- *Corresponding Author:
- Zhixiang Ming
Department of Thoracic Surgery, Nantong Hospital of Traditional Chinese Medicine, Nantong, Jiangsu Province 226001, China
E-mail: dw3579696@163.com
| Date of Received | 28 June 2022 |
| Date of Revision | 06 February 2023 |
| Date of Acceptance | 18 March 2024 |
| Indian J Pharm Sci 2024;86(2):736-741 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of ginsenoside Rd on the biological characteristics of lung adenocarcinoma H1299 cell line. Firstly, 10 μmol/l ginsenoside Rd, 20 μmol/l ginsenoside Rd, 40 μmol/l ginsenoside Rd, 80 μmol/l ginsenoside Rd, 160 μmol/l ginsenoside Rd were applied to treat H1299 cell line. 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay was used to measure the inhibitory rate of glutathione reductase on cell viability, and the half-maximal inhibitory concentration value was calculated as 48.17 μmol/l. After treating H1299 cell line with 48.17 μmol/l ginsenoside Rd for 3 d, effects of ginsenoside Rd on proliferation ability, migration ability, and apoptosis were respectively detected using Ki67 immunofluorescence, Transwell culture system, and terminal deoxynucleotidyl transferase dUTP nick end labeling assay kit. Ginsenoside Rd had inhibitory effects on the viability of H1299 cells, and the inhibition is concentration-dependent. The half-maximal inhibitory concentration value is 131.92 μmol/l; compared to the control group, treatment of H1299 cells with 130 μmol/l ginsenoside Rd induced a significant decrease in Ki67-positive cells number and migratory cells. However, there was hardly any observed apoptosis in both groups. Ginsenoside Rd can suppress activity, proliferation ability, and migration capability of H1299 cell line, which indicates a noticeable suppression of malignancy in H1299 cells. Ginsenoside Rd may have potential therapeutic effects in the treatment of lung adenocarcinoma.
Keywords
Ginsenoside Rd, lung adenocarcinoma, H1299 cell line, penicillin, streptomycin
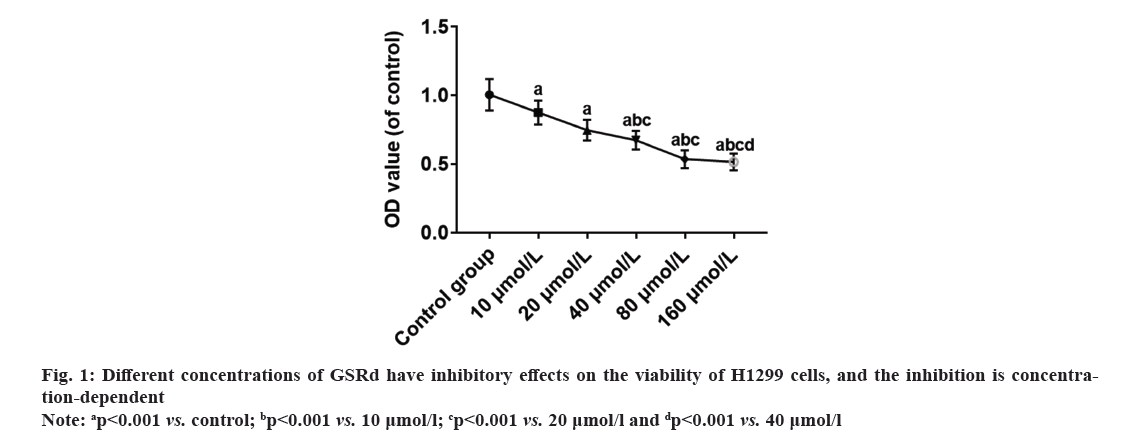
Lung cancer ranks among the prevalent malignant tumors globally, exhibiting high incidence and mortality rates. According to 2020 global cancer statistics from the World Health Organization, lung cancer ranks 2nd in terms of new cases per year (over 2.2 million cases, accounting for 11.4 % of total diagnosed cases) and first in terms of cancer-related deaths per year (over 1.79 million cases, accounting for 18 % of total deaths)[1]. Non-Small Cell Lung Cancer (NSCLC), comprising approximately 85 % of all lung cancer cases, stands as the most prevalent histological type of lung cancer[2]. Among NSCLC cases, Lung Adenocarcinoma (LUAD) has the highest incidence, accounting for about 55 %-65 % of NSCLC cases[3]. Despite significant advancements in interventions for the treatment of LUAD, the clinical efficacy is limited due to cancer metastasis, drug resistance, and adverse reactions. The 5 y survival rate for patients with LUAD is below 20 %, indicating an unsatisfactory prognosis. Further exploration of treatment methods is crucial for improving patient outcomes[4]. Therefore, it