- *Corresponding Author:
- A. V. Shitole
Department of Plant Pathology, Dr. Panjabrao Deshmukh Krishi Vidyapeeth, Akola-444 104, India
E-mail: amolsht@gmail.com
| Date of Submission | 10 July 2016 |
| Date of Revision | 02 January 2017 |
| Date of Acceptance | 05 May 2017 |
| Indian J Pharm Sci 2017;79(4):513-520 |
Abstract
Antifungal effects of methanol, acetone, dichloromethane and aqueous extracts of four flowers, marigold (Tagetes erecta), Gaillardia sp. (G. aristata), Chrysanthemum sp. (C. indicum) and Calotropis sp. (C. gigantea) were evaluated in vitro and in vivo against Sclerotium rolfsii, the causal agent of collar rot of chickpea alone and in combination with bio agents. Among all extracts tested, aqueous extract of marigold flower exhibited potential antifungal activity against S. rolfsii. Seed treatment with Pseudomonas fluorescens 10 g/kg+Trichoderma harzianum 4 g/kg+marigold aqueous extract 4% was proved to be effective in increasing seedling vigour index of chickpea (2716.91) in paper towel assay and also found effective to reduce collar rot disease incidence of chickpea (70.56%) under greenhouse conditions.
Keywords
Bio agents, collar rot, Marigold sp., Gaillardia sp., Chrysanthemum sp., Calotropis sp., S. rolfsii
In recent times, focus on plant research has increased all over the world and a large body of evidence has been accumulated to highlight the immense potential of medicinal plants used in various traditional systems of medicine. Plant extracts have been reported to possess unique antimicrobial properties. Various workers have reported that plant extracts and their secondary metabolites such as alkaloids, terpenoids, glycosides and phenolic acids have a number of medicinal properties, including antimicrobial activity and affect biological functions at very low concentrations [1].
Chickpea is known in this country since ancient times. It is a major pulse crop widely grown in India, accounts for nearly 75 percent of the total pulse production in the world. Chickpea crop is prone to many diseases such as Fusarium wilt, dry root rot, collar rot, Ascochyta blight, Verticillium wilt, black root rot, Phytophthora root rot, wet root rot, foot rot, Pythium rot and seed rot. Among these, collar rot caused by Sclerotium rolfsii, is of high importance. S. rolfsii is a pathogen of high economic impact since it affects numerous crops worldwide. It has an extensive host range; at least 500 species in 100 families are susceptible, the most common hosts are legumes, crucifers and cucurbits and commonly occur in the tropics, subtropics and other warm temperate regions [2].
S. rolfsii is a soil borne polyphagous fungal pathogen distributed in tropical and subtropical regions of the world, where high or warm temperature prevails. Mortality losses due to this pathogen vary from 10-100% [3]. Due to abundant growth of the pathogen with a capability of producing excessive sclerotia that may persist in soil for several years [4,2]. Usually, management of soil borne plant pathogens can be achieved by different fungicides, soil fumigants (methyl bromide), plant extracts and bio agents.
Plant metabolites and plant-based pesticides appear to be one of the better alternatives as they are expected to have minimal environment impact and danger to consumers in contrast to the synthetic pesticides [5]. Active principles from medicinal plants are being tried as replacements of synthetic fungicides in management of plant diseases in organic farming system. Frequent application of fungicides causes environmental pollution and there is a need to reduce the amount of chemicals applied to the soil. Thus, plant extracts and bio agents can be used as an alternative source for controlling soil-borne diseases since they are a rich source of bioactive substances. Plants extracts are eco-friendly, protective, curative and antagonistic to many diseases [6-9]. Biological control of plant diseases has been the subject of extensive research in the last two decades. Trichoderma sp. is well documented as an effective biological control agents of plant diseases [10-13]. P. fluorescens mixed with other strains of fungi or bacteria increased the efficacy of biocontrol [8]. Hence, present investigation was carried out to screen out the most compatible combinations of floral extracts, biocontrol agents and fungicides to find out efficient management practices against collar rot of chickpea caused by S. rolfsii.
Material and Methods
A study was conducted to check the efficacy of plant extracts, biocontrol agents and fungicides against S. rolfsii under in vitro and in vivo conditions. The pathogen was isolated from infected gram seedlings by hyphal tip method of fungal isolation. The morphological and microscopic identification of pathogen was carried out with the help of experts from mycology department, Dr. Panjabrao Deshmukh Krishi Vidyapeeth (Dr. PDKV), Akola. Required biocontrol agents viz., Pseudomonas fluorescens, Bacillus subtilis and Trichoderma harzianum were collected from the department of plant pathology, Dr. PDKV, Akola. Chickpea variety, ICCV-2 was used as host plant throughout study.
