- *Corresponding Author:
- Xinli Feng
Department of Gastroenterology, Affiliated Hospital of Hebei University, Baoding, Hebei 071000, China
E-mail: doctor8010@sina.cn
| Date of Received | 22 June 2020 |
| Date of Revision | 29 October 2020 |
| Date of Acceptance | 24 December 2020 |
| Indian J Pharm Sci 2020;82(6):1040-1043 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of Lactobacillus rhamnosus on intestinal mucosal barrier function in post-infectious irritable bowel syndrome mice. Fifty-four 8 w old Specific-pathogen free male sprague dawley mice were randomly divided into the control group (n=18), model group (n=18), and experimental group (n=18). The post-infectious irritable bowel syndrome model was established by the Trichinella spiralis infection. The experimental group was treated with Lactobacillus rhamnosus (1.0×107 CFU/kg, 0.5 ml), twice a day for 1 w, while the control group and model group were given the same dose of normal saline for 1 w. The changes in body weight, fecal water content, intestinal motility, and D-lactic acid levels of mice in each group were compared. The body weight of mice in the model group and the experimental group was lower than that in the control group, while that in the experimental group was higher than that in the model group (p<0.05). The fecal water content of mice in the model group and the experimental group was higher than that in the control group, while that in the experimental group was lower than that in the model group (p<0.05). The Bristoli score of the model group and the experimental group was higher than that of the control group, and the Bristoli score of the experimental group was lower than that of the model group (p<0.05). The level of D-lactic acid in the model group and the experimental group was higher than that in the control group, while that in the experimental group was lower than that in the model group (p<0.05). Lactobacillus rhamnosus can improve the intestinal motility and intestinal mucosal barrier function in post-infectious irritable bowel syndrome mice.
Keywords
Lactobacillus rhamnoides, infection, irritable bowel syndrome, intestine mucosal barrier function
Irritable bowel syndrome (IBS) was a functional gastrointestinal disease whose main symptoms were the abdominal discomfort, abdominal pain with abnormal stool characteristics and changes in defecation habits. Its specific pathogenesis was complex, and it was generally considered that its pathogenesis was related to a variety of influencing factors[1,2]. Post-infectious irritable bowel syndrome (PI-IBS) was one of the important subtypes of IBS. About 7 %-30 % of IBS patients have a history of acute intestinal infection[3,4]. In recent years, it has been found that intestinal mucosal bacteria and immune activation play an important role in mediating the pathogenesis of PI-IBS[5]. Lactobacillus rhamnosus (L. rhamnosus) belongs to the genus Bacillus lactis and was first isolated from the intestines of healthy people. In recent years, much attention has been paid to the application of L. rhamnosus in the regulation of intestinal flora[6]. Therefore, this study aims to explore the effect of L. rhamnosus on the intestinal mucosal barrier function in PI-IBS mice.
Materials and Methods
Experimental animals and drugs:
Fifty-four 8 w old Specific-pathogen free (SPF) male sprague dawley (SD) mice (20±2 g) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd and adaptive breeding for 3 d (60 % humidity, 25°). L. Rhamnosus was purchased from Shandong Onalai Biotechnology Co., Ltd.
Reagents and instruments:
The main reagents including sodium chloride injection and chloral hydrate (CR double-Crane Co. Ltd), and Trichinella spiralis cysts (Lanzhou Veterinary research institute). The main instruments including BX40 optical microscope (OLYMPUS Co., Ltd), HM-315 histotome (Leica RM201), ESJ200-4 electronic balance (Longteng Electronic Co., Ltd), and centrifuge 5417 (Eppendorf Co., Ltd).
Grouping:
Fifty-four 8 w old SPF male SD mice were randomly divided into the control group (n=18), model group (n=18), and experimental group (n=18).
Model Preparation:
The PI-IBS model was established by the Trichinella spiralis infection. The muscle larvae in SD rats infected with Trichinella spiralis was isolated by 1.5 % pepsin artificial digestion and suspended in normal saline. The mice were fed with 0.2 ml saline containing 300 larvae.
The criteria of successful modeling: at the 8th w after infection, the abdominal withdrawal reflex and colonic transit function test were obviously abnormal, and there was no obvious abnormality in intestinal histopathology.
