- *Corresponding Author:
- L. Y. Jiang
Department of Geriatrics
Zhejiang Hospital of Integrated Traditional Chinese and Western Medicine
Hangzhou, Zhejiang 310003, China
E-mail: jianglanying79@163.com
| Date of Received | 19 January 2021 |
| Date of Revision | 06 January 2022 |
| Date of Acceptance | 05 September 2022 |
| Indian J Pharm Sci 2022;84(5):1248-1256 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of Panax notoginseng saponins on atherosclerosis with type 2 diabetes mellitus in Goto-Kakizaki rats by nuclear factor-kappa B signaling pathway. Goto-Kakizaki rats fed with high fat diet and injected with N-nitro-L-arginine methyl ester for 8 w, were randomized into model group and Panax notoginseng saponins group (400 mg/kg/d). Another Goto-Kakizaki rats and Wistar rats were selected as Goto-Kakizaki group and control group which fed with normal diet and injected with saline. Serum lipid and inflammatory factors were measured. There were no statistical differences of lipid profiles between model group and Panax notoginseng saponins group (p>0.05). In Panax notoginseng saponins group, serum tumor necrosis factor-alpha, interleukin-6, intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and monocyte chemotactic protein-1 were significantly decreased (p<0.01), messenger RNA expression and protein levels of interleukin-6, tumor necrosis factor-alpha, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, monocyte chemotactic protein-1 and nuclear factor-kappa B in the Panax notoginseng saponins group were significantly lower, compared with those in model group (p<0.05, p<0.01). Some inflammatory factors in model group were higher than those in Goto-Kakizaki group which were higher than those in control group. The inflammatory reaction with nuclear factorkappa B signaling pathway as core, occurs throughout type 2 diabetes mellitus and its atherogenesis and Panax notoginseng saponins improves nuclear factor-kappa B signaling pathway, decreasing its related inflammatory factors to achieve an improvement in the diabetic atherosclerotic process.
Keywords
Panax notoginseng saponins, type 2 diabetes mellitus, atherosclerosis, nuclear factor-kappa B, interleukin-6
With lifestyle changes and accelerated population aging, the incidence of diabetes mellitus is increasing. According to statistics, there are 424.9 million adults with diabetes mellitus worldwide and the number of adults with diabetes mellitus in China has reached 114 million[1], of which more than 90 % have Type 2 Diabetes Mellitus (T2DM). Macrovascular complications are a major lethal and disabling cause in patients with diabetes mellitus. Numerous clinical studies have found[2] that glucose control alone can significantly control chronic microvascular complications, but cannot significantly reduce the macrovascular complications caused by atherosclerosis. The "common soil" doctrine[3] was thus proposed that inflammation is the common soil where atherosclerosis and T2DM occurs.
Studies have shown that some Traditional Chinese Medicine (TCM) extracts can significantly reduce blood lipids as well as inhibit the inflammatory reaction in the vessel wall, which may play an important role in Anti-Atherosclerosis (AS)[4,5]; some TCM extracts are able to reduce inflammatory factors and improve insulin resistance and diabetic macrovascular complications[6]. Therefore, there is a promising prospect to explore the roles and mechanisms of TCMs to improve diabetic macroangiopathy with the approach of modern medicine.
Panax notoginseng (P. notoginseng) (Burk.), the plant of the genus Panax in the family Araliaceae, has the major active components of P. notoginseng Saponins (PNS), including notoginsenoside R1, ginsenoside Rb1, Rg1, Re and Rd and so on. In recent years, studies have found that PNS reduces inflammatory factors and regulates lipid to improve the process of diabetic atherosclerosis[7], but the specific molecular mechanism is still unclear. Based on the Nuclear Factor-Kappa B (NF-κB) signaling pathway, this paper explores the mechanism of PNS in improving atherosclerosis in T2DM rats from the perspective of inflammation.
Materials and Methods
Experimental animals:
24 Specific Pathogen Free (SPF) grade 6 mo old male rats with spontaneous type 2 diabetic Goto-Kakizaki (GK) and 8 Wistar rats, weighing around 400 g, were selected from the Animal Center of Zhejiang Academy of Medical Sciences under the production license number SYXK (Z) 2019-0011, with temperature (22°±1°), humidity 50 %±5 % and 12 h each day of light and night time. The operations such as gavage and dissection during the experimental period were all performed within the super-clean bench and water exchange and feed addition were performed by special person responsible for the management.