The sand sorghum medium (SSM) was used for the mass multiplication (mass inoculum) of S. rolfsii in laboratory. The sorghum grains were soaked in water for overnight. These grains were spread on the clear blotter paper for drying. The grains were taken in the tray and 25 g/kg calcium carbonate was added to grains to avoid clumping. About 300 g moistened grains and 150 g dry sand was filled in each 1000 ml flask. The conical flask were plugged, wrapped with paper and autoclaved for 30 min at 1.04 kg/cm2 pressure 2 times by keeping 24 h gap between the 2 successive autoclavings. The grains in flasks were inoculated with the pure culture of S. rolfsii under aseptic condition and incubated at 28±2° for 2 w. The flasks were shaken on alternate days to avoid clumping of grains and to facilitate early growth of the fungus on the grains. The grains turned whitish due to mycelial growth of the test pathogen.
Pathogenicity determination of S. rolfsii was carried out in vitro. Plastic pots of 2 kg capacity were filled with double sterilized soil. The inocula of S. rolfsii were separately multiplied on SSM and added to each pot 100 g/kg of soil. The inoculum was thoroughly mixed with the upper 15 cm soil. Similarly 3 pots with sterilized soil without inoculum were used as control. The pots were watered lightly and incubated for 4 d. Surface sterilized chickpea seeds of ICCV-2 variety susceptible to S. rolfsii, were sown in each pot (10 seeds/pot). The pots were kept open and seedling mortality was noticed. The symptoms were noticed after 10 d of sowing.
An in vitro test was conducted to determine the effect of four extracts of four cost effective and commonly available temple waste flowers such as marigold sp. (Tagetes erecta), Gaillardia sp. (G. aristata), Chrysanthemum sp. (C. indicum) and Calotropis sp. (C. gigantea) on dry mycelial growth of S. rolfsii. Marigold sp. (Tagetes erecta), Gaillardia sp. (G. aristata), Chrysanthemum sp. (C. indicum) and Calotropis sp. (C. gigantea) flowers were collected from local temples of Akola, Maharashtra, India in October 2012. The identification and authentication of the plants was carried out at the Nagarjun medicinal plants garden, Dr. PDKV, Akola, India. All the selected flowers were thoroughly washed under tap water to remove dust and other impurities. The flowers were dried separately under shade with occasional shifting for about 3 to 4 w. The dried flowers were powdered in a grinder and stored in airtight container until further use [14].
Methanol, acetone, dichloromethane and sterilized distilled water were used as solvent for preparation of flower extracts. Forty gram powder of each flower was separately soaked in 200 ml of methanol, acetone, dichloromethane and sterilized distilled water in 500 ml conical flask and plugged tightly with cotton wrapped in paper. All conical flasks were shaken on a rotary shaker for 4 d and then allowed to stand for 5 h to settle the flower material. Supernatant from each flask was filtered separately through Whatman No. 1 filter paper and evaporated at room temperature. Residual portion of flowers was extracted three times to harvest maximum metabolites from floral parts. Air dried extracts were weighed separately and transferred into small vials and kept in refrigerator at 5° until further use.
In vitro evaluation of floral extracts was carried out by liquid bioassay method on potato dextrose broth medium (PDB), in this method, one gram residue of four flower extracts from all the solvents were diluted in 10 ml dimethyl sulphoxide (DMSO) separately and from this 250, 500, 750 and 1000 μl suspensions were poured separately into 250 ml conical flasks containing 50 ml sterilized PDB. In control set only 250, 500, 750 and 1000 μl DMSO were used. For each treatment 3 replicates (flasks) were used. All conical flasks were inoculated individually with 5 mm diameter discs of the test fungal culture of S. rolfsii and then incubated at 28±2° for 7 d. After incubation period each flask was filtered off using pre-weighted Whatman filter paper. Mycelial mat along with filter paper from each treatment were dried up to a constant dry weight at 70° for 6 h. Then dry weight of each fungus along with filter paper was noted and actual weight of each filter paper was subtracted and resultant value was used to determine the growth inhibition. The percent growth inhibition was calculated by using formula suggested by Vincent [15].
In vitro evaluation of floral water extracts was again carried out by filter paper disc method, in this method; culture suspension of test fungus was prepared separately by adding 10 ml distilled sterilized water in pure culture plates of test fungus. One ml culture suspension of test fungus was uniformly spread on PDA medium in petriplates. The filter paper disc of 5 mm diameter (Whatman paper no. 1) impregnated with the floral water extracts of different concentrations (25, 50, 75 and 100 mg/ml) were placed on the petriplates overlaid with the fungal culture. The plates were incubated at 28±2° in incubator and the inhibition zones of the extracts were examined at intervals of 24 h.
Antagonistic activity of P. fluorescens, B. subtilis and T. harzianum on the growth of S. rolfsii was studied using the dual culture technique on PDA plates. Inoculum disc of 5 mm diameter was taken from 3 d old culture of S. rolfsii. These discs were placed at the centre of respective PDA plates. Then bacterial antagonists were streaked parallel on both sides of fungal pathogens leaving 3 cm distances between them. In case of fungal antagonist, each plate was inoculated with 5 mm mycelial discs of fungal pathogen and T. harzianum were placed side by side on the medium in each plate approximately at a distance of 4 cm away from each other. Similarly, one set of plates with the fungal pathogen without any bio agent culture served as control. The inoculated plates were incubated at 28 ± 2° for 7 d. Observations regarding antagonistic effect of all these bioagents against test pathogens were recorded on 7 d after inoculation. The growth inhibition of test pathogen was calculated by using formula suggested by Vincent [15].
