- *Corresponding Author:
- H. Pan
Department of Medical Nursing, Jiangsu Vocational College of Medicine, 224005, 283 Jiefang South Road, Yancheng City, Jiangsu Province, China
E-mail: panhongning3989@126.com
| This article was originally published in a special issue, "Clinical and Experimental Studies on Drug and Intervention Repurposing in China |
| Indian J Pharm Sci 2019:81(4)spl issue1;122-128 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study was to reveal the effect of rehabilitation nursing training on nerve repair in rats with cerebral ischemic stroke as well as the effect of rehabilitation nursing training on brain protection and its potential molecular mechanism. It was also aimed to provide objective experimental basis for the implementation of clinical nursing measures. Adult male Sprague Dawley rats were randomly divided into 3 groups, the sham-operated group, non-exercise group and early-exercise group. On this basis, a rat model of middle cerebral artery occlusion was established. After 24 h of cerebral ischemia and perfusion, the rats were subjected to treadmill nursing training intervention for 30 min once a day. Finally, the neuronal apoptosis and the expression of MMP-9 and MMP-2 in rats were observed by labelled staining protein and the mechanism of rehabilitation nursing training on the repair and protection of neuronal function in rats was further discussed. The results showed that there was no significant change in neurological deficits in the sham-operated group, but neurological deficits in the middle cerebral artery occluded rats were significantly impaired. There was a significant difference between the sham-operated group and the nursing training group (p<0.01). In the ischemic control group, the recovery rate of nerve function after ischemic injury was slow, and even the neurological dysfunction was aggravated. It was most severe on the day 3 and would recover slowly thereafter. After cerebral ischemia injury, the expression of MMP-2 protein began to increase gradually until the d 3 after operation. The expression of MMP-2 in the sham-operated group remained at a low level, while the expression of MMP-2 in the ischemic control group was significantly higher than that in nursing training group. The differences between the nursing training group and the sham-operated group and the nursing training group and the ischemic control group were statistically significant (p<0.01). It can be concluded that stroke rehabilitation nursing training could improve the recovery effect of neurological function and protect brain tissue, and its mechanism might be related to inhibiting signal transduction pathway and regulating the expression of MMP-9 and MMP-2 protein.
Keywords
Rehabilitation nursing training, cerebral ischemic stroke, inflammatory factors, neurofactors, MCAO model
Cerebral ischemic stroke, commonly known as stroke, includes ischemic stroke and haemorrhagic stroke[1]. It is a disease of brain tissue or cell necrosis[2]. With the development of society, the incidence of stroke has also increased year by year, which has been one of the most fatal sudden diseases in China[3]. Cerebral ischemic stroke is characterized by focal (or holistic) neurological deficits. Ischemic stroke accounts for 75 to 90 % of all strokes, while hemorrhagic stroke accounts for a minority[4], accounting for only 10- 25 % of all strokes. Therefore, research on prevention and treatment of ischemic stroke should be the key to the treatment of stroke. In general, the incidence of ischemic stroke in males is higher than that in females, higher in the north than in the South and higher in the cities than in the countryside[5]. In addition, the mortality rate of ischemic stroke in one month after cerebral infarction is 3.5-5.0 %, while the mortality rate in three months after cerebral infarction is 8.9-9.8 %. With the breakthrough of diagnosis and treatment of stroke, the death rate of stroke has been greatly reduced[6]. There are many complications in stroke patients, including brain oedema and increased intracranial pressure, dysuria and urinary tract infections, pressure sores, nutritional disorders and post-stroke affective disorders, which have a great impact on the physical and mental state as well as living conditions of patients[7]. Cerebral ischemia injury not only damages local brain tissue and its function, but also leads to acute blood-brain barrier damage, increases the permeability of blood-brain barrier, and causes a series of molecular events[8].
