%) | SOD activity (U/mg) | MDA content (nmol/mg) |
|---|---|---|---|
| Control | 100.00±0.00 | 91.07±7.86 | 5.16±0.49 |
| High glucose | 426.69±27.82* | 32.32±3.44* | 23.34±2.02* |
| High glucose+butein-low | 353.61±16.79# | 53.65±4.63# | 15.83±1.32# |
| High glucose+butein-middle | 275.55±19.38#& | 66.97±6.27#& | 11.08±1.08#& |
| High glucose+butein-high | 181.54±13.05#&$ | 83.03±7.87#&$ | 6.38±0.56#&$ |
| F | 479.477 | 126.345 | 330.868 |
| p | 0 | 0 | 0 |
Note: $p<0.05 vs. high glucose+butein-middle group; #p<0.05 vs. high glucose group; *p<0.05 vs. control group and &p<0.05 vs. high glucose+butein-low group
Table 1: Effect of Butein on Oxidative Stress Induced by High Glucose in HRECs (x͞±s, n=9)
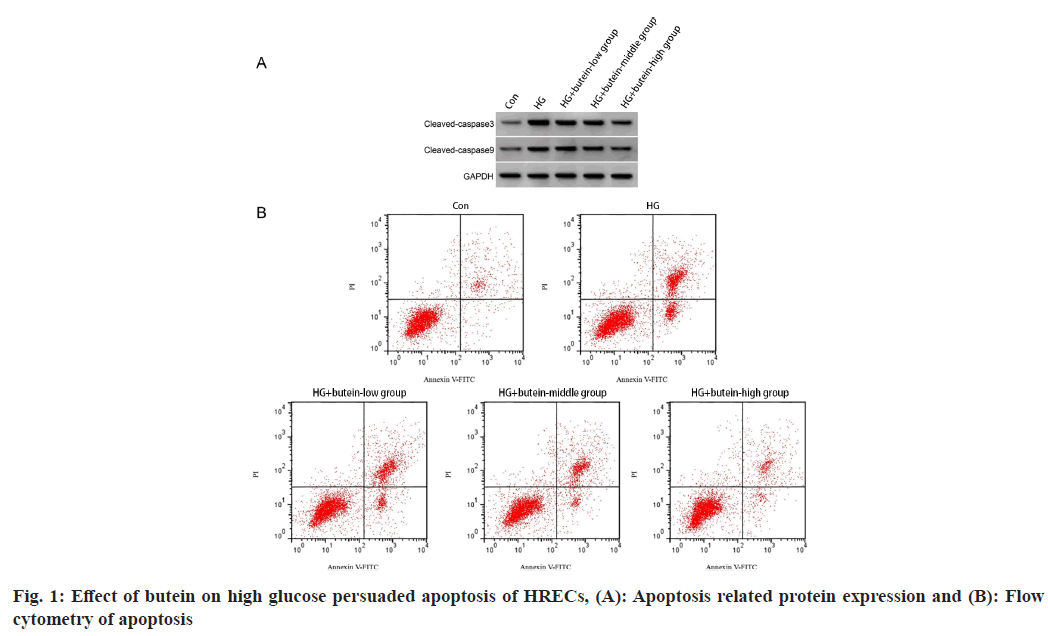
The study found that when HRECs were exposed to a high glucose environment, there was a considerable upsurge in the levels of cleaved caspase-3 protein and apoptotic rates compared to the control group. However, when HRECs were treated with dissimilar absorptions of butein (a natural flavonoid), the levels of cleaved caspase-3 protein and apoptotic rates were remarkably lower as compared to the high glucose group. The results were considerably important statistically (p<0.05) and are presented in fig. 1 and Table 2 of the study. These findings suggest that butein may have a defensive impact against high glucose persuaded apoptosis in HRECs.