Reagents and instruments:
Reagents: PNS (Lot No. 160902, purity 83.4 %, Wuhan Yuancheng Technology Development Co., Ltd.); endothelial Nitric Oxide Synthase (NOS) inhibitors; N-nitro-L-Arginine Methyl Ester (L-NAME) (Beyotime Biotech Inc., Shanghai); Total Cholesterol (TC), Triglyceride (TG), Low-Density Lipoprotein- Cholesterol (LDL-C), High-Density Lipoprotein- Cholesterol (HDL-C) levels were measured (Medicalsystem Biotechnology Co., Ltd.); rat Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-6 (IL-6), Intercellular Adhesion Molecule-1 (ICAM-1), Vascular Cell Adhesion Molecule-1 (VCAM-1), Monocyte Chemotactic Protein-1 (MCP-1) kits (Nanjing Jiancheng Bioengineering Institute); high fat diet (Shanghai Puluteng Biotechnology Co., Ltd.) normal diet 88.2 %, refined lard 10 %, cholesterol 1.5 %, porcine bile salt 0.3 % and propylthiouracil 0.2 %. Ribonucleic Acid (RNA) extraction kit (TaKaRa, Japan), Reverse Transcription (RT) kit (TaKaRa, Japan), SYBR® green fluorescent quantitative Polymerase Chain Reaction (PCR) dye (TaKaRa, Japan). IL-6, TNF-α, ICAM- 1, VCAM-1, MCP-1, NF-κB, RT-PCR primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Table 1). IL-6, TNF-α, ICAM-1, VCAM-1, MCP- 1, NF-κB p65 immunohistochemistry kit (Nanjing Jiancheng Bioengineering Institute).
| Gene (s) | Primer | Sequence | Size |
|---|---|---|---|
| Rat IL-6 | Forward | 5'-AAGCCAGAGTCATTCAGAGCAATACTG-3' | 167 |
| Rat IL-6 | Reverse | 5'-GATGAGTTGGATGGTCTTGGTCCTTAG-3' | |
| Rat TNF-α | Forward | 5'-ATACACTGGCCCGAGGCAAC-3' | 75 |
| Rat TNF-α | Reverse | 5'-CCACATCTCGGATCATGCTTTC-3' | |
| Rat ICAM-1 | Forward | 5'-GGCAGCAGGTGGGCAAGAAC-3' | 110 |
| Rat ICAM-1 | Reverse | 5'-CTGGCGGCTCAGTGTCTCATTC-3' | |
| Rat VCAM-1 | Forward | 5'-GCTGCTGTTGGCTGTGACTCTC-3' | 108 |
| Rat VCAM-1 | Reverse | 5'-GCTCAGCGTCAGTGTGGATGTAG-3' | |
| Rat MCP-1 | Forward | 5'-CACCTGCTGCTACTCATTCACTGG-3' | 92 |
| Rat MCP-1 | Reverse | 5'-CTTCTTTGGGACACCTGCTGCTG-3' | |
| Rat NF-κB p65 | Forward | 5'-GGCACAGTCAAGGCTGAGAATG-3' | 86 |
| Rat NF-κB p65 | Reverse | 5'-ATGGTGGTGAAGACGCCAGTA-3' | |
| Rat GAPDH | Forward | 5'-GGCACAGTCAAGGCTGAGAATG-3' | 143 |
| Rat GAPDH | Reverse | 5'-ATGGTGGTGAAGACGCCAGTA-3' |
Table 1: Primer Sequences
Instruments: Multifunctional micro plate reader (Thermo, USA), fluorescence quantitative PCR instrument (Ankle-Brachial Index (ABI), United States of America (USA)), Nikon eclipse 80i microscope (Nikon, Japan), Carl Zeiss Imaging Systems (Carl Zeiss, Germany), Olympus digital pathology section scanning system Olympus VS120 (Olympus, Japan).
Experimental grouping and intervention:
After the rats had been acclimated for 1 w, GK rats with random blood glucose ≥11.1 mmol/l were determined as T2DM rats on two consecutive days. 24 GK rats were randomly divided into GK group, model group, PNS group and Wistar rats were divided into normal control group; both model group and PNS group were fed daily with high-fat diet containing metabolic inhibitor propylthiouracil and intraperitoneally injected with 8 mg/kg L-NAME once a week, control group and GK group were fed with normal diet, PNS group was given 400 mg/(kg/d) PNS by gavage and the other groups were given equal normal saline by gavage for a total of 8 w[8,9].
Index tests and methods:
The rats were observed for hair, activity, mental status, body weight, food intake, etc. All rats fasted for 12 h and were injected intraperitoneally with 10 % chloral hydrate (0.3 ml/100 g). After successful anesthesia, the abdominal cavity was opened by cutting along the median line of the abdomen, the aorta was carefully separated and 8 ml of routine abdominal aortic blood was collected and placed in a centrifuge tube and the serum was separated after centrifugation at 3000 r/min in a low-speed centrifuge and stored at -20° for further use. A perfusion needle was inserted from the apical site into the left ventricle to the aorta, the rats were quickly perfused with 100 ml saline and then 500 ml saline for repeated perfusion through the heart to whitening and perfusing for about 30 min, two segments of 2-3 cm from the lower thoracic and abdominal aorta of the aortic arch were removed, a section was fixed with 10 % formaldehyde solution for 48 h, dehydrated and paraffin embedded, and then sectioned for routine Hematoxylin and Eosin (H&E) staining to observe the atherosclerotic lesions of the aorta and their extent and the other section was prepared for immunohistochemical staining and the remaining aorta was homogenized and then RNA was extracted for RT-PCR.