The efficacy of three fungicides viz., thiram (0.2%), carbendazim (0.1%) and metalaxyl (0.2%) were evaluated in vitro against S. rolfsii by liquid bioassay technique on PDB medium (Figure 1). Required quantity of fungicidal formulations were added in 250 ml conical flasks containing 50 ml sterilized PDB separately to make the desired concentration of each fungicide. In control set only 50 ml PDB was used. For each treatment 3 replicates (flasks) were used. All conical flasks were inoculated individually with 0.5 cm diameter discs of the test fungal culture and then incubated at 28±2° for 7 d. After incubation, each flask was filtered off using pre-weighed Whatman filter paper. Mycelial mats along with filter paper from each treatment were dried to a constant weight at 70° for 6 h. Then the dry weight of each fungus along with filter paper was noted and from which actual weight of each filter paper was subtracted and the resultant weight was used to determine growth inhibition. Per cent growth inhibition was calculated by using formula suggested by Vincent [15].
On the basis of in vitro growth inhibition studies greenhouse experiments was conducted to evaluate the individual and combined effect of marigold water extract at different concentrations, T. harzianum and P. fluorescens at recommended dose against chickpea collar rot caused by S. rolfsii. Chickpea ICCV-2 seeds were surface disinfected in 2% sodium hypochlorite for 30 s, rinsed in sterile distilled water and dried overnight. Ten seeds were planted per pot filled with sterilized potting soil (1.5 kg) [16]. The inoculum of fungal pathogens multiplied on sand: sorghum medium was incorporated in to the separate pots at 1:20 (w/w) ratio of pathogen and soil. In every treatment, the talc-based formulation of T. harzianum and P. fluorescens was applied as a seed treatment at 4 and 10 g/kg of seed, respectively. In marigold water extract treatment, seeds were soaked in 2, 3 and 4% solutions separately for 3 h and air dried overnight before sowing. The fungicides metalaxyl (0.2%), carbendazim (0.1%) and thiram (0.2%) were also used for comparison and inoculated pots with the pathogen alone served as control. Three replications were maintained for each treatment in a completely randomized design in a glasshouse. Incidence of collar rot in chickpea was recorded at 30 and 60 d after sowing.
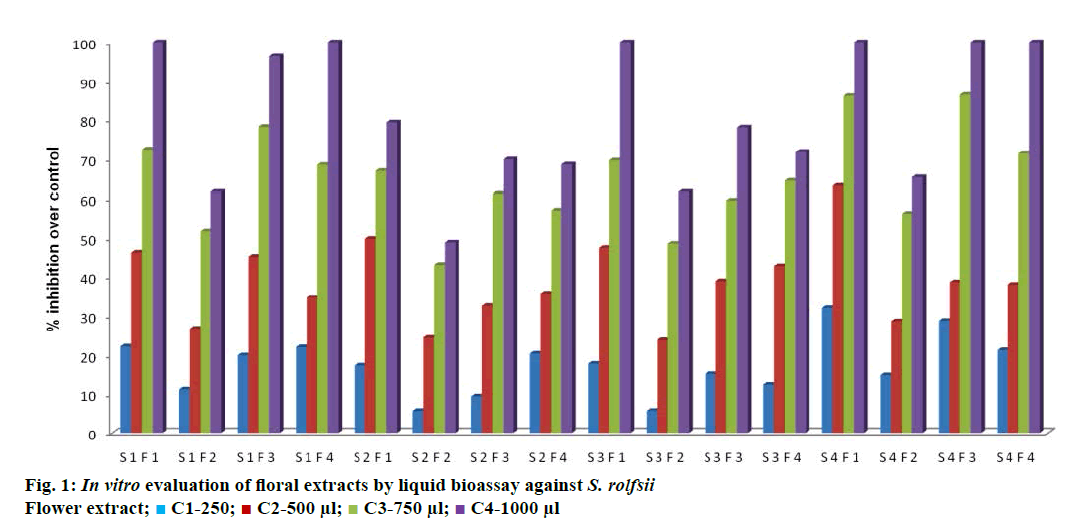
Treatment details are as follows, S1F1, methanol extract of marigold sp. flower; S1F2, methanol extract of Gaillardia sp. flower; S1F3, methanol extract of Chrysanthemum sp. flower; S1F4, methanol extract of Calotropis sp. flower; S2F1, acetone extract of marigold sp. flower; S2F2, acetone extract of Gaillardia sp. flower; S2F3, acetone extract of Chrysanthemum sp. flower; S2F4, acetone extract of Calotropis sp. flower; S3F1, dichloromethane extract of marigold sp. flower; S3F2, dichloromethane extract of Gaillardia sp. flower; S3F3, dichloromethane extract of Chrysanthemum sp. flower; S3F4, dichloromethane extract of Calotropis sp. flower; S4F1, distilled water extract of marigold sp. flower; S4F2, distilled water extract of Gaillardia sp. flower; S4F3, distilled water extract of Chrysanthemum sp. flower; and S4F4, distilled water extract of Calotropis sp. flower.