is an urgent issue in the field to search for drugs that are effective, low-toxicity, and well-tolerated for LUAD, as well as to elucidate their mechanisms of anticancer action. Traditional Chinese Medicine (TCM) has a rich history in treating tumors, and recent research has highlighted the unique advantages of Chinese herbal medicine in tumor treatment. It can regulate immunity, induce apoptosis of tumor cells, promote differentiation, inhibit Ribonucleic Acid (RNA) and Deoxyribonucleic Acid (DNA) synthesis, reverse multidrug resistance, and play a good role in clinical anti-tumor and enhancing therapeutic efficacy while reducing toxicity[5-7]. Ginsenoside Rd (GSRd) is an active ingredient extracted from ginseng. It belongs to the class of ginsenosides, which are the main components found in ginseng. An increasing number of studies have reported the broad anti-tumor activity of ginsenosides. Well-studied ginsenosides such as Rg3 and Rh2 have demonstrated strong anticancer effects in breast cancer, liver cancer, colorectal cancer, and melanoma[8,9]. The anticancer effects of GSRd have also been reported in various cancers, including tongue cancer[10], breast cancer[11], colorectal cancer[12] and gastric cancer[13]. However, the effects of GSRd on the biological characteristics of LUAD cells remain unclear. This research aimed to investigate the impact of GSRd on the biological properties of the LUAD H1299 cell line. H1299 cell line was purchased from the Chinese Academy of Sciences Shanghai Cell Bank. The cells were cultured using Roswell Park Memorial Institute (RPMI)-1640 medium (HyClone™, America)+10 % fetal bovine serum (Beyotime, China)+1 % Penicillin-Streptomycin (P/S) (HyClone, America). Log-phase H1299 cells were harvested, digested, centrifuged, and resuspended. After counting the cells using a cell counter, they were seeded in a 96-well plate (5×103 cells/ml). Plate was placed in a 37°, 5 % Carbon dioxide (CO2) incubator and cultured for 48 h. The cell culture was divided into a control group and GSRd (Chem Faces, China) groups, with the drug concentration gradient set at 10, 20, 40, 80, and 160 μmol/l. The research received approval from the Ethics Committee of Nantong Hospital of TCM. After 48 h of cell culture, an appropriate amount of trypsin was added to completely digest the cells. Added 6 times the amount of trypsin of complete medium and gently blow to transfer the suspended cells to a centrifuge tube. Centrifuged at 1000 r/min for 5 min and discard the supernatant. Added an appropriate amount of medium and gently blow to achieve a uniform state of suspended cells. Added 100 μl of 2000 cells per well. Added 10 μl of pre-prepared 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) solution (5 mg/ml) (Beyotime, China) to each well, continued incubation for 4 h. Added 100 μl of formazan solution to each well, mix properly, and continue incubation until all formazan was dissolved. Measure the absorbance at 570 nm. Calculate the Optical Density (OD) values of each group with the mean OD value of the control group as reference.
