- *Corresponding Author:
- Na Li
Department of Neurology, Fifth North Campus, Qiqihar First Hospital, Qiqihar, Heilongjiang 161005, China
E-mail: lina830923@126.com
| Date of Received | 28 September 2021 |
| Date of Revision | 04 November 2022 |
| Date of Acceptance | 12 June 2023 |
| Indian J Pharm Sci 2023;85(3):815-821 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of evodiamine on Parkinson’s disease and its possible mechanism. 6-hydroxydopamine challenged SK-N-SH cells were adopted to mimic Parkinson’s disease condition in vitro. Levels of proteins and genes were tested using Western blot and quantitative reverse transcription-polymerase chain reaction. Oxidative stress was evaluated by detecting the expression of lactate dehydrogenase and glutathione. Enzyme-linked immunosorbent assay analysis and flow cytometry were applied for the examination of inflammatory reaction and apoptosis rate. Evodiamine treatment could dosedependently inhibit lactate dehydrogenase activity and cell apoptosis, reduced the levels of inflammatory factors, but elevated glutathione content in 6-hydroxydopamine-challenged SK-N-SH cells. Evodiamine exposure led to the decline of microRNA-9-5p content. Inhibition of microRNA-9-5p was able to protect against 6-hydroxydopamine stimulated oxidative stress, elevation of apoptosis and inflammatory factors in cells. Besides that, boosting microRNA-9-5p attenuated the protective functions of evodiamine on 6-hydroxydopamine stimulated SK-N-SH cells. Evodiamine could inhibit 6-hydroxydopamine induced oxidative, inflammatory and apoptotic injury in the Parkinson’ s disease cell model via microRNA-9-5p.
Keywords
Evodiamine, Parkinson's disease, microRNA-9-5p, 6-hydroxydopamine, inflammation
As one of common neurodegenerative diseases in clinic, Parkinson's Disease (PD) clinical manifestations are cognitive dysfunction, sensory impairment, etc. It has been revealed that neuron apoptosis, oxidative stress and neuroinflammation have significant roles in the progression of PD[1-3], thus, probing the PD pathogenesis is urgent for progressing new therapeutic targets for PD.
Currently, studies have shown the important role of Traditional Chinese Medicine (TCM) in disease treatment, as the indispensable part of TCM, Herbal Medicine (HM) possesses antioxidant and antiinflammatory effects, and can be used to reduce nerve cell damage and treat PD[4-6]. The evodiamine is a frequently used HM and has been revealed to have protective effects on cardiovascular and nervous system, as well as anti-inflammatory and antioxidant effects and can be used to treat tumors, Alzheimer’s disease, dysentery, cardiovascular disorders and antimicrobials in clinical practice[7-10]. However, there are relatively few studies on the relationship between evodiamine and PD. Micro Ribonucleic Acids (miRNAs) have been uncovered to participate in many aspects of cell biology[11,12], among which, it was discovered that PD cell model showed higher miR-9-5p level and its down-regulation devastated neuronal apoptosis, oxidative stress and inflammation[13,14]. However, it is not known whether evodiamine can affect PD process via modulating miR-9-5p.
Therefore, this study adopted 6-Hydroxydopamine (6- OHDA) challenged human SK-N-SH neuroblastoma cell lines to establish a cell model of PD, then whether evodiamine could affect the PD process via miR-9-5p was explored.
Materials and Methods
Cell culture and treatment:
SK-N-SH cell lines were purchased from American Type Culture Collection (ATCC) (Manassas, Virginia, United States of America (USA)). The high glucose Dulbecco's Modified Eagle Medium (DMEM) (ATCC) with 1 % penicillin/streptomycin and 10 % Fetal Bovine Serum (FBS) (Solarbio, Shanghai, China) were applied to culture SK-NSH cells. The medium was replaced every day. Then SH-SY5Y cells were differentiated to have the biological characteristics of dopamine neurons using vitamin A and Type Plasminogen Activator (TPA) (Life Technologies)[15]. SK-N-SH cells cultured with completed DMEM medium were deemed to be the control group. In functional experiments, cells cultured with 100 μmol/l 6-OHDA in completed DMEM medium was utilized to induce PD model cells (PD group). In addition, different concentrations evodiamine (1 μmol/l, 5 μmol/l or 10 μmol/l) was applied to incubate with SK-N-SH cells overnight, which were reacted with 6-OHDA (100 μmol/l) overnight for another analysis.