Basic index test: Random blood glucose was measured every 4 w from baseline blood glucose and blood glucose levels were measured with a blood glucose monitor. The serum of 1.3 rats was obtained, and the levels of TG, TC, LDL-C and HDL-C in serum were detected by an automatic biochemical analyzer (Biobase, China).
Determination of inflammatory factors: The serum of 1.3 rats was obtained and the levels of IL-6 and TNF-α, ICAM-1, VCAM-1 and MCP-1 were measured according to the instructions of Enzyme-Linked Immunosorbent Assay (ELISA) kits.
H&E staining: It was used to observe the histopathological and morphological appearance of rat aorta. A segment of aorta in 1.3 was removed and fixed in 10 % formaldehyde solution for 48 h. After dehydration and paraffin embedding, it was sectioned with a thickness of 4 μm and conventional H&E staining was performed. The histopathological morphological manifestations of rat aorta were observed under an ordinary light microscope.
qRT-PCR was used for expression of IL-6 and TNF-α, ICAM-1, VCAM-1, MCP-1 and NF- κB p65 messenger RNA (mRNA) in aorta: Gene sequences were sought on PubMed; primer sequences (Table 1) were designed with the aid of Premier 5.0 and the primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. Total RNA of rat aortic vessels was extracted according to the instructions of RNAiso plus (TaKaRa, Japan) kit and the OD260/OD280 was measured by Ultraviolet (UV) spectrophotometer to calculate the RNA purity and concentration. RNA was reverse transcribed into complementary Deoxyribonucleic Acid (cDNA) and subjected to PCR amplification following the RT kit (TaKaRa, Japan) instructions. Reaction conditions were 95°, 3 min; 95°, 10 s; 60°, 30 s; total 40 cycles, dissolution curve from 55°, increase by 0.5° every 30 s until 95°, one cycle. All reaction information data were collected by an ABI StepOnePlusTM PCR machine and the Cycle Threshold (CT) values were measured and calculated by computer software and the transcript levels were calculated by the formula 2–ΔΔCt and by using Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) as the housekeeping gene, the CT value of each sample was corrected.
Aortic IL-6, TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB p65 immunohistochemical detection: The aortic tissue was prepared from 1.3 rats. After fixation with 4 % paraformaldehyde for 48 h, paraffin sections with a thickness of about 4 μm were prepared routinely. The sections were placed in a 60° oven and baked for 2 h; sections were deparaffinized to water and washed with distilled water for 2 min; high pressure heat repair by adding a suitable amount of tap water within a high-pressure pot, pour the repair solution into the beaker, place the beaker into a high-pressure pot and boil (the high-pressure pot does not need to be covered tightly), place the sections into the beaker containing the repair solution, cover the high-pressure pot, heat to the set pressure after gas injection for 1 min, cooling by tap water; peroxidase was blocked by 3 % Hydrogen peroxide (H2O2) solution, 10 min; washing with Phosphate Buffered Saline (PBS) for 5 min×3 times; primary antibodies were added drop wise and incubated at 37° for 60 min; washing with PBS for 5 min×3 times; secondary antibodies were added drop wise and incubated at 37° for 60 min; washing with PBS for 5 min×3 times; Diaminobenzidine (DAB) color development for 1-2 min; counterstaining, transparent posterior mounting slides; pictures were taken microscopically at the sites to be analyzed for each section and those with brownish yellow granules in the cells were observed as positive cell expression and the aortic tissue was analyzed for IL-6 and TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB p65 expression by image analysis management system (Carl Zeiss Imaging Systems).
Statistical methods:
Statistical Package for Social Sciences (SPSS) 26.0 statistical software was used for data processing and statistical analysis and the measurement data were expressed as x±s and comparisons of means among multiple groups were performed by one-way Analysis of Variance (ANOVA) and comparisons between two groups were performed by t-test, with p<0.05 considered significant.
Results and Discussion
After H&E staining, no obvious pathological changes were observed in the aortas of the control group, as the aortic intima was smooth, the endothelial cells were flat and the smooth muscle cells of the tunica media were arranged orderly and approximately interphase with the elastic plates. In the model group, the intimal defect was incomplete, smooth muscle cells were mildly proliferative and the arrangement was disordered and the elastic lamina was thick and thin, with fracture phenomenon and lots of foam cell formation. The above lesions were present in the GK group, but the extent was significantly milder than that in the model group and the degree of aortic pathology in the PNS group was milder than that in the model group.