Rate of viability inhibition=(OD control group-OD GSRd group)/OD control group×100 %
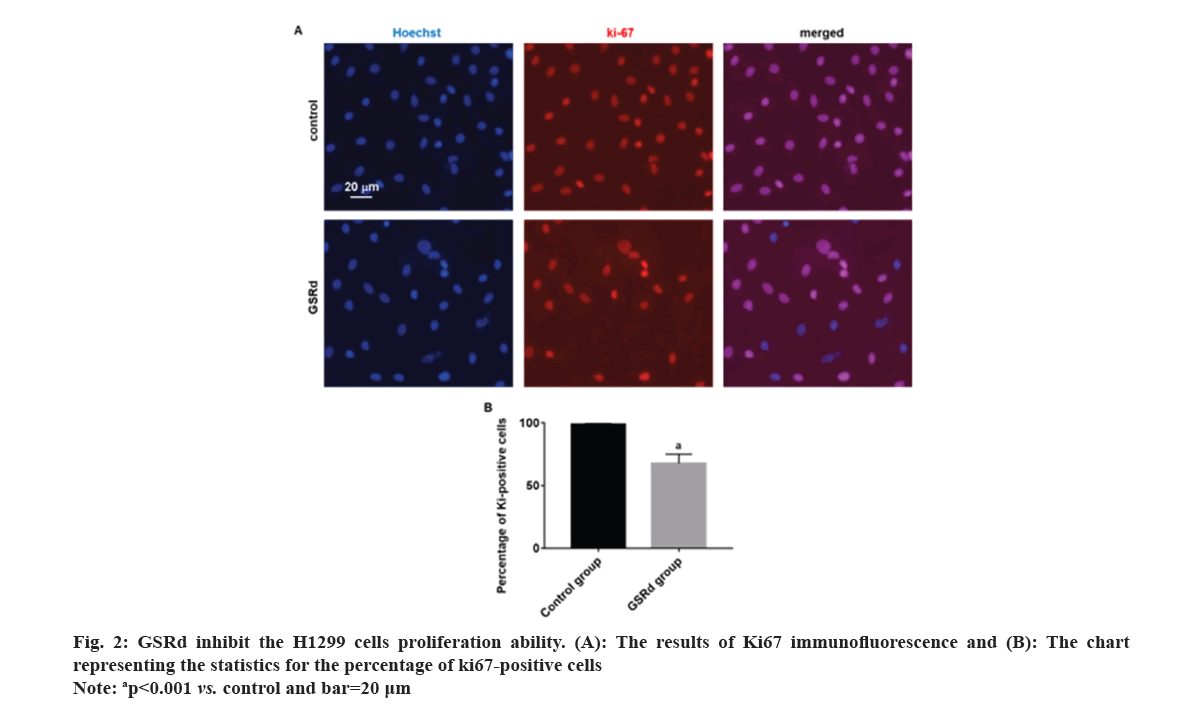
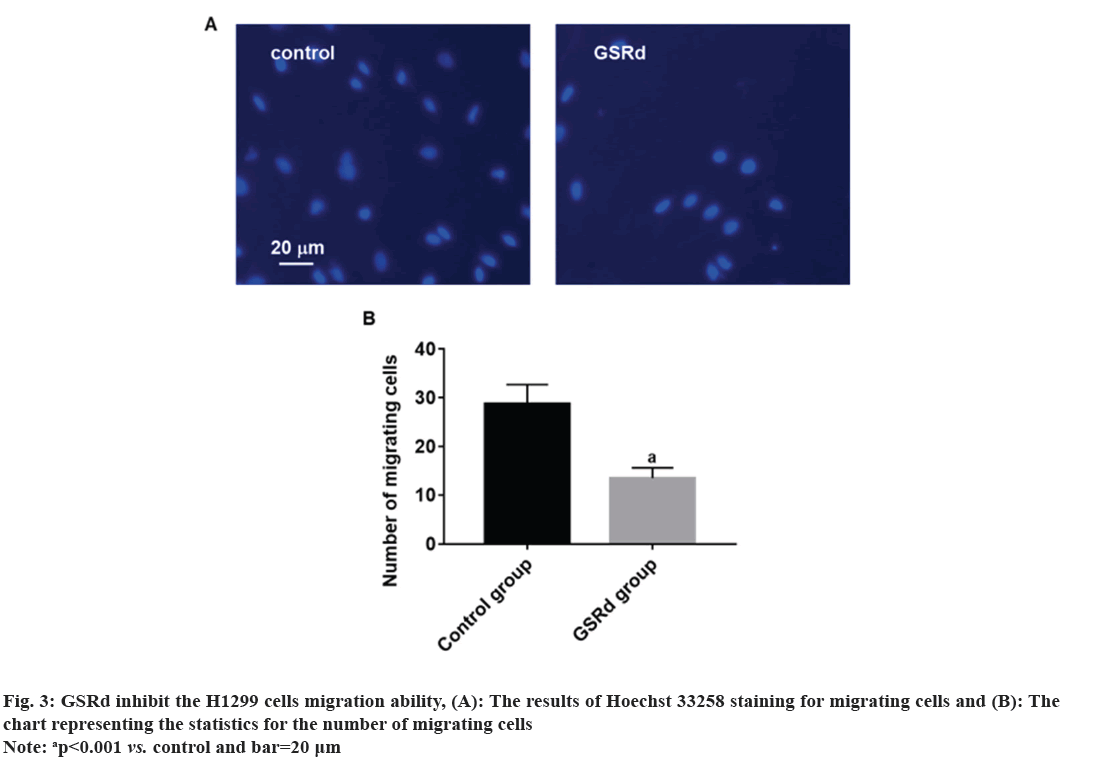
Log-phase H1299 cells were seeded in a 24-well plate (2×105 cells/ml). Control group was cultured with RPMI-1640 medium (HyClone, America)+10 % fetal bovine serum (Beyotime, China)+1 % P/S (HyClone, America). GSRd group was cultured with RPMI-1640 medium (HyClone, America)+10 % fetal bovine serum (Beyotime, China)+1 % P/S (HyClone, America) and 130 μmol/l GSRd (Chem Faces, China). Plate was placed in a 37°, 5 % CO2 incubator and cultured for 48 h. Then, discarded culture medium and added an appropriate amount of 4 % paraformaldehyde for fixation for 6 h. Discarded 4 % paraformaldehyde and washed with Phosphate Buffer Solution (PBS) three times. Discarded PBS and added an appropriate amount of blocking solution, incubated at room temperature in a humid chamber for 10 min. Discarded blocking solution and added Rabbit anti-Ki67 primary antibody (100 μl, 1:600) (Abcam, United Kingdom (UK)), incubated at room temperature in a humid chamber for 4 h. Discarded primary antibody incubation solution and washed with PBS three times. Discarded the PBS and added Goat Anti-Rabbit Immunoglobulin G (IgG) H&L (Alexa Fluor® 594) secondary antibody (100 μl, 1:800) (Abcam, UK), incubated at room temperature in a dark chamber for 3 h. Discarded the secondary antibody incubation solution and washed with PBS three times. Discarded the PBS and added Hoechst 33258 staining solution (1:3000) (Beyotime, China), incubated at room temperature in a dark chamber for 30 min. Discard the Hoechst 33258 staining solution and wash with PBS three times. The percentage of Ki67-positive cells was observed under a fluorescence microscope (Leica, German). Log-phase H1299 cells were seeded in the upper chamber of the Transwell culture plate (3×105/ml), the serum-free RPMI-1640 medium was added in the control group, and serum-free RPMI-1640 medium 130 μmol/l GSRd was added in the GSRd group. In the lower chamber of the Transwell culture plate, RPMI-1640 medium+10 % FBS was added. The Transwell culture plate was placed in incubator for 48 h. The cells that migrated to the lower surface of the upper chamber were carefully removed and fixed with 4 % paraformaldehyde for 20 min, washed three times with PBS, incubated with Hoechst 33258 staining solution (1:3000) (Beyotime, China) in a humid box at room temperature, avoided light for 30 min, washed three times with PBS, and observed migrating cells in each group under a fluorescence microscope (Leica, German). Log-phase H1299 cells were cultured in a 24-well plate (2×105 cells/ml). Control group was cultured with RPMI-1640 medium (HyClone, America)+10 % fetal bovine serum (Beyotime, China)+1 % P/S (HyClone, America). GSRd group was cultured with RPMI-1640 medium (HyClone, America)+10 % fetal bovine serum (Beyotime, China)+1 % P/S (HyClone, America) and 130 μmol/l GSRd (Chem Faces, China). Plate was placed in a 37°, 5 % CO2 incubator and cultured for 48 h. Then, abandoned the culture medium and added an appropriate amount of 4 % paraformaldehyde for 6 h fixation. Washed three times with PBS, added an appropriate amount of Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) detection solution (Beyotime, China), and incubated for 1 h. Washed three times with PBS, stained with Hoechst 33258 staining solution (1:3000) (Beyotime, China), and incubated for 30 min. Washed three times with PBS and observed apoptotic positive cells under a fluorescence microscope (Leica, German). The obtained measured data in this study follows a normal distribution, which is expressed using x̄±Standard Deviation (SD). Independent t-tests or one-way Analysis of Variance (ANOVA) were used for group comparisons. The probit regression was used to calculate the concentration of half-maximal Inhibitory Concentration (IC50). The above statistical procedures were conducted using Statistical Package for the Social Sciences (SPSS) 21.0. Graphs for statistics were created using GraphPad Prism 7.0. p<0.05 was considered statistically significant. Compared to the control group, treatment with GSRd significantly reduced the viability of H1299 cells (p<0.001). Moreover, as the concentration of GSRd increased, the decrease in cell viability became more evident. There were no significant differences between the groups treated with 10 μmol/l and 20 μmol/l, 40 μmol/l and 80 μmol/l, or 80 μmol/l and 160 μmol/l (p>0.05). However, significant differences were observed in pairwise comparisons between the other groups (p<0.001) (fig. 1). Probit regression analysis showed the concentration of IC50 occurred was 131.92 μmol/l. A concentration of 130 μmol/l was used in the following experiment. In the control group, almost all cells were Ki67-positive. In the GSRd group, there was a significant decrease in the number of Ki67-positive cells, with the percentage being notably lower compared to the control group (p<0.001) (fig. 2). The control group showed numerous migrating cells, while the GSRd group had only a small amount of migrating cells. The disparity in the number of migrating cells between the two groups showed statistical significance (p<0.001) (fig. 3). Nearly no apoptotic cells were found in both the control and GSRd groups. Currently, LUAD is the predominant subtype of lung cancer, comprising 40 % of all lung cancer cases, and its incidence is increasing year by year[14]. Current treatment options encompass surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapy[15]. Nevertheless, due to high heterogeneity, rapid