| Group | Apoptosis rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|
| Control | 6.22±0.56 | 0.25±0.02 | 0.17±0.02 |
| High glucose | 34.28±3.04* | 0.82±0.05* | 0.65±0.05* |
| High glucose+butein-low | 25.28±2.11# | 0.67±0.04# | 0.53±0.04# |
| High glucose+butein-middle | 16.75±1.39#& | 0.51±0.04#& | 0.38±0.03#& |
| High glucose+butein-high | 9.15±0.82#&$ | 0.35±0.03#&$ | 0.24±0.03#&$ |
| F | 363.08 | 344.571 | 282.357 |
| p | 0.000 | 0.000 | 0.000 |
Note: $p<0.05 vs. high glucose+butein-middle group; #p<0.05 vs. high glucose group; *p<0.05 vs. control group and &p<0.05 vs. high glucose+butein-low group
Table 2: Impact of Butein on the Apoptosis of HRECs Induced by High Glucose (x͞±s, n=9)
The study found that there was a remarkable difference (p<0.05) in the expression levels of miR-146a in HRECs between the high glucose and control groups. The expression levels of miR-146a have been considerably lower in the high glucose group compared to the control group. However, when the HRECs were treated with butein (a type of flavonoid), the expression levels of miR-146a increased considerably (p<0.05) in a dose-dependent manner. The high glucose+butein-low, high glucose+butein-middle, and high glucose+butein-high groups, all showed a substantial increase in miR-146a expression levels compared to the high glucose group. These findings are presented in Table 3.
| Group | miR-146a |
|---|---|
| Control | 1.000±0.00 |
| High glucose | 0.31±0.035* |
| High glucose+butein-low | 0.53±0.04# |
| High glucose+butein-middle | 0.73±0.05#& |
| High glucose+butein-high | 0.92±0.07#&$ |
| F | 363.954 |
| p | 0.000 |
Note: $p<0.05 vs. high glucose+butein-middle group; #p<0.05 vs. high glucose group; *p<0.05 vs. control group and &p<0.05 vs. high glucose+butein-low group
Table 3: Effect of Butein on miR-146a Expression in HRECs Induced by High Glucose (x͞±s, n=9)
In comparative study, the group treated with high glucose and miR-NC, the high glucose+miR-146a group demonstrated a substantial increase in miR-146a expression level and SOD activity while a significant reduction in ROS level and MDA content in HRECs as shown in Table 4.
| Group | miR-146a | ROS level (%) | SOD activity (U/mg) | MDA content (nmol/mg) |
|---|---|---|---|---|
| High glucose+miR-NC | 1.00±0.00 | 441.02±27.82 | 31.35±3.43 | 24.83±2.16 |
| High glucose+miR-146a | 2.86±0.26* | 226.97±21.97* | 72.08±5.72* | 9.74±0.68* |
| F | 21.462 | 18.115 | 18.32 | 19.991 |
| p | 0 | 0 | 0 | 0 |
Note: Compared with high glucose+miR-NC group, *p<0.05
Table 4: Effects of miR-146a Over-Expression on High Glucose Induced Oxidative Stress in HRECs (x͞±s, n=9)
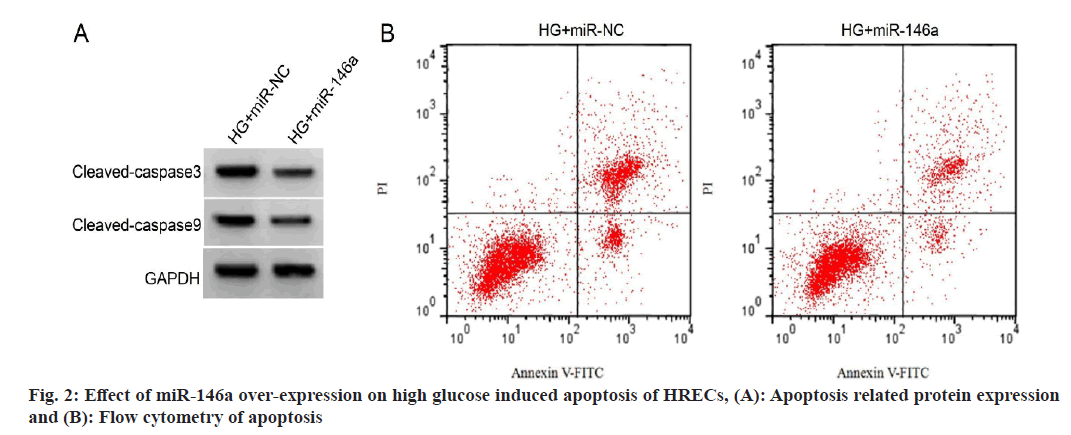
In the HRECs of the high glucose+miR-146a group compared to the high glucose+miR-NC group, cleaved caspase-3 and cleaved caspase-3 induced apoptosis protein levels were considerably lower (with no miRNA treatment). The results shown in fig. 2 and Table 5 indicate that this difference was statistically important (p<0.05).