In vitro effect of floral solvent extract on test pathogen was done by using completely randomized block design (FRBD) with three factors having four levels in each factor. Pot culture studies were carried out by using completely randomized design and each treatment had three replications. The statistical analysis of the data was done by statistical method as suggested by Panse and Sukhatme [17]. ‘F’ test of significance was used to know whether observed treatment effects were real or not from the data in which the treatment effects were significant. The standard error (SE) and critical difference (CD) at 1% level of probability were calculated.
Results and Discussion
The pathogenicity of S. rolfsii was tested on susceptible variety ICCV-2 of chickpea by soil inoculation technique. The initiation of symptoms were observed after 15 d of inoculation of S. rolfsii. Infected plants turned slightly yellow, collar region became constricted and started rotting after 15 d of inoculation. Fungal strands were seen growing over the attached tissues. Sclerotia were small, round and appeared like mustard seed. Similar results were reported by Maurya et al. [18], in chickpea. Observations on interaction effect of solvents, flowers and concentrations on dry mycelial weight of S. rolfsii were recorded and percent inhibition were determined and presented in Table 1.
| S×F×C (solvent×flower×concentration) |
%Inhibition over control | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |||
| S1F1 | 22.21 (27.92)* | 46.32 (42.88) | 72.45 (58.43) | 100.00 (90.00) | ||
| S1F2 | 11.17 (18.81) | 26.71 (31.05) | 51.63 (45.94) | 61.62 (51.80) | ||
| S1F3 | 19.98 (26.35) | 45.25 (42.27) | 78.20 (62.33) | 96.37 (79.09) | ||
| S1F4 | 22.05 (27.83) | 34.67 (35.95) | 68.52 (55.90) | 100.00 (90.00) | ||
| S2F1 | 17.43 (24.49) | 49.76 (44.86) | 67.05 (55.04) | 79.32 (63.03) | ||
| S2F2 | 5.51 (12.81) | 24.45 (29.53) | 42.96 (40.93) | 48.84 (44.33) | ||
| S2F3 | 9.39 (17.33) | 32.57 (34.76) | 61.15 (51.49) | 69.92 (56.86) | ||
| S2F4 | 20.41 (26.73) | 35.75 (36.72) | 57.04 (49.08) | 68.66 (55.98) | ||
| S3F1 | 17.88 (24.93) | 47.51 (43.56) | 69.68 (56.63) | 100.00 (90.00) | ||
| S3F2 | 5.52 (12.51) | 23.82 (29.14) | 48.52 (44.15) | 61.60 (51.81) | ||
| S3F3 | 15.06 (22.49) | 38.90 (38.55) | 59.52 (50.52) | 78.05 (62.10) | ||
| S3F4 | 12.37 (20.53) | 42.67 (40.77) | 64.59 (53.52) | 71.86 (57.98) | ||
| S4F1 | 32.04 (34.44) | 63.36 (52.77) | 86.23 (68.34) | 100.00 (90.00) | ||
| S4F2 | 14.75 (22.59) | 28.63 (32.20) | 56.21 (48.61) | 65.47 (54.06) | ||
| S4F3 | 28.76 (32.38) | 38.62 (38.40) | 86.56 (68.50) | 100.00 (90.00) | ||
| S4F4 | 21.31 (27.40) | 38.01 (38.05) | 71.48 (57.77) | 100.00 (90.00) | ||
| Control | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | ||
| Source | SE (M)± | CD at (P=0.01) | ||||
| Solvent (S) | 0.51 | 1.87 | ||||
| Flowers (F) | 0.51 | 1.87 | ||||
| Concentrations (C) | 0.51 | 1.87 | ||||
| Solvent×flowers (S×F) | 1.01 | 3.74 | ||||
| Solvent×concentrations (S×C) | 1.01 | 3.74 | ||||
| Flowers×concentrations (F×C) | 1.01 | 3.74 | ||||
| Solvent×flowers×concentrations (S×F×C) | 2.03 | 7.48 | ||||
*Fig in parenthesis is arc sin transformed values average of three replications. S1-methanol, S2-acetone, S3-dichloromethane, S4-distilled water; F1-marigold sp. flower extract, F2-Gaillardia sp. flower extract, F3-Chrysanthemum sp. flower extract, F4-Calotropis sp. flower extract; C1-250 μl, C2-500 μl, C3-750 μl, C4-1000 μl
Table 1: In vitro evaluation of floral extracts by liquid bioassay against S. rolfsii
Results presented in Table 1 indicated that the concentrations of the tested floral extracts against S. rolfsii had a positive effect in inhibiting dry mycelial growth. Marigold sp. by using distilled water at 250 μl (C1) concentration gave maximum 32.04% reduction in test fungal growth in terms of dry mycelial weight, followed by S4F3, which showed 28.76%. Similar treatment gave maximum inhibition of (63.36%) of S. rolfsii. Whereas, 86.56% inhibition of test pathogen was recorded in Chrysanthemum sp. extract by using distilled sterile water as solvent at 750 μl concentration. Complete inhibition was recorded at 1000 μl concentration of marigold sp. extract in water as solvent. Subramaniam et al. [19] reported that organic fractions of Parthenium, Jatropha and Annona biowash at 0.5% concentration inhibited the biomass production of S. rolfsii by 85, 87 and 78%, respectively, compared to control. In present investigation maximum reduction in biomass production of S. rolfsii was observed. This clearly indicates that the flower extract has fungicidal property.