It can be seen in Table 2, blood glucose in all three groups of GK rats before and after intervention was higher than that in the control group (p<0.01) and there was no difference among the three groups. Compared with the control group, the levels of serum TC, LDL-C and HDL-C in the GK group, the model group and the PNS group were all significantly increased (p<0.05), while serum TG in the model group was decreased (p<0.05); compared with the GK group, the levels of TC, LDL-C and HDL-C in the model group were significantly increased (p<0.05) and those of TC and LDL-C in the PNS group were significantly increased (p<0.05); compared with the model group, there were no statistical differences in TC, TG, LDL-C and HDL-C in the PNS group (p>0.05).
| Indicators | Control group | GK group | Model group | PNS group |
|---|---|---|---|---|
| TC (mmol/l) | 1.74±0.17 | 2.15±0.17* | 2.91±0.40*# | 2.89±0.68*# |
| TG (mmol/l) | 0.96±0.19 | 0.69±0.22 | 0.62±0.09* | 0.76±0.18 |
| LDL-C (mmol/l) | 0.19±0.02 | 0.24±0.04* | 0.47±0.11*# | 0.48±0.15*# |
| HDL-C (mmol/l) | 0.56±0.09 | 0.78±0.07* | 0.99±0.10*# | 0.87±0.15* |
| Fasting plasma glucose (mmol/l) | 5.18±0.65 | 13.86±1.89** | 14.75±1.80** | 15.85±1.89** |
| After the intervention (mmol/l) | 5.05±0.59 | 14.18±2.12** | 13.66±3.17** | 14.51±1.93** |
Note: *p<0.05 compared with the normal control group; **p<0.01 compared with the normal control group and #p<0.05 compared with the GK group
Table 2: Comparison of Serum Lipids, Fasting Plasma Glucose and Body Weight among Rats in Each Group