| Group | Apoptosis rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|
| High glucose+miR-NC | 33.13±3.54 | 0.84±0.06 | 0.67±0.05 |
| High glucose+miR-146a | 12.92±1.11* | 0.44±0.04* | 0.32±0.03* |
| F | 16.343 | 16.641 | 18.007 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with high glucose+miR-NC group, *p<0.05
Table 5: Effects of miR-146a Over-Expression on High Glucose Induced Apoptosis of HRECs (x͞±s, n=9)
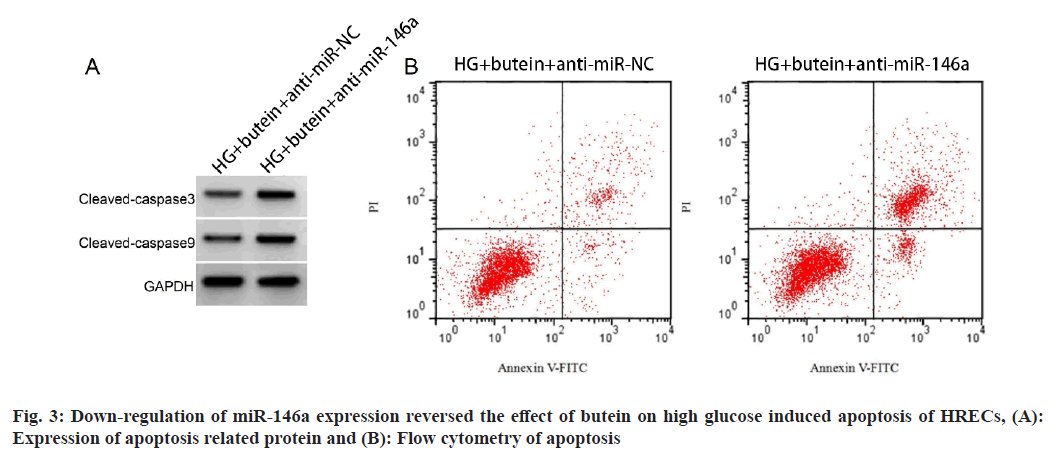
In HRECs exposed to high glucose+butein+anti-miR-NC as compared to the high glucose+butein+anti-miR-NC group, the expression and activity of miR-146a was remarkably reduced, although levels of ROS, apoptotic rate, MDA content and protein levels of cleaved-caspase-3 and cleaved caspase-9 were significantly elevated. Fig. 3 and Table 6 show these results, which were statistically significant (p<0.05). The primary pathogenic factor of DR is hyperglycemia and chronic hyperglycemia can alter ROS production, which causes an imbalance in the antioxidant and oxidative systems of HRECs when ROS generation exceeds the scavenging limit and exacerbates the production of MDA, an end product of lipid peroxidation, which causes HREC damage and apoptosis[1]. Thus, it is crucial to lessen the oxidative stress and cell death that high glucose levels cause in HRECs. Butein has been shown to lessen cardiomyocyte oxidative stress and cell apoptosis and ameliorate cardiac ischemia-reperfusion damage[13]. By boosting the enzymes that act as antioxidants, preventing oxidative stress and inflammatory responses, and ultimately suppressing neuronal death, butein had neuroprotective benefits[14]. Butein's impact on the oxidative damage to HRECs brought on by high glucose levels is yet unknown. SOD is a crucial antioxidant enzyme that guards against oxidative cell damage. In order to comprehend how butein defends against high glucose-induced oxidative damage in HRECs, this research assessed ROS production, MDA content, cell death rate and SOD activity. The results showed that high glucose therapy raised levels of ROS, cell apoptosis, MDA, reduced SOD activity, and pro-apoptotic proteins caspase-3 and caspase-9 cleavage in HRECs. The fact that butein treatment significantly reduced the oxidative stress and apoptosis brought on by high glucose levels in HRECs suggests that butein may act as a DR inhibitor.
