- *Corresponding Author:
- N. Marasini
Department of Pharmacy, Maharajgunj Medical Campus, Institute of Medicine, Tribhuvan University, Kathmandu, Nepal
E-mail: marasininirmal@iom.edu.np
| Date of Submission | 06 June 2016 |
| Date of Revision | 12 September 2016 |
| Date of Acceptance | 25 September 2016 |
| Indian J Pharm Sci 2016;78(5):624-630 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Diazepam ingestion along with ethanol is encountered commonly in drug overdose cases. In the present study, the effect of pH and ethanol on the adsorption of diazepam in the simulated gastric fluid and the simulated intestinal fluid onto activated charcoal was determined in vitro. The adsorption behaviors of diazepam in both simulated gastric and intestinal pH onto activated charcoal were studied. In the adsorption study with ethanol, some of the gastric or intestinal fluid was replaced with an equivalent volume of 10 and 25% ethanol, respectively. The unabsorbed diazepam in simulated gastric fluid and simulated intestinal fluid (with and without ethanol) was determined by UV spectrophotometer at wavelengths 289 and 256 nm, respectively. The maximum adsorption capacities (at 95% confidence limits) of activated charcoal were 25 (18.42; 31.5) mg and 200 (175.158; 224.84) mg diazepam/g activated charcoal at pH values 1.2 and 6.8, respectively. In case of 10 and 25% ethanol, adsorption in the simulated gastric fluid were 19.20 (21.23; 18.00) and 0.268 (0.286; 0.26), respectively. Surprisingly, in the simulated intestinal fluid, the adsorption patterns were not affected due to presence of 10% ethanol while it was reduced to 76.92 (78.179; 74.84) mg at 25% ethanol concentration. Under the simulated gastrointestinal environmental condition, the activated charcoal adsorbed a sufficient amount of diazepam (200 mg/g-25 mg/g) with maximum at intestinal pH. Our results show that standard dose of 50 g of activated charcoal as provided in general poisoning cases is sufficient to prevent diazepam intoxication with or without ethanol.
Keywords
Activated charcoal, drug overdose, diazepam, flumazenil, gastrointestinal decontamination
In developing countries like Nepal, suicidal poisoning is considered as a major problem [1]. The most commonly used chemicals for self-harm or poisoning include pesticides (organophosphates), rodenticides (zinc phosphide), drugs (benzodiazepines, paracetamol, phenytoin, fluoxetine, amitriptyline) and alcohol [1,2]. Among the drugs, benzodiazepines were one of the most commonly used [1]. The percentage of intentional poisoning was found to be higher than accidental poisoning [2,3]. A comparative study carried out in central, zonal and district hospitals of Nepal showed that sedative and hypnotics are the most common types of drugs (43%) used for intentional attempt [3]. Another study conducted by Lohani between 1997 and 2007 showed that the hypnosedative drugs ranked first on list followed by analgesic/antipyretics among different pharmaceutical agents responsible for drug overdose or poisoning [4]. Many studies have shown benzodiazepines as one of the major drugs responsible for self-poisoning throughout the world [1-8]. Among the benzodiazepines, diazepam is one of the most widely used drugs for different clinical purposes such as anxiety, acute alcohol withdrawal, status epilepticus and other convulsive states. Because of wider therapeutic uses, diazepam is also the most common benzodiazepine in poisoning [8].
In diazepam intoxication, difficulties occur when other central nervous system (CNS) depressants such as tricyclic antidepressants, opioids or alcohol are taken in addition, which is seen especially among poly drug abusers [9]. Thus, the antagonist of diazepam i.e. flumazenil may not be appropriate in intentional or accidental diazepam overdose as flumazenil is not as effective when multiple drug overdoses are present [9]. Thereby, the first priority goes to activated charcoal (AC) for gastrointestinal decontamination even if the specific antidote is available [9]. Besides, flumazenil has been reported to show complications like the development of seizures in cases of multiple drug overdose of benzodiazepine along with cyclic antidepressants, cocaine, lithium, methyl xanthine, isoniazid, propoxyphene, monoamine oxidase inhibiter, bupropion hydrogen chloride and cycloserine [9-11]. In addition, it can precipitate ventricular arrhythmia in those who have co-ingested tricyclic antidepressants [9]. It may show withdrawal symptoms and is contraindicated in the patients with elevated intracranial pressure [12]. AC may adsorb a large amount of diazepam as well as other toxic agents co-ingested if given within 30 min to 1 h [9,13]. Thus the best measure for diazepam overdose is administration of AC if it can be given within a short time after intoxication.