Cell transfection:
MiR-9-5p mimic (miR-9-5p) and the inhibitor (anti-miR-9-5p) with the controls (miR-NC or anti-miR- NC) were provided by Genema (Shanghai, China), the transient transfection was performed adopting Lipofectamine 2000.
Detection of Lactate Dehydrogenase (LDH) and Glutathione (GSH):
The culture of SK-N-SH cells was gathered, then active of LDH were tested using 2,4-Dinitrophenylhydrazine test. Besides, cells in different groups were lysed by repeated freeze-thaw cracking, then the supernatant was gathered and GSH content was tested by the GSH Peroxidase assay kit.
Enzyme-Linked Immunosorbent Assay (ELISA):
ELISA Kits (Abcam, Cambridge, Massachusetts) were adopted to test levels of inflammatory cytokines. SK-N-SH cell supernatant were gathered by centrifugation, after the reaction with antibody cocktail, substrate and Stop solution, the absorption was read by a microplate reader.
Flow cytometry:
After indicated treatment, 500 μl binding buffer suspended cells were added into the cell precipitation. Then Annexin V-Fluorescein Isothiocyanate (FITC) (5 μl) and Propidium Iodide (PI) (5 μl) were added respectively according to the instructions of the apoptosis kit, followed by a flow cytometry analysis.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR):
After the isolation of total RNA via the Trizol reagent (Beyotime, Shanghai, China), reverse transcription was performed for complementary Deoxyribonucleic Acid (cDNA) generation, and qRT-PCR amplification using cDNA as template was conducted. The content of miR-9-5p expression was calculated through the Cycle threshold (Ct) value with U6 as the reference control.
Western blot:
The total protein was extracted by Radioimmunoprecipitation Assay (RIPA) buffer (Beyotime). After being transferred into Polyvinylidene Difluoride (PVDF) membrane[16], the protein was enclosed with 5 % skim milk for 2 h at 37°. The diluent of B-cell lymphoma 2 (Bcl-2) (1:1000) and Bcl-2-Associated X Protein (BAX) (1:1000) primary antibody and the diluent of internal reference Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:2000) were added and incubated at 4° for 12 h. The diluent of the second antibodies was added (1:3000) and incubated at 37° for 1 h. ImageJ software to analyze the gray value of each band after analyzing by Electrochemiluminescence (ECL) system.
Statistical analysis:
The data were manifested as (x͞ ±s). The comparison between groups were executed using Statistical Package for the Social Sciences (SPSS) 21.0 statistical software with the t-test or analysis of variance. p<0.05 indicated statistically significant.
Results and Discussion
Compared with the untreated SK-N-SH cells, 6-OHDA treatment markedly increased LDH active and the content of Interleukin (IL)-1 beta (β), IL-6 and IL-18, but decreased GSH content in SK-N-SH cells (Table 1). Besides that, evodiamine treatment dose-dependently reduced the contents of LDH, IL-1β, IL-6 and IL-18, and increased GSH level in SK-N-SH cells treated with 6-OHDA as shown in Table 1.