| Group | miR-146a | ROS level (%) | SOD activity (U/mg) | MDA content (nmol/mg) | Apoptosis rate (%) | Cleaved caspase-3 protein | Cleaved caspase-9 protein |
|---|---|---|---|---|---|---|---|
| High glucose+butein+anti-miR-NC | 1.00±0.00 | 178.39±12.72 | 84.21±7.22 | 6.14±0.55 | 8.94±0.67 | 0.33±0.03 | 0.23±0.02 |
| High glucose+butein+anti-miR-146a | 0.41±0.05* | 360.88±29.48* | 46.11±4.32* | 16.65±1.28* | 23.05±2.27* | 0.74±0.06* | 0.57±0.05* |
| F | 35.4 | 17.051 | 13.585 | 22.632 | 17.885 | 18.336 | 18.941 |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Notes: *p<0.05, compared with high glucose+butein+anti-miR-NC
Table 6: Down-Regulation of miR-146a Expression Reversed the Effect of Butein on High Glucose Induced Oxidative Damage in HRECs (x͞±s, n=9)
miR-146a is a protective miRNA, and research has shown that it may reduce the cardiotoxicity caused by doxorubicin by thermostatically controlling autophagy by inhibiting apoptosis[15]. Moreover, miR-146a prevents ischemia/reperfusion damage to the small intestine and liver by inhibiting the apoptosis that is brought on by ischemia-reperfusion in intestinal epithelial cells and hepatocytes[16,17].
Moreover, miR-146a enhances the long-term neurological function of the mouse brain and mitigates the consequences of traumatic brain damage[18]. We discovered that high glucose treatment resulted in down-regulation of miR-146a expression in HRECs, and over-expression of miR-146a significantly inhibited high glucose caused apoptosis of HRECs. These findings are similar to the findings of Gao et al.[11], who have investigated the effects of high glucose. This study also showed that over-expression of miR-146a reduced the rise in ROS and MDA levels brought on by high glucose and increased SOD activity, indicating that miR-146a protects HRECs from high glucose-induced oxidative damage by reducing ROS accumulation and enhancing antioxidant enzyme activity. Recent studies have shown that some TCM substances help prevent DR by using tiny molecules called miRNAs. For instance, by lowering the expression of miR-34a[19], which reduces oxidative stress and cell death, dihydromyricetin might lessen the detrimental effects of high hyperglycemia on human retinal pigment epithelium cells. By regulating the DLX6-AS1/miR-145-5p axis, another substance, Marinol A, may prevent high glucose-induced cell death in retinal endothelial cells[20]. According to the research, butein therapy inhibited the expression of miR-146a from declining in response to high glucose levels. The research also discovered that butein and over-expressed miR-146a had comparable protective effects against apoptosis and oxidative stress brought on by high glucose, suggesting that butein may slow the development of DR through miR-146a. Subsequent research revealed that lower expression of miR-34a interfered with butein's capacity to suppress apoptosis and oxidative stress in HRECs under high glucose circumstances.
The outcomes of the study suggest that butein has the potential to shield HRECs against damage caused by high glucose levels. The study found that butein treatment led to increased expression of miR-146a, a molecule that plays a crucial role in protecting cells from oxidative stress and cell death. This mechanism of action may contribute to butein's ability to prevent DR. These findings provide empirical evidence supporting the use of butein as a clinical therapy for the prevention and management of DR. Butein shows promise as a potential treatment option for DR, and further studies may be needed to explore its full therapeutic potential in this area.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lu J. Effects of FOXO4 on oxidative stress and apoptosis of retinal vascular endothelial cells under high glucose environmental conditions. Int Eye Sci 2018:2146-50.
- Gao X, Tong X. Research progress of butein in malignant tumor. World Latest Med Info 2020;20(36):65-71.
- Farias-Pereira R, Zhang Z, Park CS, Kim D, Kim KH, Park Y. Butein inhibits lipogenesis in Caenorhabditis elegans. Biofactors 2020;46(5):777-87.