Observations on zone of inhibition of tested fungus by water extract of different flowers at different concentrations are presented in Table 2. Among all the water extracts, maximum 19.7 mm inhibition zone was recorded in water extract of marigold flower at 100 mg/ml, followed by 18.3 and 18.0 mm in water extract of Calotropis sp. and Chrysanthemum sp. flowers.
| Distilled water extract | Concentrations (mg/ml) | Zone of inhibition (mm) of S. rolfsii |
|---|---|---|
| Marigold flower extract | 25 | 5.7 |
| 50 | 11.3 | |
| 75 | 15.3 | |
| 100 | 19.7 | |
| Gaillardia flower extract | 25 | 2.3 |
| 50 | 5.0 | |
| 75 | 9.7 | |
| 100 | 11.7 | |
| Chrysanthemum flower extract | 25 | 5.3 |
| 50 | 6.3 | |
| 75 | 14.7 | |
| 100 | 18.0 | |
| Calotropis flower extract | 25 | 3.7 |
| 50 | 6.7 | |
| 75 | 12.7 | |
| 100 | 18.3 | |
| Control | 25 | 0.0 |
| 50 | 0.0 | |
| 75 | 0.0 | |
| 100 | 0.0 | |
| SE (M)± | 1.00 | |
| CD at (P=0.01) | 3.93 |
Average of three replications
Table 2: Effects of different floral extracts using water as solvent on growth of S. rolfsii by filter paper disc method
In vitro studies on antagonistic activities of three bioagents against S. rolfsii revealed that there was a significant difference in percent inhibition of mycelial growth of S. rolfsii (Table 3). T. harzianum recorded maximum (79.52%) inhibition of S. rolfsii, followed by P. fluorescens (67.68%). Least inhibition was observed in B. subtilis (61.59%). Madhavi et al. [20] observed 57.5 and 40.7% mycelial inhibition of S. rolfsii with T. harzianum and P. fluorescens, respectively. This might be due to production of antibiotics, which diffused air filled pores, which are detrimental to the growth of S. rolfsii [21].
| Treatment | Radial mycelial growth (mm) | Percent inhibition | |
|---|---|---|---|
| T. harzianum | 18.43 | 79.52 (63.14) | |
| P. fluorescens | 29.00 | 67.78 (55.45) | |
| B. subtilis | 34.57 | 61.59 (51.73) | |
| Control | 9 0.00 |
0.00 (0.00) | |
| SE (M)± | 0.76 | 0.53 | |
| CD at (P=0.01) | 2.99 | 2.05 | |
*Figures in parenthesis are arc sin transformed values, average of five replications
Table 3: Efficacies of bioagents on mycelial growth of S. rolfsii
In vitro results indicated that Trichoderma isolates had competition, mycoparasitic and lysis effect on the pathogen. T. harzianum produces antibiotics such as gliotoxin, viridin and some cell wall degrading enzymes [22] and also certain biologically active heat stable metabolites such as ethyl acetate [23]. These substances may involve in suppression of fungal pathogens. The bioagents used in the present study are easily producible, biodegradable, less expensive and cause no environmental hazards to human health. These are ecologically safe and culturally more acceptable among the farmers.
Three different fungicides were evaluated to check their efficacy against S. rolfsii by liquid bioassay method and results were depicted in Table 4. Complete inhibition of dry mycelial weight S. rolfsii, was observed in T1 (carbendazim 0.1%), followed by 80.95% inhibition in T3 (thiram 0.2%), whereas, T2 (metalaxyl 0.2%) showed least inhibition (12.03%) compared to control (Table 4). Prabhu and Hiremath [24] found 55.6 and 74.45% inhibition of S. rolfsii in carbendazim and thiram, respectively. Madhavi et al. [20] observed 94% mycelial inhibition of S. rolfsii with combined treatment of carbendazim+dithane M-45.