Administration method:
The experimental group was treated with L. rhamnosus (1.0×107 CFU/kg, 0.5 ml), twice a day for 1 w, while the control group and model group were given the same dose of normal saline for 1 w.
Detection indicators:
Detection of changes in the body weight of mice in each group. The changes in fecal water content of mice in each group were detected and the feces of mice were collected every 2 h for 8 h continuously. The dry and wet weight of feces was weighed and the water content in the feces of mice was calculated. The changes in intestinal motility of mice in each group were detected and evaluated by Bristoli score. The scores of normal feces, soft or unformed feces and water feces were 1, 2 and 3 scores respectively. Detection of D-lactic acid levels changes of intestinal mucosal barrier in mice of each group by spectrophotometry.
Statistical analysis:
SPSS22.0 was used for statistical analysis. T-test was used to analyze the measurement data between the two groups, F-test was used to analyze the measurement data between groups, and x2 test was used to analyze the counting data. p<0.05 indicates that the difference is statistically significant.
Results and Discussion
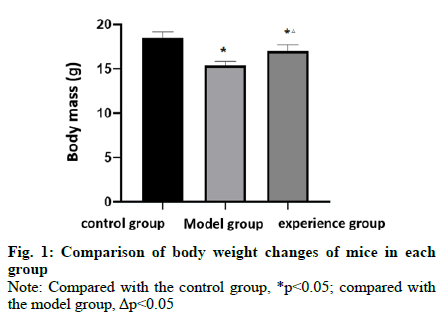
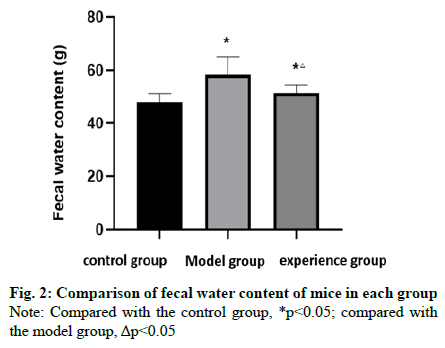
Table 1 showed that the body weight of the model group and the experimental group was lower than that of the control group, while that of the experimental group was higher than that of the model group (fig. 1). Table 2 showed that the fecal water content of the model group and the experimental group was higher than that of the control group while that of the experimental group was lower than that of the model group (fig. 2).
| Group | n | Body weight (g) |
|---|---|---|
| Control group | 18 | 18.54±0.64 |
| Model group | 18 | 15.41±0.45* |
| Experimental group | 18 | 16.98±0.71*? |
Note: Compared with the control group, *p<0.05; compared with the model group, ?p<0.05
Table 1: Comparison of Body Weight in Each Group (X¯±S)
| Group | n | Fecal water content (g) |
|---|---|---|
| Control group | 18 | 47.85±3.24 |
| Model group | 18 | 58.38±6.57* |
| Experimental group | 18 | 51.32±2.98*? |
Note: Compared with the control group, *p<0.05; compared with the model group, ?p<0.05
Table 2: Comparison of Fecal Water Content in Each Group (X¯±S)
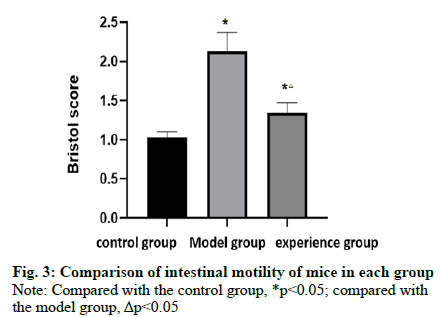
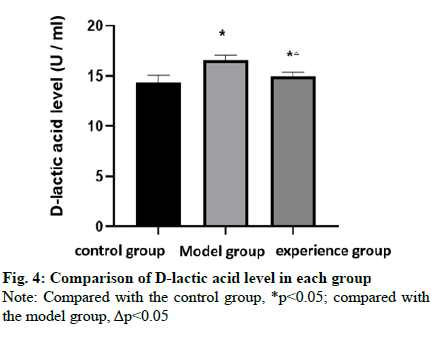
Table 3 showed that the Bristoli score of the model group and the experimental group was higher than that of the control group, and the Bristoli score of the experimental group was lower than that of the model group (fig. 3). Table 4 showed that the D-lactic acid levels of the model group and the experimental group was higher than that of the control group, while that of the experimental group was lower than that of the model group (fig. 4).