Diazepam overdose frequently involves co-ingestion of ethanol [14]. In spite of high prevalence of diazepam overdose along with ethanol, the effect of the pH of the gastrointestinal tract and ethanol on the adsorption of diazepam (oral formulation) onto AC has not yet been studied. In vitro drug adsorption method that simulates in vivo conditions is usually carried out to determine the effect of pH and ethanol on the adsorption of drugs to AC [15,16]. Thus, an in vitro study was undertaken to determine the effects of the gastrointestinal pH and different concentrations of ethanol (10% and 25%) on the adsorption of diazepam onto AC.
Materials and Methods
Reference standard of diazepam was obtained from National Medicine Laboratory, Government of Nepal. AC powder (D-Tox; Asian Pharmaceuticals Pvt. Ltd, Nepal) and diazepam (Noten 5 mg; Asian Pharmaceuticals Pvt. Ltd, Nepal) tablets were purchased from a pharmacy. All the reagents such as potassium dihydrogen phosphate (KH2PO4), sodium hydroxide (NaOH), hydrochloric acid (HCl), ethanol (100%) used were of analytical grade. The simulated gastric and intestinal fluid environments (pH 1.2 and pH 6.8) without enzymes were prepared as per pharmacopoeial standards (United States Pharmacopeia). The simulated gastric fluid (SGF), pH 1.2 was prepared by dissolving sodium chloride (NaCl) 2 g, concentrated HCl 7 ml in 1000 ml distilled water and the simulated intestinal fluid (SIF), pH 6.8 was prepared by dissolving 68.05 g KH2PO4 and 8.96 g NaOHin 10 l water.
Adsorption study
Twenty diazepam tablets were crushed in a mortar using a pestle. The weight of powder equivalent to 2 mg diazepam was taken and dissolved in 90 ml in both SGF and SIF. Different amounts of AC ranging from 2 mg to 50 mg were weighed and added to the drug suspension to get charcoal-to-drug ratio varying from 1:1 up to 25:1. Incubation of the samples was performed for 15 min in water bath at 37° with constant stirring by magnetic stirrer. After completion of adsorption time (15 min), 10 ml 100% ethanol was added to each of ACdiazepam suspensions and was shaken well to dissolve the remaining unadsorbed diazepam. The liquid phase from the incubated trials was allowed to remain still for 10 min. After that, the mixtures were filtered through a filter paper by discarding the first 10 ml portion to mitigate the effect due to adsorption of diazepam by the filter paper. Blanks were prepared for the entire samples under analyses under the same conditions without diazepam. UV absorbencies (UVa) were measured for each of the solutions at their respective wavelength (SIF-ethanol mixtures 256 nm; SGF-ethanol mixtures 289 nm). The adsorption experiments were performed in triplicate for each study.
Effect of ethanol on adsorption
The ethanol used was pure (100% w/v). SGF and SIF were sequentially diluted with ethanol to yield (a) simulated fluid-ethanol (90/10 v/v.) i.e. simulated fluid 90% and ethanol 10% and (b) simulated fluid-ethanol (75/25 v/v) i.e. simulated fluid 75% and ethanol 25% respectively. Assuming the stomach contains 500 ml fluid, 10% v/v ethanol corresponds to the ingestion of 100 ml of strong spirit (containing 50% v/v ethanol) [15]. 25% v/v ethanol in the simulated fluid corresponds to 250 ml of strong spirit.
In the experiments with the ethanol, the suspensions of AC-diazepam were prepared in 100 ml of abovementioned simulated fluid-ethanol solution (with ethanol 10 and 25%, respectively) and then incubated for adsorption in the same way as was done for the experiments at pH 1.2 and pH 6.8 without ethanol.