It can be seen in Table 3, compared with the control group, serum levels of IL-6, ICAM-1, VCAM-1 and MCP-1 in the GK group, the model group and the PNS group all increased significantly (p<0.05, p<0.01) and serum levels of TNF-α in the model group were significantly higher (p<0.05, p<0.01); compared with GK group, the levels of TNF-α, VCAM-1 and MCP- 1 were significantly increased (p<0.05, p<0.01) in the model group, while the levels of VCAM-1 and MCP-1 were decreased (p<0.05) in the PNS group; compared with the model group, the levels of serum TNF-α, IL- 6, ICAM-1, VCAM-1 and MCP-1 were decreased significantly (p<0.01) in the PNS group.
| Indicators | Control group | GK group | Model group | PNS group |
|---|---|---|---|---|
| TNF-α (ng/l) | 126.58±24.55 | 130.08±24.49 | 169.26±21.93**## | 133.53±23.21◆◆◆◆ |
| IL-6 (ng/l) | 24.58±6.50 | 61.42±15.08** | 70.13±11.41** | 45.96±14.39**◆◆◆◆ |
| ICAM-1 (ng/l) | 3.50±1.16 | 8.37±2.55** | 23.94±5.16**## | 9.96±2.63**◆◆◆◆ |
| VCAM-1 (ng/l) | 13.64±0.93 | 16.65±2.00** | 18.72±0.90**# | 14.04±1.72#◆◆◆◆ |
| MCP-1 (ng/l) | 45.98±7.24 | 74.66±17.95** | 129.18±22.48**## | 54.75±17.02#◆◆◆◆ |
Note: *p<0.05 compared with control group; **p<0.01, #p<0.05, ##p<0.01 compared with GK group and ♦p<0.05 compared with model group
Table 3: Comparison of Serum Inflammatory Factors among Rats in Each Group
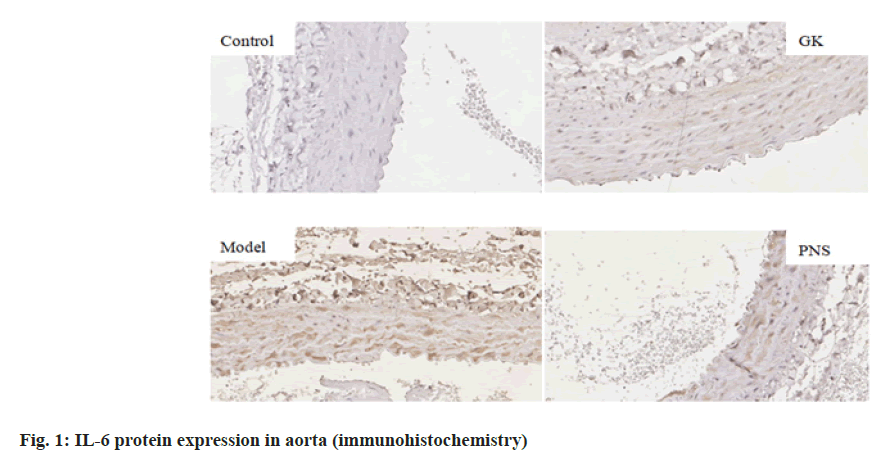
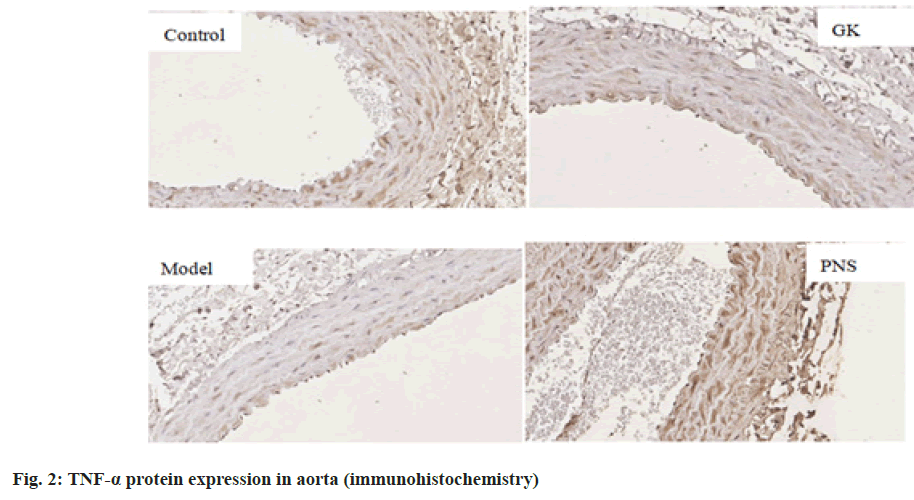
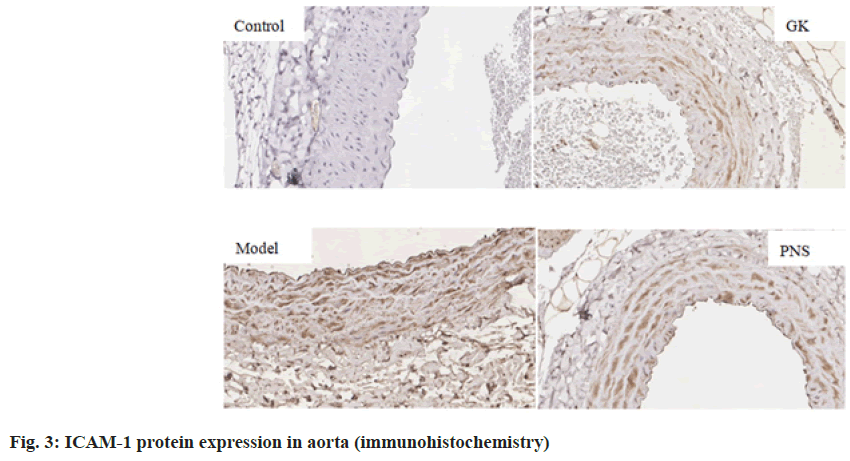
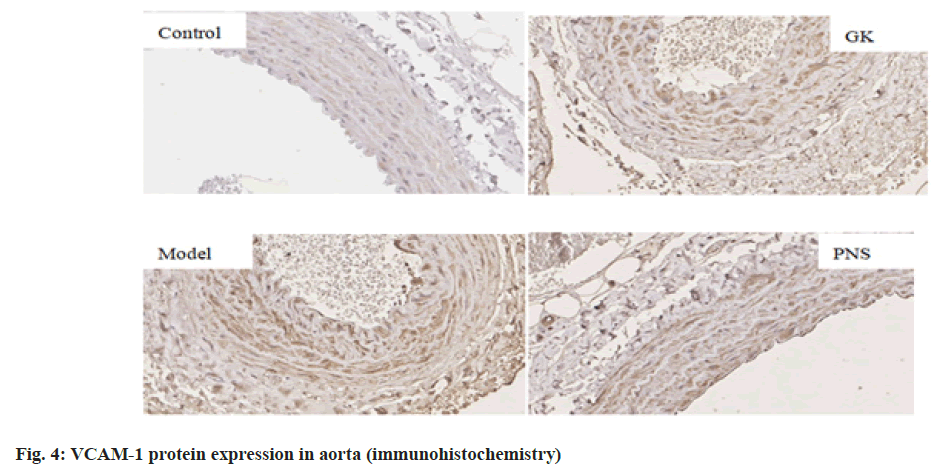
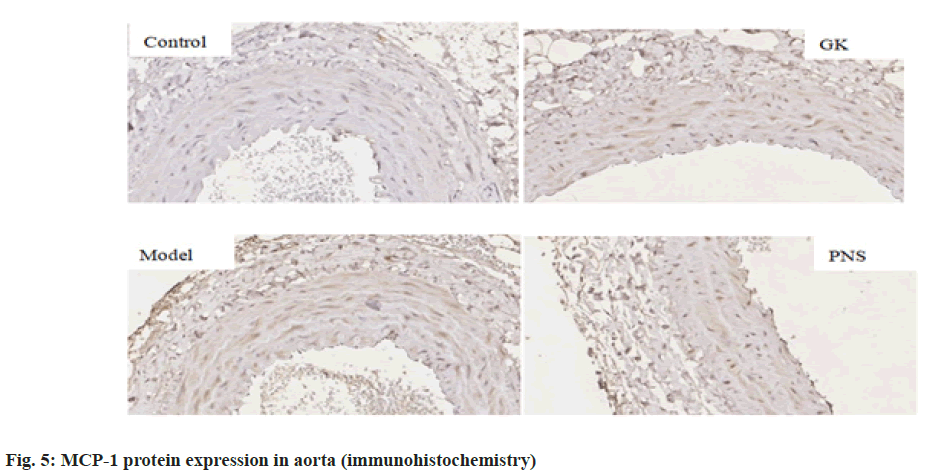
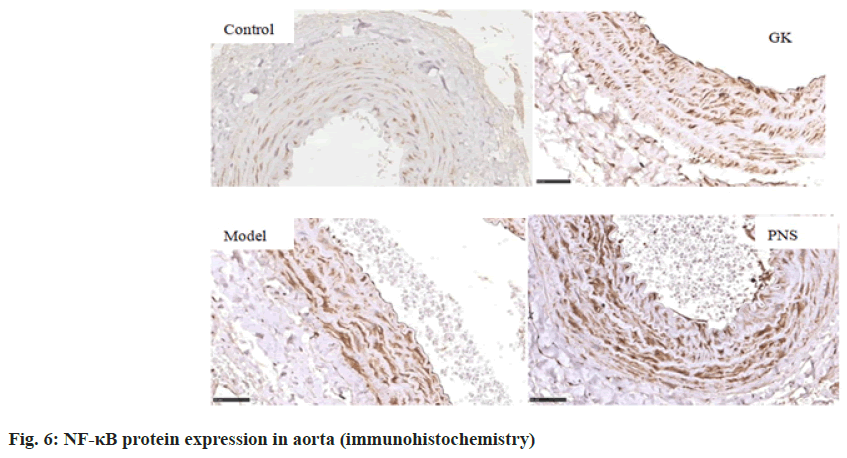