| Group | LDH (U/l) | GSH (mg/l) | IL-1β (pg/ml) | IL-6 (pg/ml) | IL-18 (pg/ml) |
|---|---|---|---|---|---|
| Control | 13.36±1.27 | 87.40±3.61 | 2.80±0.57 | 8.68±0.49 | 9.81±0.78 |
| PD | 58.85±4.60a | 33.83±3.18a | 23.83±2.31a | 37.80±3.41a | 49.88±4.00a |
| 1 μmol/L EVO | 42.00±2.07b | 56.03±1.68b | 17.68±0.54b | 28.85±1.30b | 32.92±2.54b |
| 5 μmol/L EVO | 24.13±4.86bc | 65.31±2.97bc | 15.11±0.77bc | 22.62±2.40bc | 21.26±2.38bc |
| 10 μmol/L EVO | 18.12±0.65bcd | 78.89±1.40bcd | 10.03±0.40bcd | 14.55±1.81bcd | 13.79±1.01bcd |
| F | 312.724 | 534.005 | 423.7 | 263.208 | 397.402 |
| p | 0 | 0 | 0 | 0 | 0 |
Table 1: Effects of Evodiamine on the Oxidative Stress and Inflammation of 6-OHDA Challenged SK-N-SH Cells (x͞±s, n=9)
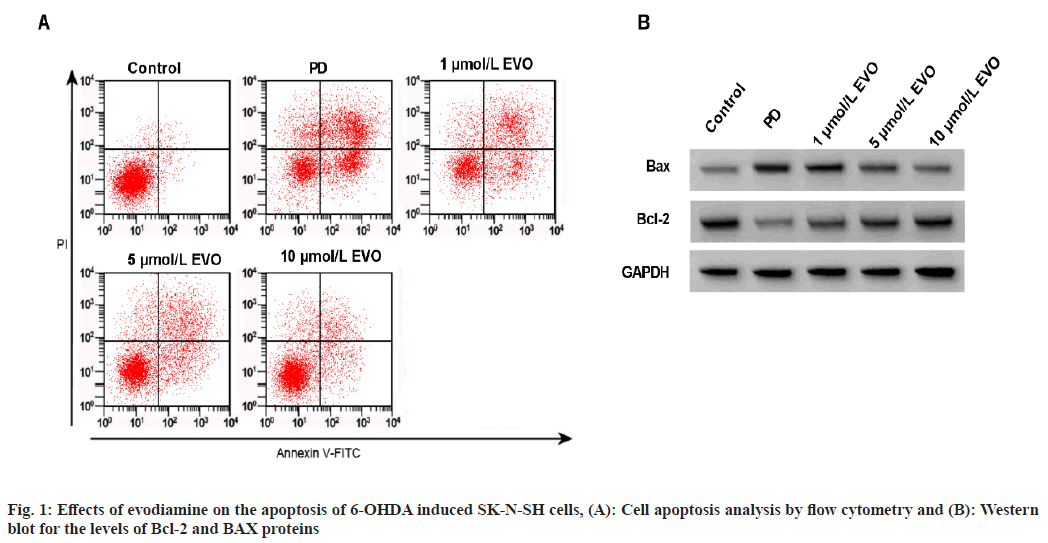
As shown in fig. 1, 6-OHDA treatment induced apoptosis, accompanied with the declined Bcl-2 protein and elevated BAX protein in SK-N-SH cells (fig. 1A, fig. 1B and Table 2). In addition, evodiamine treatment could dose-dependently suppress SK-N-SH cell apoptosis and increased Bcl-2 protein as well as decreased BAX protein under 6-OHDA stimulation as shown in fig. 1A and fig. 1B.
| Group | Apoptosis rate (%) | BAX | Bcl-2 |
|---|---|---|---|
| Control | 6.10±1.14 | 0.24±0.02 | 0.93±0.05 |
| PD | 30.93±3.64a | 0.85±0.03a | 0.27±0.05a |
| 1 μmol/L EVO | 20.11±1.44b | 0.59±0.11b | 0.50±0.04b |
| 5 μmol/L EVO | 14.98±0.71bc | 0.44±0.03bc | 0.67±0.03bc |
| 10 μmol/L EVO | 10.94±0.64bcd | 0.33±0.02bcd | 0.79±0.03bcd |
| F | 232.601 | 176.173 | 353.143 |
| p | 0 | 0 | 0 |
Table 2: Effects of Evodiamine on the Apoptosis of 6-OHDA Induced SK-N-SH Cells (x͞±s, n=9)
Levels of miR-9-5p in the PD group was increased relative to control group. Moreover, miR-9-5p content was decreased after 1 μmol/l, 5 μmol/l and 10 μmol/l evodiamine treatment as shown in Table 3.