Diazepam analysis
The amount of diazepam unadsorbed in each trial of studies was calculated from the calibration curves obtained by simultaneous analysis of seven calibrators (range 20 μg/ml-0.0625 μg/ml). Twenty μg/ml diazepam stock solutions were prepared by dissolving 20 mg in 1000 ml each of the gastric fluid and the intestinal fluid containing 10% ethanol. Then diazepam standard solutions of 15, 10, 5, 2.5, 1.125 and 0.0625 μg/ ml of diazepam stock solutions were prepared for both the gastric fluid and the intestinal fluid, and placed into individual vial cap. The UV detection wavelength at 256 nm for the intestinal fluid-stock solutions and 289 nm for the gastric fluidstock solutions were adjusted. UV absorbencies were plotted against the concentrations of diazepam and calibration curves for the simulated gastric fluid and the simulated intestinal fluid solution with ethanol were constructed.
Equilibrium isotherms
Equilibrium isotherm equations are used to describe adsorption experiments. The two most common isotherms used to describe solid-liquid sorption are Langmuir and Freundlich isotherms [17,18]. Langmuir isotherm can be expressed as qe=(qmax×bCe)/(1+bCe). This equation can be further simplified as:
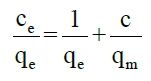
Where, qe is milligram of adsorbate accumulated per gram of the adsorbent at equilibrium (mg/g), Ce is the equilibrium concentration of adsorbate (mg/l), qm the maximum adsorption capacity (MAC) of AC and b is the Langmuir adsorption equilibrium constant (l/mg).
Freundlich isotherm can be expressed as qe=KCe1/n, in logarithmic form:
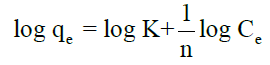
Where, ‘K’ and ‘n’ are the Freundlich constants which are considered to be the relative indicators of adsorption capacity and adsorption intensity.
Statistical analysis was done by using analysis of variance (ANOVA) paired Student’s t-test using IBM SPSS Statistics for Windows, Version 20.0. The statistical analysis of all data was expressed as mean value of three independent experiments at 95% confidence interval. Multiple group comparisons were analyzed by using one-way ANOVA followed by posthoc Tukey test. Paired Student’s t-test was used to evaluate the adsorption capacity of AC in SGF and SIF. The value set for statistical significance was P<0.05 measured using two-sided test at 95% confidence limit. Multiple group comparison was adopted to compare adsorption of diazepam onto AC in three different environments: adsorption in SGF/SIF, adsorption in SGF/SIF with 10% ethanol followed by adsorption in SGF/SIF with 25% ethanol.
Results and Discussion
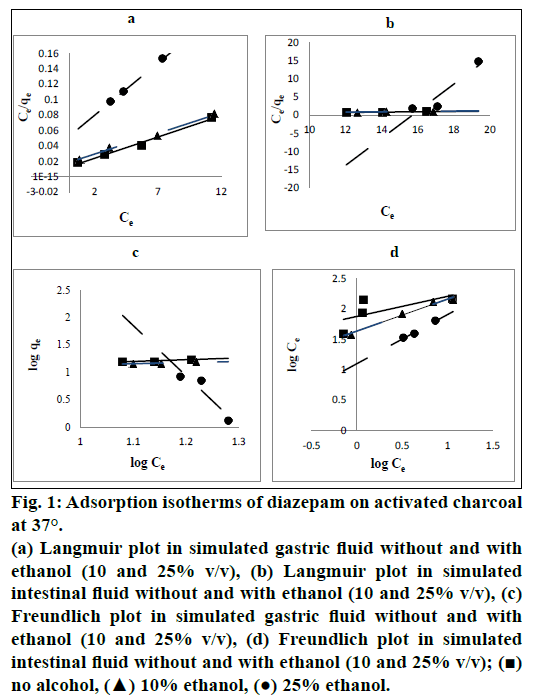
Langmuir plots showed excellent coefficient of determination (R2) compared to Freundlich isotherm for each trial of studies at both pH values (pH 1.2 of SGF and pH 6.8 of SIF) and mixtures with ethanol (10% and 25% v/v) indicating excellent fitting of the model to the experimental data. Fig. 1 shows Langmuir and Freundlich plots at both pH values of 1.2 and 6.8 and mixtures with ethanol. Table 1 shows the values of R2 for each trial of isotherm studies.