| Group | n | Bristoli score (scores) |
|---|---|---|
| Control group | 18 | 1.03±0.07 |
| Model group | 18 | 2.13±0.24* |
| Experimental group | 18 | 1.34±0.13*? |
Note: Compared with the control group, *p<0.05; compared with the model group, ?p<0.05
Table 3: Comparison of Intestinal Motility in Each Group (X¯±S)
| Group | n | D-lactic acid levels(U/ml) |
|---|---|---|
| Control group | 18 | 14.35±0.71 |
| Model group | 18 | 16.58±0.45* |
| Experimental group | 18 | 14.98±0.36*? |
Note: Compared with the control group, *p<0.05; compared with the model group, ?p<0.05
Table 4: Comparison of D-Lactic Acid Levels in Each Group (X¯±S)
IBS was a common disorder of gastrointestinal function, but the specific etiology and pathogenesis had not been fully elucidated[7-9]. In recent years, investigations have shown that the incidence of IBS is gradually increasing, and it often occurs in young and middle-aged people, which seriously affects the quality of life and physical and mental health of patients[10]. PI-IBS means that the patient was infected by enteroviruses, parasites or bacteria and develops IBS symptoms after mucosal inflammation gradually fades and pathogens were cleared[11-13]. Therefore, how to prevent and treat PI-IBS is of great significance.
L.rhamnosus was first isolated from the intestines of healthy people in the 1980s. Because L. rhamnosus can maintain the balance of human intestinal micro ecology, L. rhamnosus has become one of the most concerned probiotics in the world in recent years[14]. Pharmacological studies have shown that L. rhamnosus has the function of stabilizing and maintaining intestinal mucosal barrier, stimulating the non-specific and specific immune response, antagonizing pathogenic or conditional pathogenic bacteria and fostering normal flora[15-16]. This study showed that the body weight of mice in the model group and the experimental group was lower than that in the control group, while that in the experimental group was higher than that in the model group, indicating that L. rhamnosus could add mouse body weight. The fecal water content of the model group and the experimental group was more than that of the control group, while that of the experimental group was less than that of the model group, indicating that L. rhamnosus could increase the fecal water content of mice. The Bristoli score of the model group and the experimental group was higher than that of the control group, and the Bristoli score of the experimental group was lower than that of the model group, indicating that L. rhamnosus could improve the intestinal motility of mice.
There were different degrees of intestinal mucosal barrier injury in IBS. D-lactic acid was mainly found in the mucosa or the upper villi of mammals, most of which were found in the intestinal mucosal villi, and only a few in the endometrial villi of the viscera[17]. D-lactic acid was a highly active intracellular enzyme. Its activity was closely related to the height of the villi and the synthesis of protein and nucleic acid in mucosal cells. It can be used as an ideal index to reflect the structural and functional integrity of intestinal mucosa. When the intestinal mucosal epithelial cells were necrotic or damaged, D-lactic acid was released into the blood or along with the necrotic and exfoliated intestinal mucosal cells into the intestinal cavity, resulting in an increase in the activity of D-lactic acid in the intestinal cavity and plasma, and a decrease in the activity of D-lactic acid in the intestinal mucosa[18]. This study showed that the level of D-lactic acid in the model group and the experimental group was higher than that in the control group, while that in the experimental group was lower than that in the model group, indicating that L. rhamnosus could improve the intestinal mucosal barrier function by reducing the level of D-lactic acid. Because the PI-IBS model mice do not have a complete intestinal mucosal barrier, the high level of D-lactic acid may be the reason for the increase of intestinal mucosal permeability of PI-IBS, or it may be due to the increase of fecal water content in mice. Thus, L. rhamnosus can reduce fecal water content, and improve the intestinal mucosal barrier function.
To sum up, L. rhamnosus can improve the intestinal motility and intestinal mucosal barrier function in PIIBS mice, which has important research value.
Author’s contributions
XINLI FENG and D. ZHANG conceived and designed the experiments; Q. DI and S. LOU performed the experiments; YING CHANG and JIE MENG analyzed the data and wrote the paper.