progression, early metastasis, and the absence of precise biomarkers, the prognosis for most patients is bleak. Ginseng is one of the most widely used traditional herbal medicines. It is a perennial plant; ginseng has been employed as a traditional medicine for more than 5000 y in Asia [16]. The primary active compounds found in ginseng are ginsenosides, which exhibit various pharmacological effects, including anti-inflammatory, anti-atherosclerotic, anti-diabetic, anti-aging, immune regulatory, and neuroprotective effects. In addition, ginsenosides also have anti-cancer effects[17]. The primary way TCM exerts its anti-tumor effect is by inhibiting tumor cell proliferation, promoting apoptosis, curbing invasion and metastasis, regulating the tumor microenvironment, preventing angiogenesis, and overcoming drug resistance etc.,[5-7]. Our research results showed GSRd can significantly inhibit the activity, proliferation ability, and migration capability of H1299 cell line. Under normal circumstances, cell proliferation is a controlled process, regulated by both internal and external factors of the organism to maintain the normal structure and function of tissues. Tumor formation can be caused by various factors, including genetic mutations, environmental exposure, lifestyle, and others. These factors may disrupt the normal regulatory mechanisms of cells, leading to unrestricted proliferation of abnormal cells. These abnormal cells may evade the body’s natural defense mechanisms, continuing to grow and spread, forming tumors[18]. In this study, we used MTT to assess the H1299 cells viability, and the results showed after treatment with GSRd, viability of H1299 cells experienced a notable decrease. Additionally, as concentration of GSRd increased, the decrease in cell viability became more pronounced. Probit regression analysis showed the concentration of IC50 occurred was 131.92 μmol/l. It indicated that GSRd can inhibit the H1299 cells viability. The results of Ki67 immunofluorescence showed after treatment with GSRd, number of Ki67-positive cells in H1299 cells was significantly reduced. It indicated GSRd can inhibit the H1299 cells proliferation ability. The migration ability of tumor cells refers to their ability to detach from the primary tumor tissue by altering characteristics such as morphology, motility, and adhesiveness, and then entering other parts of the body through the bloodstream or lymphatic system. This migration ability is typically regulated by multiple factors, including interactions between cells and the extracellular matrix, the expression and function of cell adhesion molecules, cellular motility, and plasticity. Through these mechanisms, tumor cells can penetrate blood vessel walls or lymph nodes and spread to tissues or organs distant from the primary