| Group | miR-9-5p |
|---|---|
| Control | 1.00±0.05 |
| PD | 4.53±0.22a |
| 1 μmol/l EVO | 3.15±0.10b |
| 5 μmol/l EVO | 2.35±0.15bc |
| 10 μmol/l EVO | 1.25±0.05bcd |
| F | 1096.02 |
| p | 0 |
Table 3: The Effects of Evodiamine on miR-9-5P Content In 6-OHDA Challenged SK-N-SH Cells (x͞±s, n=9)
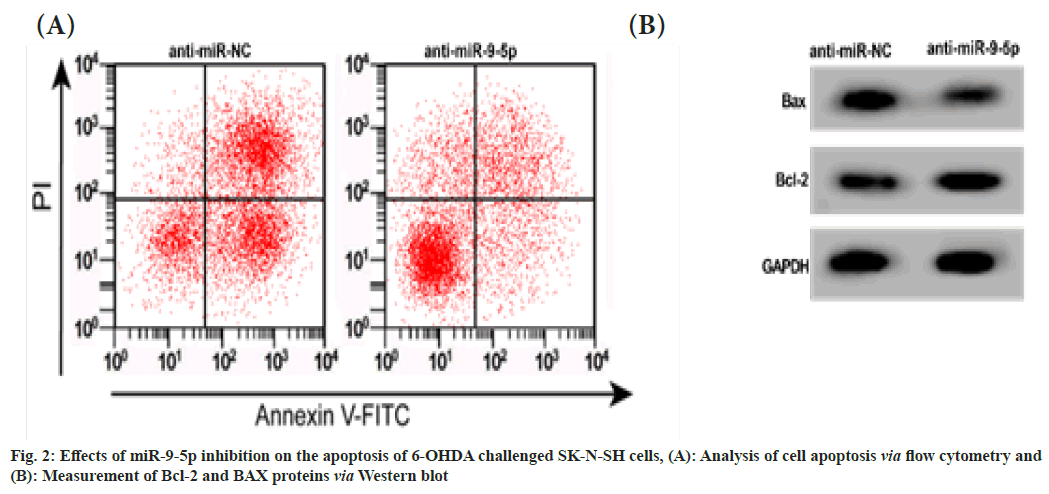
The 6-OHDA induced SK-N-SH cells in antimiR- 9-5p group exhibited reduced LDH activity, levels of IL-1β, IL-6 and IL-18, apoptosis rate and BAX protein, but elevated GSH content and Bcl-2 protein level relative to cells in anti-miRNC group as shown in fig. 2A, fig. 2B and Table 4.
| Group | miR-9-5p | LDH (U/l) | GSH (mg/l) | IL-1β (pg/ml) | IL-6 (pg/ml) | IL-18 (pg/ml) | Apoptosis rate (%) | BAX | Bcl-2 |
|---|---|---|---|---|---|---|---|---|---|
| Anti-miR-NC | 1.00±0.07 | 58.14±5.08 | 33.55±5.68 | 23.90±2.88 | 37.96±1.24 | 49.45±5.19 | 30.37±2.06 | 0.86±0.04 | 0.27±0.03 |
| Anti-miR-9-5p | 0.27±0.04a | 24.31±2.34a | 65.34±2.03a | 15.06±0.93a | 22.57±1.85a | 21.88±2.04a | 14.63±1.01a | 0.44±0.04a | 0.68±0.03a |
| t | 27.164 | 18.146 | 15.811 | 8.763 | 20.731 | 14.832 | 20.582 | 22.274 | 28.991 |
| p | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 4: The Effects of miR-9-5P Inhibition on 6-OHDA Induced SK-N-SH Cells
Compared with the 10 μmol/l+miR-NC group, the expression level of miR-9-5p, the LDH activity and the levels of IL-1β, IL-6 and IL-18 in the 10 μmol/l+miR-9-5p group were increased as shown in Table 5.