| Treatment | Dry mycelial weight (mg) | Per cent inhibition |
|---|---|---|
| Carbendazim (0.1%) | 0.00 | 100.00 (90.00) |
| Metalaxyl (0.2%) | 202.57 | 12.03 (20.14) |
| Thiram (0.2%) | 43.86 | 80.95 (64.13) |
| Control | 230.43 | 0.00 (0.00) |
| SE (M)± | 1.61 | 0.47 |
| CD at (P=0.01) | 6.28 | 1.85 |
*Figures in parenthesis are arc sin transformed values, average of five replications
Table 4: Efficacies of fungicides on dry mycelial weight of S. rolfsii
Effect of seed treatment with marigold water extract and bio agents alone and in combination on growth parameters of chickpea variety ICCV-2 was evaluated by paper towel method. Three seed dressing fungicides were also tested for comparing results of marigold water extract and bio agents. Observations on percent germination, mean shoot length, mean root length and seedling vigour index were recorded and presented in Table 5. The per cent germination, root length, shoot length and seedling vigor index of chickpea seedlings of cultivar ICCV-2 were significantly different (P<0.05) when treated with marigold distilled water extract, T. harzianum, P. fluorescence and fungicides. Seedlings of chickpea cultivar ICCV-2 treated with P. fluorescens 10 g/kg seed+ T. harzianum 4 g/kg seed+marigold water extract 4% had the maximum seed germination (95.33%), shoot length (11.00 cm), root length (17.50 cm) and SVI (2716.91) compared with control and other treatments. While, marigold water extract alone (2%) exhibited 73.33% seed germination, 7.00 cm shoot length, 5.00 cm root length and 879.96 SVI. These results clearly indicate that, the plant extracts used are not phytotoxic to chickpea seedlings. The current findings suggest that combined application of P. fluorescens, T. harzianum and marigold water extract can be used as seed treatments for the control of S. rolfsii in chickpea seeds and for improving chickpea seedling growth and are safe and eco-friendly on chickpea compared to synthetic chemicals. Shahnaz et al. [25] reported that application of Cynodon dactylon extract resulted in maximum germination per cent in cowpea and okra. Rajput et al. [3] recorded an increase in seedling vigour index of chickpea seedlings with application of Trichoderma sp. and P. fluorescence.
| S. No. | Treatment | %Germination | Mean shoot length (cm) |
Mean root length (cm) | SVI |
|---|---|---|---|---|---|
| T1 | P. fluorescens alone (10 g/kg) | 82.33 | 7.00 | 10.00 | 1399.61 |
| T2 | T. harzianum alone (4 g/kg) | 88.67 | 8.00 | 9.00 | 1507.39 |
| T3 | Marigold water extract alone 2% | 73.33 | 7.00 | 5.00 | 879.96 |
| T4 | Marigold water extract alone 3% | 75.67 | 7.00 | 5.00 | 908.04 |
| T5 | Marigold water extract alone 4% | 78.33 | 7.00 | 8.00 | 1174.95 |
| T6 | P. fluorescens (10 g/kg)+T. harzianum (4 g/kg) | 90.33 | 10.50 | 14.00 | 2213.09 |
| T7 | P. fluorescens (10 g/kg)+marigold water extract 2% | 82.67 | 7.50 | 9.50 | 1405.39 |
| T8 | P. fluorescens (10 g/kg)+marigold water extract 3% | 83.50 | 8.00 | 9.00 | 1419.50 |
| T9 | P. fluorescens (10 g/kg)+marigold water extract 4% | 85.33 | 9.00 | 11.00 | 1706.60 |
| T10 | T. harzianum (4 g/kg)+marigold water extract 2% | 86.00 | 8.00 | 12.00 | 1720.00 |
| T11 | T. harzianum (4 g/kg)+marigold water extract 3% | 87.33 | 8.00 | 14.00 | 1921.26 |
| T12 | T. harzianum (4 g/kg)+marigold water extract 4% | 89.50 | 9.50 | 13.00 | 2013.75 |
| T13 | P. fluorescens (10 g/kg)+T. harzianum (4 g/kg)+marigold water extract 4% | 95.33 | 11.00 | 17.50 | 2716.91 |
| T14 | Metalaxyl (0.2%) | 83.33 | 5.00 | 6.00 | 916.63 |
| T15 | Thiram (0.2%) | 84.67 | 7.00 | 8.00 | 1270.05 |
| T16 | Carbendazim (0.1%) | 77.67 | 8.00 | 10.00 | 1398.06 |
| T17 | Control | 69.33 | 4.00 | 3.00 | 485.31 |
SVI is seedling vigour index, average of five replications
Table 5: Effects of marigold water extract, psudomonas fluorescens and trichoderma harzianum alone and in combination on seedling growth parameters of chickpea variety iccv-2 by paper towel method
Maximum seed germination (94.00%) was observed in T13 (P. fluorescens 10 g/kg seed+T. harzianum 4 g/kg seed+marigold water extract 4%) and was significantly superior over all the treatments. Lowest per cent germination (67.67%) was reported in treatment T17 (control). Minimum collar rot incidence (10.33%) at 30 DAS was exhibited in T16 (carbendazim 0.1%), followed by 11.67% and 13.67% in T15 (thiram 0.2%) and T13 (P. fluorescens 10 g/kg+T. harzianum 4 g/kg+marigold water extract, 4%, Table 6). Maximum collar rot incidence (56.33%) at 30 DAS was observed in control treatment, followed by 46.33% in T14 (metalaxyl, 0.2%) [26]. T16 (carbendazim, 0.1%) exhibited minimum collar rot incidence (16.33%) at 60 DAS and was significantly superior over control and other treatments. This is the first report of use of marigold flower extract against S. rolfsii. Maximum collar rot incidence (82.67%) was recorded in T17 (control).