tumor site. Once tumor cells successfully metastasize to other sites and form metastatic foci, the prognosis and treatment outcomes for patients are usually more unfavorable[19,20]. Therefore, studying the migration ability of tumor cells and its relationship with metastasis is of significant importance for understanding the mechanisms of cancer metastasis and identifying new treatment strategies. In this study, we used Transwell culture system to assess the H1299 cells migration ability, and the results showed after treatment with GSRd, the number of migrating cells decreased significantly. It indicated that GSRd can inhibit the H1299 cells migration ability. Apoptosis, also referred to as programmed cell death, is a physiological process occurring within human cells to eliminate damaged cells that cannot be successfully repaired. It plays a crucial role in maintaining tissue homeostasis and overall health. Apoptosis helps clear aging, damaged, or abnormal cells, as well as controls cell proliferation and maintains tissue balance[21]. There is a close relationship between cancer and apoptosis. Cancer is a condition marked by the uncontrolled growth and survival of abnormal cells. Inhibition of apoptosis is one of key mechanisms driving cancer development. Under normal circumstances, when cells experience DNA damage, cellular stress, or encounter biological signals, they activate the apoptotic pathway to eliminate abnormal cells. However, in cancer, cancer cells often lose responsiveness to apoptotic signals or bypass the apoptosis mechanism through mutations, genetic changes, and activation of abnormal signaling pathways. This allows cancer cells to proliferate indefinitely, evade programmed cell death, and ultimately form tumors[21]. In this study we used TUNEL to detect the apoptosis; however the results showed GSRd has no effect on apoptosis of H1299 cells. In a summary, GSRd significantly suppress activity, proliferation ability, and migration capability of H1299 cell line, which indicates a noticeable suppression of malignancy in H1299 cells. GSRd may have potential therapeutic effects in the treatment of LUAD. However, the mechanism of GSRd's anti-tumor effect in LUAD still requires further investigation in our subsequent research.
Author’s contributions:
The study was designed by Zhixiang Ming. Wei Ding drafted the paper. Experimental work and data analysis were conducted by Wei Ding, Xudong Qin, Lingjie Zhou, and Zhixiang Ming. All authors reviewed and approved the final manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin 2023;73(1):17-48.
[Crossref] [Google Scholar] [PubMed]
- Wang A, Han C, Zhao H, Zheng Z, Ye X, Shan R. Progress in the knowledge on the transformation of lung adenocarcinoma to small-cell lung cancer. J Cancer Res Ther 2023;19(1):14-9.