It can be seen from Table 4, compared with the control group, the expressions of IL-6, TNF-α, ICAM-1, VCAM-1 and NF-κB, mRNA in the aorta tissues of rates in the GK group and model group were significantly higher (p<0.05, p<0.01), the expression of MCP-1, mRNA was significantly higher (p<0.01) in the model group and the expressions of IL-6 and TNF-α mRNA were significantly higher in the PNS group (p<0.01); the expressions of IL-6, ICAM-1, VCAM-1, MCP-1 and NF-κB, mRNA in aortic tissue of model rats was significantly higher (p<0.05, p<0.01) in the model group compared with the GK group; in comparison with the model group, the expressions of IL-6 and TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB, mRNA in the PNS group were significantly decreased (p<0.05). It can be seen from Table 5, compared with the control group, IL-6, TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB protein levels in the aortic tissues of rats in GK group and model group were significantly higher (p<0.05, p<0.01); compared with GK group, IL-6, ICAM-1, VCAM-1, MCP-1 and NF-κB protein levels in aortic tissue of rats in model group was significantly higher (p<0.05, p<0.01), TNF-α protein level was significantly higher (p<0.01); in comparison with the model group, IL-6 and TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB protein levels were decreased significantly (p<0.05, p<0.01) in the PNS group. IL-6, TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB immunohistochemical staining, with positive expression mainly located in the cytoplasm of intimal endothelial cells and smooth muscle of the tunica media, visible brown staining granules; intima and media are negative, or scattered brown staining granules may be seen in intima and media. Strong positive, dense or densely tiled brown staining granules may be seen in both intima and media (fig. 1-fig. 6).