Acknowledgements
This work was supported by The Youth Research Fund Project of Affiliated Hospital of Hebei University (No. 2016Q003), The Baoding Science and Technology Plan Project (No. 2041ZF181). YING CHANG and JIE MENG are contributed equally to this work, XINLI FENG and D. ZHANG are considered co-corresponding in this paper.
Conflicts of interest
The authors report no conflicts of interest.
References
- Maharshak N, Ringel Y, Katibian D. Fecal and Mucosa-associated Intestinal Microbiota in Patients with Diarrhea-predominant Irritable Bowel Syndrome. Dig Dis Sci 2018;63:1-10.
- Xing PP, Wang M, Dong LN. Effect of intestinal cleaning on clinical symptoms and intestinal flora in patients with diarrhea irritable bowel syndrome. Chin J Dig 2018;38:341-4.
- Liu HN, Wu H, Chen YZ. Altered Molecular Signature of Intestinal Microbiota in Irritable Bowel Syndrome Patients Compared with Healthy Controls: a Systematic Review and Meta-analysis. Dig Liver Dis 2017;49:331-7.
- Chen Y, Xiao RP, Yuan Y. Research progress on overlap of gastroesophageal reflux disease and irritable bowel syndrome. J Med Res 2017;46:186-8.
- Han JM, Pan JY, Ma J. Herbal prescription Jiechangling repairs intestinal mucosal barrier in rats with postinflammation irritable bower syndrome. Jilin J Chin Med 2017;37:941-4.
- Chen J, Qiu ZB, Luo ZG. Small intestinal bacterial overgrowth and low-grade systemic inflammation in 50 patients with irritable bowel syndrome. Chin J Dig 2018;38:769-73.
- Moore JS, Gibson PR, Perry RE. Endometriosis in patients with irritable bowel sndrome: Specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol 2017;57:20-205.
- Qu T S, Wang F C, Cai YY. Influences of Helicobacter pylori infection on irritable bowel syndrome and its typing. Chin J Clin Res 2018;31:64-6.
- Niranga, Manjuri, Devanarayana. Irritable bowel syndrome in children: Current knowledge, challenges and opportunities. World J Gastroenterol 2018;24:2211-35.
- Zhang DG, Luo S, Xiong F. The therapeutic effect of Bifidobacteriumexpressing human IL-10 on post-infectious irritable bowel syndrome. Chin J Microecol 2019;31:20-7.
- Zhao J, Zhao Y. Clinical effect of Weichang'an Pill combined with Live bacterial preparation in the treatment of Post-infection Irritable Bowel Syndrome. Nei Mongol J Traditional Chin Med 2017;36:79.
- Huang HK, Luo JW, Li XT. Efficacy of quercetin combined with 5-aminosalicylic acid in collaborative treatment of irritable bowel syndrome rats with infections. Chin J Nosocomiol 2017;27:5053-6.
- Dai YH, Lan C, Liu D. Clinical Features and Expression of Cytokine in Post-infectious Irritable Bowel Syndrome patients. J Sun Yat-sen University 2017;38:260-6.
- Jin X, Ge YZ, Jing P. The Effects of Probiotics on the Restoration of Intestinal Microflora in a Mouse Model of Antibiotic-induced Diarrhea. Modern Food Sci Technol 2017;12:17-24.
- Huang JW, Jin X. The promoting and regulating effects of triple probiotics on the intestine and intestinal peristalsis in mice with constipation. Chin J Microecol 2018;30:402-8.
- Yuan H, Nie GM, Ke JJ. Research progress of Lactobacillus rhamnosus in the prevention of antibiotic-associated diarrhea in children. Military Med J South Chin 2018;32:76-9.
- Ye JJ, Zhao M. Clinical Efficacy of Guchang Capsule Combined with Bifidobacterium Tetrabacterial Tablets in the Treatment of IBS-D and its Effect on Intestinal Flora and Intestinal Mucosal Barrier Function. Chin J Pharmacoepidemiol 2019;28:15-9.
- Yan MZ, Shen MR, Cui Y. Treatment of intestinal barrier function in patients with diarrhea predominant irritable bowel syndrome. Laboratory Med Clin 2019;16:33-6.