[Google Scholar] [PubMed]
- Sun R, Hou Z, Zhang Y, Jiang B. Drug resistance mechanisms and progress in the treatment of EGFR-mutated lung adenocarcinoma. Oncol Lett 2022;24(5):1-6.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Feiyue Z, Gaofeng L. Traditional Chinese medicine and lung cancer-from theory to practice. Biomed Pharmacother 2021;137:111381.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Yang S, Wang K, Lu J, Bao X, Wang R, et al. Cellular senescence and cancer: Focusing on traditional Chinese medicine and natural products. Cell Prolif 2020;53(10):e12894.
[Crossref] [Google Scholar] [PubMed]
- Su XL, Wang JW, Che H, Wang CF, Jiang H, Lei X, et al. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin Med J 2020;133(24):2987-97.
[Crossref] [Google Scholar] [PubMed]
- Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3. Int J Mol Med 2017;39(3):507-18.
[Crossref] [Google Scholar] [PubMed]
- Li X, Chu S, Lin M, Gao Y, Liu Y, Yang S, et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur J Med Chem 2020;203:112627.
[Crossref] [Google Scholar] [PubMed]
- Chang L, Wang D, Kan S, Hao M, Liu H, Yang Z, et al. Ginsenoside Rd inhibits migration and invasion of tongue cancer cells through H19/miR-675-5p/CDH1 axis. J Appl Oral Sci 2022;30:e20220144.
[Crossref] [Google Scholar] [PubMed]
- Wang P, Du X, Xiong M, Cui J, Yang Q, Wang W, et al. Ginsenoside Rd attenuates breast cancer metastasis implicating derepressing microRNA-18a-regulated Smad2 expression. Sci Rep 2016;6(1):33709.
[Crossref] [Google Scholar] [PubMed]
- Phi LT, Sari IN, Wijaya YT, Kim KS, Park K, Cho AE, et al. Ginsenoside Rd inhibits the metastasis of colorectal cancer via epidermal growth factor receptor signaling axis. IUBMB Life 2019;71(5):601-10.
[Crossref] [Google Scholar] [PubMed]
- Tian YZ, Liu YP, Tian SC, Ge SY, Wu YJ, Zhang BL. Antitumor activity of ginsenoside Rd in gastric cancer via up-regulation of caspase-3 and caspase-9. Pharmazie 2020;75(4):147-50.
[Google Scholar] [PubMed]
- Wu P, Zheng Y, Wang Y, Wang Y, Liang N. Development and validation of a robust immune-related prognostic signature in early-stage lung adenocarcinoma. J Transl Med 2020;18(1):380.
[Crossref] [Google Scholar] [PubMed]
- Hutchinson BD, Shroff GS, Truong MT, Ko JP. Spectrum of lung adenocarcinoma. Semin Ultrasound CT MR 2019;40(3):255-64.
[Crossref] [Google Scholar] [PubMed]
- Kim YJ, Zhang D, Yang DC. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv 2015;33(6):717-35.
[Crossref] [Google Scholar] [PubMed]
- Liu CY, Zhou RX, Sun CK, Jin YH, Yu HS, Zhang TY, et al. Preparation of minor ginsenosides C-Mc, CY, F2, and CK from American ginseng PPD-ginsenoside using special ginsenosidase type-I from Aspergillus niger g. 848. J Ginseng Res 2015;39(3):221-9.
[Crossref] [Google Scholar] [PubMed]
- Coelho M, Soares-Silva C, Brandão D, Marino F, Cosentino M, Ribeiro L. β-Adrenergic modulation of cancer cell proliferation: Available evidence and clinical perspectives. J Cancer Res Clin Oncol 2017;143(2):275-91.
[Crossref] [Google Scholar] [PubMed]
- Zanotelli MR, Zhang J, Reinhart-King CA. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab 2021;33(7):1307-21.
[Crossref] [Google Scholar] [PubMed]
- Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol 2022;15(1):129.
[Crossref] [Google Scholar] [PubMed]
- Kashyap D, Garg VK, Goel N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv Protein Chem Struct Biol 2021;125:73-120.
[Crossref] [Google Scholar] [PubMed]