| S. No. | Treatment | Germination (%) | Collar rot incidence (%) | %Disease control | |
|---|---|---|---|---|---|
| 30 d | 60 d | ||||
| T1 | P. fluorescens alone (10 g/kg) | 80.00 (63.43)* | 27.67 (31.73)* | 41.33 (40.01)* | 50.00 |
| T2 | T. harzianum alone (4 g/kg) | 85.33 (67.48) | 23.00 (28.66) | 34.67 (36.07) | 58.06 |
| T3 | Marigold water extract alone 2% | 72.67 (58.48) | 38.33 (38.25) | 57.33 (49.22) | 30.65 |
| T4 | Marigold water extract alone 3% | 73.00 (58.69) | 36.67 (37.27) | 54.33 (47.49) | 34.27 |
| T5 | Marigold water extract alone 4% | 74.00 (59.34) | 34.00 (35.67) | 50.33 (45.19) | 39.11 |
| T6 | P. fluorescens (10 g/kg)+T. harzianum (4 g/kg) | 89.00 (70.63) | 21.33 (27.51) | 31.33 (34.04) | 62.10 |
| T7 | P. fluorescens (10 g/kg)+marigold water extract 2% | 81.67 (64.65) | 27.33 (31.52) | 41.00 (39.82) | 50.40 |
| T8 | P. fluorescens (10 g/kg)+marigold water extract 3% | 82.67 (65.40) | 25.67 (30.44) | 38.67 (38.45) | 53.23 |
| T9 | P. fluorescens (10 g/kg)+marigold water extract 4% | 84.00 (66.42) | 23.67 (29.11) | 35.67 (36.67) | 56.85 |
| T10 | T. harzianum (4 g/kg)+marigold water extract 2% | 85.67 (67.75) | 22.33 (28.20) | 34.00 (35.67) | 58.87 |
| T11 | T. harzianum (4 g/kg)+marigold water extract 3% | 86.33 (68.30) | 21.33 (27.51) | 32.00 (34.45) | 61.29 |
| T12 | T. harzianum (4 g/kg)+marigold water extract 4% | 87.67 (69.44) | 19.67 (26.33) | 28.67 (32.37) | 65.32 |
| T13 | P. fluorescens (10 g/kg)+T. harzianum (4 g/kg)+marigold water extract 4% | 94.00 (75.82) | 13.67 (21.70) | 24.33 (29.56) | 70.56 |
| T14 | Metalaxyl (0.2%) | 76.00 (60.67) | 46.33 (42.90) | 69.33 (56.37) | 16.13 |
| T15 | Thiram (0.2%) | 84.33 (66.68) | 11.67 (19.97) | 22.00 (27.97) | 73.39 |
| T16 | Carbendazim (0.1%) | 81.33 (64.40) | 10.33 (18.75) | 16.33 (23.83) | 80.24 |
| T17 | Control (pathogen inoculated) | 67.67 (55.35) | 56.33 (48.64) | 82.67 (65.40) | 0.00 |
| SE (M)± | 1.02 | 0.61 | 1.30 | - | |
| CD at (p=0.01) | 3.77 | 2.25 | 4.81 | - | |
Days after sowing, *Figures in parenthesis are arc sin transformed values, average of three replications
Table 6: Effects of P. fluorescens, T. harzianum and marigold water extract alone and in combination on collar rot of chickpea caused by S. rolfsii
Maximum reduction in chick pea collar rot caused by S. rolfsii (80.24%) was observed in T16 (carbendazim 0.1%), followed by 73.39 and 70.56% in T15 (thiram 0.2%) and T13 (P. fluorescens 10 g/kg seed+ T. harzianum 4 g/kg seed+marigold water extract 4%), respectively (Table 6). These results clearly indicate that biocontrol agents in combination with marigold flower extract provided protection to chickpea against collar rot. Maurya et al. [18] reported 30-40% reduction in collar rot of chickpea with foliar application of T. harzianum and P. fluorescens.
The current status of research suggests that there are indeed alternatives to replace the synthetic fungicides for management of this notorious soil as well as seed borne fungi: S. rolfsii, which causes loss of multimillion dollars. It is possible that by combining these approaches (use of plant extracts, antagonistic microorganisms and plants solvent extract) an economically viable alternative for crop production system can be developed.
Conflict of interest
Nil.
Financial support and sponsorship
Nil.
References
- Singh SK, Sarma BK, Srivastava JS, Singh UP, Ray AB. Antifungal activity of ¢3-Alstovenine, a plant alkaloid isolated from Alstonia venenata. Folia Microbiol 1999;44:510-12.