Fig. 1: Adsorption isotherms of diazepam on activated charcoal at 37°.
(a) Langmuir plot in simulated gastric fluid without and with ethanol (10 and 25% v/v), (b) Langmuir plot in simulated intestinal fluid without and with ethanol (10 and 25% v/v), (c) Freundlich plot in simulated gastric fluid without and with ethanol (10 and 25% v/v), (d) Freundlich plot in simulated intestinal fluid without and with ethanol (10 and 25% v/v); (?) no alcohol, (?) 10% ethanol, (?) 25% ethanol.
| Ethanol concentration (%) | Coefficient of determination (R2) value for different isotherms | |||
|---|---|---|---|---|
| SGI | SIF | |||
| Langmuir | Freundlich | Langmuir | Freundlich | |
| No. ethanol (0%) | 0.934 | 0.789 | 0.986 | 0.422 |
| Ethanol 10% v/v | 0.944 | 0.496 | 0.993 | 0.972 |
| Ethanol 25% v/v | 0.890 | 0.874 | 0.999 | 0.987 |
Table 1: R2 For Both Langmuir and Freundlich Isotherms
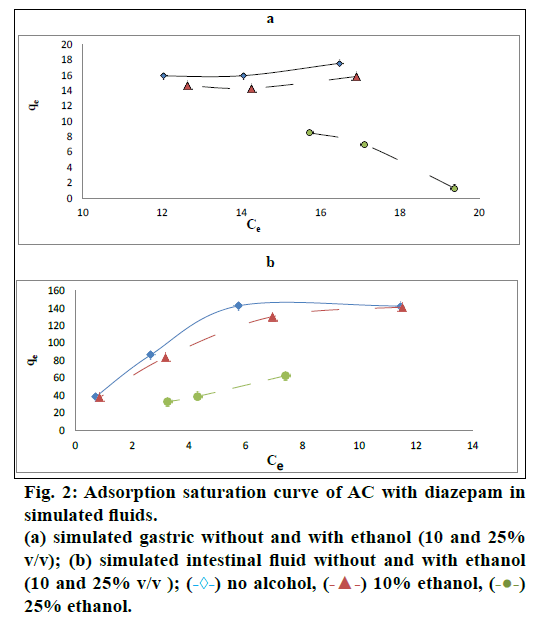
The maximum adsorption capacity (MAC) of AC in the SGF and the SIF, with pH values 1.2 and 6.8 respectively, is shown in Table 2. The graphical representation of the amount of diazepam adsorbed per gram of AC in relation to concentration of diazepam in (a) SGF and (b) SIF respectively without and with ethanol (10% v/v and 25% v/v) is expressed in fig. 2. The MAC of AC for diazepam in the SGF and the SIF was different (25 mg/g and 200 mg/g in the SGF and SIF, respectively) as shown in Table 3. The significant difference in adsorption capacity of AC in two different pH; pH 1.2 and pH 6.8 was assessed using paired Student’s t-test (Table 3). It can be concluded that diazepam was effectively adsorbed onto AC at both pH values 1.2 and 6.8 with significantly higher adsorption at pH at 6.8.
| Simulated fluid ethanol concentration qm (mg diazepam adsorbed/g AC) | ||
|---|---|---|
| Simulated gastric fluid (pH 1.2) |
No ethanol | 25.000 (18.420; 31.5) |
| 10% ethanol 25% ethanol |
19.200 (21.233; 18.0) 0.268 (0.286; 0.26) |
|
| No ethanol | 200.00 (175.158; 224.84) | |
| Simulated intestinal fluid (pH 6.8) |
10% ethanol 25% ethanol |
200.00 (219.40; 191.00) 76.92 (78.179; 74.84) |
MAC and qm in SGF, SIF without and with ethanol (10% and 25%) respectively. All data are mean of three trials, (95% confidence limit)
Table 2: Maximum Adsorption Capacity of AC in Sgf and Sif with and Without Ethanol
| pH | Mean difference | P value |
|---|---|---|
| 6.8 and 1.2 | 175.00* (165.53602; 184.46398) | 0.000 |
*The mean difference is significant at the 0.05 level
Table 3: Difference in Maximum Adsorption Capacity of AC Due to Ph
The phenomenon of adsorption in presence of ethanol (10% and 25%) in SGF and SIF is presented in the Table 2. In the experiments performed in the presence of ethanol, a significant difference (P<0.05) in MAC of AC was seen with both 10 and 25% ethanol in the SGF as shown in Table 4. In the case of the SIF, the significant effect of ethanol on the MAC was observed only at the concentration level of 25% ethanol as shown in Table 5. The significant differences in the adsorption of diazepam onto AC in SGF and SIF, with or without ethanol of different concentrations were assessed using one-way ANOVA followed by post-hoc Tukey test.