| Indicators | Control group | GK group | Model group | PNS group |
|---|---|---|---|---|
| IL-6 | 1.02±0.19 | 1.33±0.25* | 1.80±0.33**## | 1.60±0.38**◆◆ |
| TNF-α | 1.00±0.08 | 1.42±0.34* | 1.80±0.56** | 1.27±0.17**◆◆ |
| ICAM-1 | 1.02±0.20 | 1.40±0.41* | 1.82±0.34**# | 1.35±0.43◆◆ |
| VCAM-1 | 1.02±0.20 | 1.30±0.26* | 1.74±0.41**# | 1.32±0.34◆◆ |
| MCP-1 | 1.01±0.13 | 1.11±0.35 | 1.65±0.27**# | 1.27±0.35◆◆ |
| NF-κB | 1.04±0.29 | 1.44±0.36* | 1.97±0.50*# | 1.25±0.25◆◆ |
Note: *p<0.05 compared with control group; **p<0.01, #p<0.05, ##p<0.01 compared with GK group and ♦p<0.05 compared with model group
Table 4: Comparison of the Expression of IL-6, TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB mRNA in Aortic Tissue of Each Group
| Indicators | Control group | GK group | Model group | PNS group |
|---|---|---|---|---|
| IL-6 | 111±27 | 1517±291** | 8731±2484**## | 1242±389**◆◆◆◆ |
| TNF-α | 5611±706 | 8350±1577** | 21556±6253**## | 8070±2301**◆◆◆◆ |
| ICAM-1 | 41±12 | 3273±868** | 8195±2470**## | 3434±863**◆◆◆◆ |
| VCAM-1 | 3780±1307 | 22865±2732** | 33135±5751**## | 21731±2744**◆◆◆◆ |
| MCP-1 | 1444±279 | 5412±1613** | 13119±3865**## | 5139±1633**◆◆◆◆ |
| NF-κBp65 | 32838±6495 | 47546±5740* | 65780±12390*# | 45078±7931*◆◆ |
Note: *p<0.05 compared with control group; **p<0.01, #p<0.05, ##p<0.01 compared with GK group; ♦p<0.05 and ♦♦p<0.01 compared with model group
Table 5: Comparison of the Expressed IL-6, TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB Protein Levels in Aortic Tissue of Each Group
NF-κB is a multiple dominant transcription factor widely present in cells and belongs to the NF-κB/Rel family, mainly heterodimers composed of p50/p65, has pleiotropic transcriptional regulation and it has been implicated in the transcription of a variety of genes. NF-κB has long been considered an atherogenic factor, which is implicated in multiple pathological processes during atherosclerosis, including foam cell formation, vascular inflammation, vascular smooth muscle cell proliferation, arterial calcification and plaque progression; conversely, inhibition of NF-κB signaling protects against atherosclerosis[10].
It is the most common macrovascular complication of T2DM. In prediabetes, the majority of patients have mild vacuities. The accompanying hyperglycemia, hyperlipidemia and hyperinsulinemia in T2DM make the body more oxidative capable and damage vascular endothelial cells. Vascular endothelial dysfunction is a hallmark of most conditions associated with diabetes mellitus and atherosclerosis. Dysfunction of endothelial cells leads to the abnormal secretion of the cytokine IL-6 as well as TNF-α[11,12]. At the same time, the above factors activate NF-κB. Activated NF-κB enters the nucleus and regulates the expression of adhesion molecules involved in this process such as ICAM- 1, VCAM-1 and MCP-1, which mediate monocyte adhesion, transmigration to the sub endothelium, aggregation and retention and enhance the role of circulating monocytes[13-15]. Inflammation is a common soil for the development of atherosclerosis and T2DM and NF-κB is a core regulator of inflammation[16].
In this study, we found that the serum levels of IL- 6, ICAM-1, VCAM-1 and MCP-1 were significantly increased in GK rats; expression and protein levels of IL-6, TNF-α, ICAM-1, VCAM-1, MCP-1 and NF-κB mRNA were significantly higher in the aortic tissues of GK rats, whereas serum TNF-α, VCAM- 1, MCP-1 levels increased more significantly in the atherosclerotic model group with dyslipidemia and, the expression and protein levels of IL-6, ICAM-1, VCAM- 1, MCP-1, NF-κB, mRNA in aortic tissues increased more significantly with the increased protein levels of TNF-α more pronounced. The results indicate that the inflammatory reaction extends throughout the course of T2DM and its macrovascular complications and the gene regulatory network with NF-κB as the core link constantly contributes to the increase of inflammatory factors and the aggravation of lesions.
In recent years, studies have found[17,18] that the PNS and its components can inhibit the expression of pro-inflammatory factors such as NF-κBp65, IL-6, TNF-α proteins by inhibiting NF-κB signaling pathway of Apolipoprotein E (ApoE) rat, showing that PNS inhibits the progression of atherosclerotic lesions through anti-inflammatory biological properties. In addition, PNS can inhibit inflammatory factors; improve insulin resistance as well as glucose uptake to achieve glucose lowering effects[19].