| Group | miR-9-5p | LD (HU/l) | GSH (mg/l) | IL-1β (pg/ml) | IL-6 (pg/ml) | IL-18 (pg/ml) |
|---|---|---|---|---|---|---|
| 10 μmol/l EVO+miR-NC | 1.00±0.05 | 18.04±1.37 | 78.44±4.94 | 10.08±0.75 | 14.24±0.76 | 13.75±0.85 |
| 10 μmol/l EVO+miR-9-5p | 2.17±0.10a | 40.02±4.01a | 51.06±4.19a | 17.21±1.38a | 27.64±2.64a | 32.82±3.40a |
| t | 31.394 | 15.561 | 12.681 | 13.619 | 14.633 | 16.324 |
| p | 0 | 0 | 0 | 0 | 0 | 0 |
Table 5: The Overexpression of miR-9-5P Reversed the Effects of Evodiamine on Inflammation and Oxidative Stress in SK-N-SH Cells Induced by 6-OHDA (x͞±s, n=9)
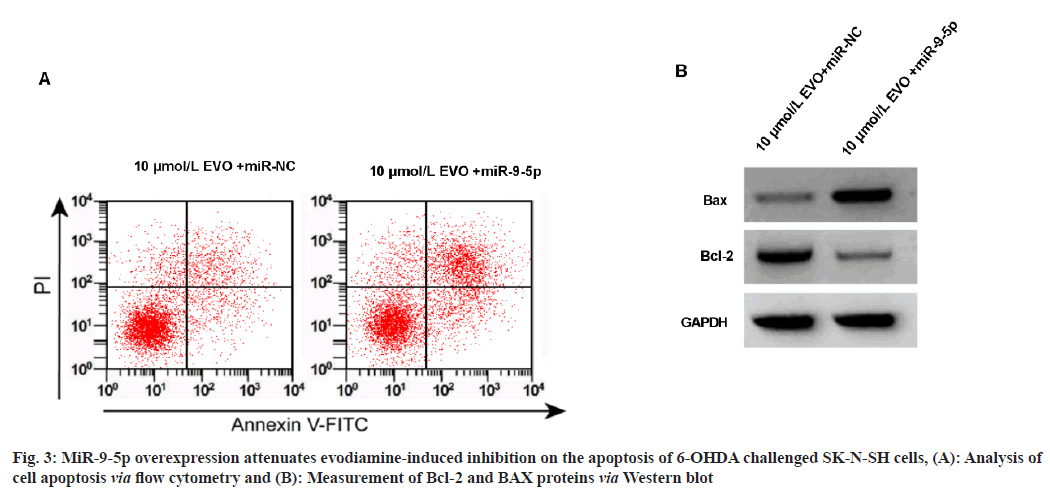
Relative to the 10 μmol/l+miR-NC group, the apoptosis of 6-OHDA induced SK-N-SH cells were reduced in the 10 μmol/l+miR-9-5p group, accompanied with the increased BAX and decreased Bcl-2 protein as shown in fig. 3A, fig. 3B and Table 6.
| Group | Apoptosis rate (%) | BAX | Bcl-2 |
|---|---|---|---|
| 10 μmol/l EVO+miR-NC | 10.53±0.92 | 0.32±0.04 | 0.80±0.04 |
| 10 μmol/l EVO+miR-9-5p | 21.96±1.73a | 0.60±0.05a | 0.50±0.03a |
| t | 17.5 | 13.119 | 18 |
| p | 0 | 0 | 0 |
Table 6: miR-9-5P Boost Abated the Effects of Evodiamine on 6-OHDA Evoked Apoptosis in SK-N-SH Cells
The inflammatory reaction of neurons can aggravate the oxidative stress of cells, thereby inducing the apoptosis of neurons and promoting neuron injury. Research shows that HM can treat PD via multiple targets and multiple ways[17]. Additionally, research have shown that miRNA is abnormally expressed in the cell model of PD[18,19]. Whereas, whether Chinese HM can be used in combination with miRNA for PD therapy.