| Mean difference | P value | |
|---|---|---|
| SGF SGF- ethanol (90/10, v/v) | 5.80*(2.60; 8.99) | 0.004 |
| SGF SGF- ethanol (75/25, v/v) | 24.73*(21.53; 27.92) | 0.000 |
| SGF- ethanol( 90/10, v/v) SGF- ethanol (75/25, v/v) | 18.93*(15.73; 22.12) | 0 .000 |
*The mean difference is significant at P=0.05* level, when an equivalent amount of simulated gastric fluid (SGF) was replaced with ethanol such that 10% v/v and 20% v/v total ethanol was resulted respectively. Data are shown as mean difference of three trials (95% confidence limits)
Table 4: Difference in Maximum Adsorption Capacity of AC in Simulated Gastric Environment Under Influence of Ethanol
| Mean difference | P value | |
|---|---|---|
| SIF SIF - ethanol (90/10, v/v) | 0.00 (-14.68; 14.68) | 1.0 |
| SIF SIF - ethanol (75/25, v/v) | 124.46* (109.77; 139.15) | 0.0 |
| SIF-ethanol (90/10, v/v) SIF- ethanol (75/25, v/v) | 124.46* (109.77; 139.15) | 0.0 |
*The mean difference is significant at P=0.05* level when an equivalent amount of simulated intestinal fluid (SIF) is replaced with ethanol such that 10% v/v and 25% v/v total ethanol resulted respectively. Data are shown as mean difference of three trials (95% confidence limits)
Table 5: Difference in Maximum Adsorption Capacity Of AC in Simulated Intestinal Environment Under Influence of Ethanol
The adsorption studies were performed at two pH values to simulate the environments in the gastrointestinal tract (stomach and intestine). To achieve an adsorption saturation of AC with the studied drug, the amount of AC in each of the tests was varied so that the ratios of the mass of AC to diazepam varied from 1:1 to 25:1. Diazepam was added in powder form after crushing the tablets in order to simulate in vivo intoxication conditions when the patient takes the tablets available in the market.
Equilibrium isotherms were used to describe the experimental adsorption data. The data were plotted among two most commonly used adsorption isotherms, Langmuir isotherm and Freundlich isotherm [17,18] and their R2 values were compared to get the best adsorption isotherm equation to produce highly accurate results. In our study, R2 values for each trial of studies are greater for Langmuir isotherm than those of Freundlich isotherm. In recognition of the value of R2, Langmuir isotherm was used. Also, R2 values for both Langmuir and Freundlich isotherm models increase with an increase in ethanol concentration in the SIF. This shows that the isotherm plot gets fitted better with an increase in ethanol concentration in the SIF composition. This can be explained on the basis that an excellent logarithmic relationship was observed between the adsorption affinity and solubility of diazepam in the SIF-ethanol mixtures [19]. Earlier investigators have explained this type of relationship by showing the linear relationship between the differential free energy change of displacement and the differential free energy change of solution [19]. The R2 value decreased with increase in concentration of ethanol from 10 to 25% for Langmuir in SGF. It might be due to some sorts of physicochemical incompatibilities among SGF-ethanol-AC and high alteration in the polarity of SGF on addition of ethanol or some type of sorption (adsorption and/or desorption) phenomenon which was also revealed in our research at the SGF-ethanol mixture, 25% (75/25 v/v).