[Crossref] [Google Scholar] [PubMed]
- Zheng W, Zhang H, Jin Y, Wang Q, Chen L, Feng Z, et al. Butein inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and slows the progression of osteoarthritis in mice. Int Immunopharmacol 2017;42(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Lee DS, Jeong GS. Butein provides neuroprotective and anti-neuroinflammatory effects through Nrf2/ARE-dependent haem oxygenase 1 expression by activating the PI3K/Akt pathway. Br J Pharmacol 2016;173(19):2894-909.
[Crossref] [Google Scholar] [PubMed]
- Zhu Y, Wang K, Ma Z, Liu D, Yang Y, Sun M, et al. SIRT1 activation by butein attenuates sepsis-induced brain injury in mice subjected to cecal ligation and puncture via alleviating inflammatory and oxidative stress. Toxicol Appl Pharmacol 2019;363:34-46.
[Crossref] [Google Scholar] [PubMed]
- Tian T, Yao R, Peng J. Effect of miR-106 regulating CC chemokine ligand 2 on human retinal microvascular endothelial cell proliferation, angiogenesis and inflammatory responses in proliferative diabetic retinopathy. Recent Adv Ophthalmol 2021;41(9):831-7.
- Chen P, Miao Y, Yan P, Wang XJ, Jiang C, Lei Y. miR-455-5p ameliorates HIGH GLUCOSE-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells. J Cell Physiol 2019;234(12):21915-24.
[Crossref] [Google Scholar] [PubMed]
- Su J, Jiao Y, Wei H. Curcumin protecting human retinal pigment epithelial cells against high glucose induced cell injury by down-regulating microRNA-125b. Chin J Comp Med 2020;30(12):30-5.
- Ma J, Wang Q, Niu H. Cryptogenin alleviates high glucose induced retinal vascular endothelial cell injury by up-regulating miR-1247-3p expression. J Prac Med 2021;37(11):1397-402.
- Gao F, Wu Z, Ruan Y. The inhibiting effect of miR-146a on high glucose induced apoptosis in human retinal microvascular endothelial cells and the underlying mechanism. Chin J Exp Ophthalmol 2021;39(5):398-403.
- Zhang M. Protective effect of gigantolon human retinal microvascular endothelial cells induced by high glucose. Int Eye Sci 2019;19(2):209-13.
[Crossref] [Google Scholar] [PubMed]
- Liu K, Duan J, Su J. Protective effect of butein against oxidative stress injury in PC12 cells and its effect on mitochondrial function study. Chin Pharm J 2020;31(24):2974-81.
- Duan J, Zhi M, Mou F. Butein inhibiting myocardial ischemia-reperfusion injury by modulating AMPK/GSK-3β signaling pathway. Prog Mod Biomed 2017;17(14):2616-21.
- Pan JA, Tang Y, Yu JY, Zhang H, Zhang JF, Wang CQ, et al. miR-146a attenuates apoptosis and modulates autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced cardiotoxicity. Cell Death Dis 2019;10(9):668-78.
[Crossref] [Google Scholar] [PubMed]
- He X, Zheng Y, Liu S, Shi S, Liu Y, He Y, et al. miR-146a protects small intestine against ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB pathway. J Cell Physiol 2018;233(3):2476-88.
[Crossref] [Google Scholar] [PubMed]
- Jiang W, Kong L, Ni Q, Lu Y, Ding W, Liu G, et al. miR-146a ameliorates liver ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS One 2014;9(7):e101530.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Zhao L, Zhu W, Ding Y, Chen H, Chi N. miR-146a mimics ameliorates traumatic brain injury involving JNK and NF-κB signaling pathway. Neuromolecular Med 2020;22(4):484-92.
[Crossref] [Google Scholar] [PubMed]
- Li W, Xiao H. Dihydromyricetin alleviates high glucose-induced oxidative stress and apoptosis in human retinal pigment epithelial cells by downregulating miR-34a expression. Diabetes Metab Syndr Obes 2021;14(1):387-97.
- Wang X, Liu W, Yang L. Effect of Marinol A on the proliferation and apoptosis of human retinal vascular endothelial cells induced by high glucose in vitro. China Pharm J 2021;24(4):683-8.