- Punja ZK. The biology, ecology and control of Sclerotium rolfsii. Ann Rev Phytopathol 1985;23:97-127.
- Rajput VA, Konde SA, Thakur MR. Evaluation of bioagents against chickpea wilt complex. J Soils Crops 2010;20:155-8.
- Chet I, Henis Y. The response of two type of Sclerotium rolfsii to factors affecting Sclerotium formation. J Gen Microbol 1972;73:483-6.
- Varma J, Dubey NK. Prospectives of botanical and microbial products as pesticides of tomorrow. Curr Sci 1999;76:172-9.
- Kandasamy D, Keseran R, Ramasamy K, Rrasad N. Occurrence of microbial inhibitors in the exudates of certain leguminous seeds. Indian J Microbiol 1974;14:25-30.
- Hale CN, Mathers DJ. Toxicity of white clover seed diffusate and its effect on the survival of Rhizobium trifolii. New Zealand. J Agric Res 1977;20:69-73.
- Rahber-Bhatti MH. Control of Phakopsora grewia with plant diffusates. Pak J Bot 1986;18:329-33.
- Kalo F, Taniguchi T. Properties of a virus inhibitor from spinach leaves and mode of action. Ann Phytopath Sec Japan 1987;53:159-67.
- Harman GE, Mattick LR, Nash G, Nedrow BL. Stimulation of fungal conidia germination and inhibition of sporulation in fungal vegetation thalli by fatty acids and their volatile peroxidation products. Canadian J Botany 1980;58:1541-7.
- Sivan A, Elad Y, Chet I. Biological control effects of a new isolate of Trichoderma harzianum on Pythium aphanidermatum. J Phytopathol 1984;74:498-501.
- Coley-Smith JR, Ridout CJ, Mitchell CM, Lynch JM. Control of button rot disease of lettuce (Rhizoctonia solani) using preparations of Trichoderma viride, T. harzianum or tolclofosmethy. Plant Pathol 1991;40:359-66.
- Duffy BK, Simon A, Weller DM. Combination of Trichoderma oningii with Fluorescent pseudomonads for control of take-all on wheat. Phytopathology 1996;86:188-94.
- Thenmozhi M, Bhavya PK, Rajeshwari Sivaraj. Compounds identification using HPLC and FTIR in Eclipta alba and Emilia sonchifolia. Int J Engin Sci and Technol 2011;3:292-8.
- Vincent JH. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 1947;159:850.
- Latha P, Anand T, Ragupathi V, Prakasam R, Samiyappan R. Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plant by mixture of PGPR strains and zimmu leaf extract against Alternaria solani. Biol Control 2009;50:85-93.
- Panse VG, Sukhatme PV. Statistical methods for Agricultural Workers. New Delhi: ICAR Publication; 1978.
- Maurya S, Sing R, Singh DP, Singh HB, Singh UP, Srivastava JS. Management of collar rot of chickpea (Cicer arietinum) by Trichoderma harzianum and plant growth promoting rhizobacteria. J Plant Protec Res 2008;48:347-54.
- Subramaniam G, Iyer GKK, Gottumukkala A, Pagidi H, Meesala SV, Deepthi K. Efficacy of Jatropha, Annona and Parthenium biowash on S. rolfsii, Fusarium oxysporum f.sp. ciceri and Macrophomina phaseolina, pathogens of chickpea and sorghum. African J Biotech 2010;9:8048-57.
- Madhavi G, Bindu, Bhattiprolu SL. Integrated disease management of dry root rot of chilli incited by S. rolfsii (Sacc.). Int J Plant Animal and Environ Sci 2011;1:31-7.
- Dange V. Studies on root rot of chilli caused by S. rolfsii Sacc. M. Sc. Thesis (Unpub.) University of Agriculture sciences, Dharwad; 2006.
- Bello DK, Wells HD, Morkhan CR. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Ecol Epidemiol 1997;72:579.
- Claydown KL, Emerson OH, Sauthwell RJ. The isolation of a toxic substance from the culture filtrates of Trichoderma. Phytopathology 1987;36:1068.
- Prabhu HV, Hiremath PC. Bio-efficacy of fungicides against collar rot of cotton caused by Sclerotium rolfsii Sacc. Karnataka J Agric Sci 2003;16:576-9.
- Shahnaz D, Sadia K, Marium T. Comparative effect of plant extract of Datura alba Nees and Cynodon dactylon (L.) Pers. alone or in combination with microbial antagonists for the control of root rot disease of cowpea and okra. Pak J Bot 2010;42:1273-9.
- Lokesha NM, Benagi VI. Biological management of pigeon pea dry root rot caused by Macrophomina phaseolina. Karnataka J Agr Sci 2007;20:456.
 C1-250;
C1-250;  C2-500 μl;
C2-500 μl;  C3-750 μl;
C3-750 μl;  C4-1000 μl
C4-1000 μl