Previous research article has demonstrated that the adsorption of drugs primarily occurs in their un-ionized form [20]. Diazepam is a weakly basic or neutral drug [19]. As the unionized fraction of diazepam is higher at pH 6.8 than at pH 1.2, the MAC is expected to be at pH 6.8. Our study showed high adsorption of diazepam onto AC at pH 6.8 and is significantly different (P<0.05) compared to pH 1.2. The MAC of AC was found to range from 200 to 25 mg/g in SIF and SGF, respectively. Our result also complies with earlier studies regarding the change in MAC with change in pH [21,22]. A great difference in adsorption capacity of AC for diazepam has been observed at the two pH values in this study. Such type of result was also found by Dawling et al. for in vitro study on adsorption of aspirin onto two form of AC [23].
AC can adsorb up to 10 g diazepam (2000 tablets of diazepam 5 mg) at intestinal environment and up to 1.25 g diazepam (250 tablets of diazepam 5 mg) at gastric environment at its standard dose of 50 g. This shows that a standard dose of 50 g AC is highly sufficient to prevent the severity of orally ingested diazepam intoxicated with ethanol and there is no need to depend on flumazenil if AC can be provided within 0.5-1 h of overdose.
Adsorption of diazepam onto AC has been studied by earlier investigators in acidic pH 1.2 (gastric pH) [24], but not in pH similar to the intestine. They have shown higher adsorption of diazepam onto AC than ours at pH 1.2. This might be because their study was performed with pure diazepam in contrast to this study where it was performed with diazepam tablets and excipients might have affected the adsorption capacity. Also, the authors might have used AC with a higher surface area or the physicochemical properties of AC to adsorb drug might differ than ours.
Another physicochemical change on addition of ethanol in the simulated gastric and intestinal fluids upon the adsorption pattern of diazepam onto AC was also studied. Our findings demonstrate that the simulated solutions containing ethanol lowers the adsorption of diazepam at concentrations of 10 and 25% compared to adsorption without ethanol in both the simulated gastric fluid and the simulated intestinal fluid which are statistically significant (P<0.05) except for simulated intestinal fluid-ethanol, 10% v/v (P>0.05). The MAC gets lowered significantly (P<0.05) with an increase in ethanol concentration from 10 to 25% in all cases of our study for both simulated fluids. The observed in vitro effect of ethanol is probably due to the environmental change in the trials. Water and ethanol are polar molecules, although ethanol is less so. The addition of ethanol changes the polarity of the test solution which is a purely aqueous solution. The water-ethanol solution is less polar than pure water [15]. This study is supported by Cooney statement that the decreased polarity of the solution containing ethanol compared to a pure aqueous solution makes drugs less adsorbable to activated charcoal [20].
In conclusion, the extrapolation of this in vitro study shows that if AC is given at a standard treatment dose of 50 g within a short time after diazepam intoxication, it has the capacity to adsorb sufficient amounts of diazepam. AC must be administered as quickly as one can because of the fact that diazepam is rapidly absorbed from the gastrointestinal tract. In addition, it can also enhance the elimination of most of the coadministered toxins.
The efficacy of AC as an antidote lowers as the concentration of ethanol increases. Since cases of diazepam and ethanol co-ingestion are frequent, knowledge regarding this finding is clinically significant. There is an increasing need for calculating the appropriate amount of AC to be administered during acute poisoning since overloading the patient with AC is not free from side effects. Though diazepam has a specific antidote, flumazenil, it is not used as very severe effects may arise if flumazenil is used in cases of intoxication of diazepam along with other drugs. So, AC can be considered as a boon for diazepam overdose cases.
Financial support and sponsorship
The research was supported by the Department of Pharmacy, Maharajgunj Medical Campus, Tribhuvan University, Nepal..
Conflict of interest
There are no conflict of interest mentioned.
References
- Ghimire RH, Sharma SP, Pandey KR. A retrospective study of changing trends of acute poisoning cases at Tribhuwan University Teaching Hospital, Nepal between 1990-1992 and 2000-2002. J Nepal Health Res Counc 2003;1:38-41.
- Khadka SB. A study of poisoning cases in emergency Kathmandu Medical College Teaching Hospital. Kathmandu Univ Med J 2005;3:388-91.