Previous study manifested that evodiamine can slow down the development of atherosclerosis by inhibiting infectious and inflammatory reaction[20]. Evodiamine alleviated renal ischemia-reperfusion injury in rats[21]. Evodiamine reduced the arthritis in rats by inhibiting inflammatory reaction[22]. However, the functions of evodiamine on PD remain unknown. Here, this work manifested that 6-OHDA exposure induce the activation of LDH but declined the content of GSH in neurons, suggesting that the cell model of PD was successfully induced. Functionally, we found that evodiamine treatment could dose-dependently arrest LDH activity and elevated GSH content in 6-OHDA-induced neurons, implying the potential inhibitory function of evodiamine on oxidative stress in neurons during PD. In addition, the contents of inflammatory cytokines were declined by 6-OHDA in neurons, which were dose-dependently rescued by evodiamine treatment, suggesting that evodiamine was able to inhibit the inflammatory reaction in neurons. Moreover, we also discovered that 6-OHDA stimulation led to the increase of cell apoptosis, accompanied with the increased BAX and decreased Bcl- 2 protein; moreover, evodiamine could protect neurons from 6-OHDA stimulated apoptosis. In all, evodiamine could inhibit 6-OHDA induced damage in the PD cell model.
MiR-9-5p is a functional miRNA. MiR-9-5p in podocytes induced by high glucose was increased and inhibition of its expression could promote podocyte proliferation and inhibit cell apoptosis induced by high glucose[23]. MiR- 9-5p exhibited high level in hypoxic induced cardiomyocytes and could expedite oxidative and apoptotic injury of cardiomyocytes[24]. Inhibiting miR-9-5p suppressed neuron apoptosis induced glucose deprivation/reoxygenation[25]. Herein, we discovered an up-regulated miR-9-5p in 6-OHDA stimulated neurons, moreover, inhibition of miR-9-5p abated stimulated apoptotic, oxidative and inflammatory injury in neurons treated with 6-OHDA. Subsequently, it was found that evodiamine treatment resulted in miR-9-5p descend in 6-OHDA-stimulated neurons; moreover, its elevation abrogated the protective effects of evodiamine on 6-OHDA stimulated neurons, indicating that evodiamine could reduce the neuron damage via miR-9-5p.
In conclusion, evodiamine could inhibit 6-OHDA induced apoptotic, oxidative and inflammatory injury in the PD cell model via miR-9-5p, implying the potential application of evodiamine in PD therapy.
Conflict of interests:
The authors declared no conflict of interests.
References
- Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Progress Neurobiol 2013;106:17-32.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Liu W, Yang H. Balancing apoptosis and autophagy for Parkinson’s disease therapy: Targeting BCL-2. ACS Chem Neurosci 2018;10(2):792-802.
- Gelders G, Baekelandt V, van der Perren A. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J Immunol Res 2018;2018:4784268.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Zhang Y, Li R, Zhu L, Fu B, Yan T. Salidroside ameliorates Parkinson's disease by inhibiting NLRP3-dependent pyroptosis. Aging 2020;12(10):9405-26.
[Crossref] [Google Scholar] [PubMed]
- Yan J, Yang Z, Zhao N, Li Z, Cao X. Gastrodin protects dopaminergic neurons via insulin-like pathway in a Parkinson’s disease model. BMC Neurosci 2019;20(1):31.
[Crossref] [Google Scholar] [PubMed]
- Zhi Y, Jin Y, Pan L, Zhang A, Liu F. Schisandrin A ameliorates MPTP-induced Parkinson’s disease in a mouse model via regulation of brain autophagy. Arch Pharm Res 2019;42(11):1012-20.
[Crossref] [Google Scholar] [PubMed]
- Li M, Wang C. Traditional uses, photochemistry, pharmacology, pharmacokinetics and toxicology of the fruit of Tetradium ruticarpum: A review. J Ethnopharmacol 2020;263:113231.
[Crossref] [Google Scholar] [PubMed]
- Tian KM, Li JJ, Xu SW. Rutaecarpine: A promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol Res 2019;141:541-50.
[Crossref] [Google Scholar] [PubMed]
- Shan QY, Sang XN, Hui H, Shou QY, Fu HY, Hao M, et al. Processing and polyherbal formulation of Tetradium ruticarpum (A. Juss.) Hartley: Phytochemistry, pharmacokinetics and toxicity. Front Pharmacol 2020;11:133.
[Crossref] [Google Scholar] [PubMed]
- Shan Q, Tian G, Han X, Hui H, Yamamoto M, Hao M, et al. Toxicity of Tetradium ruticarpum: Subacute toxicity assessment and metabolomics identification of relevant biomarkers. Front Pharmacol 2022;13:135.