Based on NF-κB signaling pathway, this study explores the progression of diabetic macroangiopathy by PNS from the perspective of inflammation and the results showed that the levels of serum TNF-α, IL-6, ICAM-1, VCAM-1, MCP-1 of PNS treated rats with diabetes mellitus and atherosclerosis were significantly decreased, while the expression and protein levels of IL-6, TNF-α, ICAM-1, VCAM-1, MCP-1, NF-κB, mRNA of aorta decreased significantly; however, there were no statistical differences in body weight, blood lipid and blood glucose when compared with the untreated model group. The results showed that PNS improved the progression of atherosclerosis in rats with diabetes mellitus not by regulating blood glucose and blood lipid levels, but by reducing the level of inflammatory factors with NF-κB signaling pathway as the core to achieve the improvement of the diabetic atherosclerosis process.
In conclusion, this study found that inflammation extends through the development and progression of T2DM and its macroangiopathy and then PNS attenuates the progression of diabetic macroangiopathy by decreasing the level of inflammatory factors with the NF-κB signaling pathway as the core to achieve the improvement of the diabetic atherosclerosis process. This study provides a new theoretical basis for further elucidating the mechanism of action of PNS in improving atherosclerosis in T2DM at the molecular level.
Funding:
This work was supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2016KYB246 and No. 2018KY601)
Conflict of interests:
The authors declared no conflict of interests.
References
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271-81.
[Crossref] [Google Scholar] [PubMed]
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27(3):813-23.
[Crossref] [Google Scholar] [PubMed]
- Ross R. Atherosclerosis-An inflammatory disease. N Engl J Med 1999;340(2):115-26.
[Crossref] [Google Scholar] [PubMed]
- Lin K, Chen H, Chen X, Qian J, Huang S, Huang W. Efficacy of curcumin on aortic atherosclerosis: A systematic review and meta-analysis in mouse studies and insights into possible mechanisms. Oxid Med Cell Longev 2020;2020:1520747.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y. New evidence for the anti-atherosclerosis effects of traditional Chinese medicine. Chin J Cardiol 2019;47(5):339-40.
- Du X, Qiao Z, Yang S. Effects and mechanisms of Polysaccharides from Schisandra chinensis on inflammatory factors in serum of type 2 diabetic rats. J Jilin Univ 2020;46(1):50-5.
- Zhang J, Li Z, Sun K. Effects of Panax notoginseng saponins on inflammatory factors and aortic lipid metabolism in high fat diet diabetic rats. Zhejiang Med 2018;40:1662-6.
- Zhong H, Li Q, Zhang Y. Construction of aortic sclerosis model in rats with T2DM. Prog Mod Biomed 2008;893:401-3.
- Heng X, Huang S, Cheng X. Glycolipid metabolism and oxidative stress in diabetic atherosclerotic rats pretreated with Dankuo formula. Chin J Integr Tradit West Med 2013;33:244-50.
- Yu XH, Zheng XL, Tang CK. Nuclear factor-κB activation as a pathological mechanism of lipid metabolism and atherosclerosis. Adv Clin Chem 2015;70:1-30.
[Crossref] [Google Scholar] [PubMed]
- Yang J, Ding X, He H, Tan B, Chen L. Action mechanism of the factors of IL-17, IL-6 and IL-10 in acute coronary syndrome. Genomics Appl Biol 2019;38(9):4329-34.
- Jiang Y, Jie L, Liang D. Expression and meaning of Lp-PLA2, TNF-α and CRP in acute ischemic stroke patients. Med J West China 2019;31(11):1709-14.
- Shen A, Peng D, Shi H. Experimental study of Taohongsiwu decoction in protecting against vascular injury in model rats with T2DM. J Anhui Tradit Chin Med Coll 2018;37(3):67-70.
- Qi Z, Liao G, Zhang J. High glucose activates HUVECs IκB-α/NF-κB pathway to induce MCP-1 expression. Chin J Diabetes 2008;16(11):685-7.
- Nitenberg A. Endothelial dysfunction in patients with diabetes: Identification, pathogenesis and treatment. Presse Med 2005;34(21):1654-61.
[Crossref] [Google Scholar] [PubMed]
- La Sala L, Prattichizzo F, Ceriello A. The link between diabetes and atherosclerosis. Eur J Prev Cardiol 2019;26(2):15-24.
[Crossref] [Google Scholar] [PubMed]
- Yang H, Liu Z, Hu X, Liu X, Gui L, Cai Z, et al. Protective effect of Panax notoginseng saponins on apolipoprotein-E-deficient atherosclerosis-prone mice. Curr Pharm Des 2022;28(8):671-7.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Feng R, Zou J, Xia F, Wan JB. 20 (S)-protopanaxadiol saponins mainly contribute to the anti-atherogenic effects of Panax notoginseng in ApoE deficient mice. Molecules 2019;24(20):3723.
[Crossref] [Google Scholar] [PubMed]
- Uzayisenga R, Ayeka PA, Wang Y. Anti‐diabetic potential of Panax notoginseng saponins (PNS): A review. Phytother Res 2014;28(4):510-6.
[Crossref] [Google Scholar] [PubMed]