- Pokhrel D, Pant S, Paudel R, Mishra S, Ojha P. A comparative retrospective study of poisoning cases in central zonal and district hospitals. Kathmandu Univ J Sci Eng Technol 2008;1:40-8.
- Lohani SP. An epidemiological study of poisoning cases reported to the Nepal Drug and Poison Information Center, Kathmandu. 2007.
- Cardozo LJ, Mugerwa RD. The pattern of acute poisoning in Uganda. East Afr Med J 1972;49:983-8.
- Jaiprakash H, Sarala N, Venkatarathnamma PN, Kumar TN. Analysis of different types of poisoning in a tertiary care hospital in rural South India. Food Chem Toxicol 2011;49:248-50.
- Jamil H. Acute poisoning, a review of 1900 cases. J Pak Med Assoc 1990;40:131-3.
- Buckley NA, Dawson AH, Whyte IM, O'Connell DL. Relative toxicity of benzodiazepines in overdose. BMJ 1995;310:219-21.
- Boon AB, Colledge NR, Walker BR, Hunter JA. Poisoning. In: Britton R, Penman ID, Ralston S, editors. Davidson’s Practice and Principle of Medicine. 22nd ed. New York: Churchill Livingstone; 2006. p. 203-27.
- Spivey WH. Flumazenil and seizures: analysis of 43 cases. Clin Ther 1992;14:292-305.
- Veiraiah A, Dyas J, Cooper G, Routledge PA, Thompson JP. Flumazenil use in benzodiazepine overdose in the UK: A retrospective survey of NPIS data. Emerg Med J 2012;29:565-69.
- Rosen P, Barkin RM, Hayden SR, Schaider JJ, Wolfe R. Benzodiazepine poisoning. In: The 5 minutes emergency consult. New York: Wolters Kluwer; 1999. p. 128-9.
- Lapatto-Reiniluoto O, Kivisto KT, Neuvonen PJ. Effect of activated charcoal alone or given after gastric lavage in reducing the absorption of diazepam, ibuprofen and citalopram. Br J Clin Pharmacol 1999;48:148-53.
- Bailey DN. Comprehensive toxicology screening: the frequency of finding other drugs in addition to ethanol. J Toxicol Clin Toxicol 1984;22:463-71.
- Hoegberg LC, Angelo HR, Christophersen AB, Christensen HR. Effect of ethanol and pH on the adsorption of acetaminophen (paracetamol) to high surface activated charcoal, in vitro studies. J Toxicol Clin Toxicol 2002;40:59-67.
- Bailey DN, Briggs JR. The effect of ethanol and pH on the adsorption of drugs from simulated gastric fluid onto activated charcoal. Ther Drug Monit 2003;25:310-13.
- Gupta VK, Gupta B, Rastogi A, Agarwal S, Nayak A. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-Acid Blue 113. J Hazard Mater 2011;186:891-901.
- Tan IA, Ahmad AL, Hameed BH. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 2009;164:473-82.
- Wurster DE, Alkhamis KA, Matheson LE. Prediction of the adsorption of diazepam by activated carbon in aqueous media. J Pharm Sci 2003;92:2008-16.
- Cooney DO. Activated charcoal in medical applications. New York: Marcel Dekker; 1995.
- Neuvonen PJ, Olkkola KT, Alanen T. Effect of ethanol and pH on the adsorption of drugs to activated charcoal: studies in vitro and in man. Acta Pharmacol Toxicol (Copenh) 1984;54:1-7.
- Tsitoura A, Atta-Politou J, Koupparis MA. In vitro adsorption study of fluoxetine onto activated charcoal at gastric and intestinal pH using high performance liquid chromatography with fluorescence detector. J Toxicol Clin Toxicol 1997;35:269-76.
- Dawling S, Chand S, Braithwaite RA, Crome P. In vitro and in vivo evaluation of two preparations of activated charcoal as adsorbents of aspirin. Hum Toxicol 1983;2:211-6.
- Sellers EM, Khouw V, Dolman L. Comparative drug adsorption by activated charcoal. J Pharm Sci 1977;66:1640-1.
 ) no alcohol, (
) no alcohol, ( ) 10% ethanol, (
) 10% ethanol, ( ) 25% ethanol.
) 25% ethanol.