[Crossref] [Google Scholar] [PubMed]
- Ye J, Xu M, Tian X, Cai S, Zeng S. Research advances in the detection of miRNA. J Pharm Anal 2019;9(4):217-26.
[Crossref] [Google Scholar] [PubMed]
- Tang J, Li Z, Zhu Q, Wen W, Wang J, Xu J, et al. MiR-204-5p regulates cell proliferation, invasion and apoptosis by targeting IL-11 in esophageal squamous cell carcinoma. J Cell Physiol 2020;235(3):3043-55.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Sun L, Jia K, Wang H, Wang X. miR-9-5p modulates the progression of Parkinson’s disease by targeting SIRT1. Neurosci Lett 2019;701:226-33.
[Crossref] [Google Scholar] [PubMed]
- Tolosa E, Botta-Orfila T, Morató X, Calatayud C, Ferrer-Lorente R, Martí MJ, et al. MicroRNA alterations in iPSC-derived dopaminergic neurons from Parkinson disease patients. Neurobiol Aging 2018;69:283-91.
[Crossref] [Google Scholar] [PubMed]
- Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Protoc 2013;1078:9-21.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Wu J, Li Y, Jiang Y, Wang L, Chen Y, et al. Circ_0000527 promotes the progression of retinoblastoma by regulating miR-646/LRP6 axis. Cancer Cell Int 2020;20:301.
[Crossref] [Google Scholar] [PubMed]
- Ma HJ, Gai C, Chai Y, Feng WD, Cheng CC, Zhang JK, et al. Bu-Yin-Qian-Zheng formula ameliorates MPP+-induced mitochondrial dysfunction in Parkinson’s disease via Parkin. Front Pharmacol 2020;11:577017.
[Crossref] [Google Scholar] [PubMed]
- Tao H, Liu Y, Hou Y. miRNA-384-5p regulates the progression of Parkinson's disease by targeting SIRT1 in mice and SH-SY5Y cell. Int J Mol Med 2020;45(2):441-50.
[Crossref] [Google Scholar] [PubMed]
- Zeng R, Luo DX, Li HP, Zhang QS, Lei SS, Chen JH. MicroRNA-135b alleviates MPP+-mediated Parkinson’s disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci 2019;65:125-33.
[Crossref] [Google Scholar] [PubMed]
- Liao JF, Chiou WF, Shen YC, Wang GJ, Chen CF. Anti-inflammatory and anti-infectious effects of Evodia rutaecarpa (Wuzhuyu) and its major bioactive components. Chin Med 2011;6(1):1-8.
- Eraslan E, Tanyeli A, Polat E, Yetim Z. Evodiamine alleviates kidney ischemia reperfusion injury in rats: A biochemical and histopathological study. J Cell Biochem 2019;120(10):17159-66.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Yin L, Lu M, Wang J, Li YT, Gao WL, et al. Evodiamine attenuates adjuvant induced arthritis in rats by inhibiting synovial inflammation and restoring the Th17/Treg balance. J Pharm Pharmacol 2020;72(6):798-806.
[Crossref] [Google Scholar] [PubMed]
- Li F, Dai B, Ni X. Long non-coding RNA cancer susceptibility candidate 2 (CASC2) alleviates the high glucose induced injury of CIHP-1 cells via regulating miR-9-5p/PPARγ axis in diabetes nephropathy. Diabetol Metab Syndr 2020;12:68.
[Crossref] [Google Scholar] [PubMed]
- Xiao Y, Zhang Y, Chen Y, Li J, Zhang Z, Sun Y, et al. Inhibition of microRNA-9-5p protects against cardiac remodeling following myocardial infarction in mice. Hum Gene Ther 2019;30(3):286-301.
[Crossref] [Google Scholar] [PubMed]
- Yan Q, Sun SY, Yuan S, Wang XQ, Zhang ZC. Inhibition of microRNA-9-5p and microRNA-128-3p can inhibit ischemic stroke-related cell death in vitro and in vivo. IUBMB Life 2020;72(11):2382-90.
[Crossref] [Google Scholar] [PubMed]