Cerebral ischemia injury can also result in lipid peroxidation of membranes, thereby impairing the structure of membranes and the function of sodium pump. The degree of cerebral ischemic injury is directly related to the duration of ischemia. Within a few minutes after ischemic injury, incomplete ischemia causes reversible damage, and most cells enter a slow and reversible apoptotic process[9]. Prolonged ischemia would lead to infarction, which is necrosis of local brain tissue due to ischemia. Neuronal apoptosis is the main factor in brain tissue and cell death. Chronic hypertension might result in cerebral ischemia damage. This is due to the over excited mood, extremely fast heart rate and high blood pressure leading to cerebral vascular rupture[10]. After cerebral ischemia injury, the active substances released by inflammatory cytokines can cause vasoconstriction, decrease blood flow in brain area, damage endothelial cells in brain area, and result in primary brain edema[11]. Rehabilitation nursing training can overcome the inadequate exercise and improve the upper limb motor dysfunction after stroke[12]. Commonly, rehabilitation nursing training is mainly divided into 2 aspects. On the one hand, it is necessary to restrict the movement of the healthy side of the body. Furthermore, a large amount of repetitive and intensive training must be performed on the unhealthy side of the body[13,14]. Based on the above background, the effect of rehabilitation nursing training on nerve repair in rats with ischemic stroke, the protective mechanism of rehabilitation nursing training on brain tissue of rats with cerebral ischemic injury, and the effect on neuron apoptosis were studied, so as to provide a strong theoretical basis for the clinical prevention and treatment of ischemic stroke.
Materials and Methods
Animals and grouping:
Adult male Sprague Dawley rats (SD rats, 240-290 g, 6-9 w old) were used in the experiment. All rats were purchased through regular channels. After purchasing, the rats were fed with standard rodent feed for about 1 w in the animal room. Rats were provided free access to food and water. The indoor temperature was controlled at 20±2° and the relative humidity was controlled at 54 to 60 %. The daily light time was controlled between 8 and 12 h. Rats allowed to adapt to the environment as soon as possible. After adapting to the environment, rats were randomly divided into the sham group, non-exercise group and early-exercise group.
Solution preparation:
Firstly, double-distilled water was mixed with 1.0 % physiological saline in a solution of 1000 ml. After completion, high temperature disinfection was carried out. Mixed solution A was obtained by adding 28.5 g of disodium hydrogen phosphate and 2.6 g of sodium dihydrogen phosphate into water. The volume of mixed solution A was 1000 ml. Then, 2.97 g of disodium hydrogen phosphate, 0.26 g of sodium dihydrogen phosphate and 8.3 g of sodium chloride were added into the water to obtain a mixed solution B of 1000 ml. Polyformaldehyde was added into the mixed solution A. After the water temperature was adjusted to 65°, the solution was kept in a water bath. The solution was cooled and stored at room temperature with mixed solution A at a constant volume of 1000 ml. Fifteen grams of sucrose was dissolved in the polyformaldehyde solution to obtain 100 ml of mixed solution C. Similarly, 25 g of sucrose was dissolved in solution A to obtain 100 ml of mixed solution D. The solution D was sterilized under high pressure for 12 min. Three hundred and twenty grams of sucrose was then dissolved in 500 ml of mixed solution A. After 12 min of high-pressure sterilization, 280 ml of ethylene glycol was added. Then, the volume of the sterile mixed solution A was set to 1000 ml. At this time, the obtained solution was referred as in situ hybridization protection solution. Next, 0.3 g of Evans blue dye was dissolved in the mixed solution B to obtain a 2 % Evans blue solution. The solution was stored in the dark at room temperature of 35°±2°. Tris HCl (2.36 g) was dissolved in 150 ml of deionized water. The pH value of the solution was adjusted to 7.6 and the final volume was 200 ml with deionized water. To obtain 0.1 M Tris-HCI solution. Phenylmethylsulphonyl chloride (PMSF, 17.4 mg) was dissolved in 1 ml of isopropanol and stored at -20° to obtain a 100 mM PMSF solution. Tissue lysate was obtained by diluting a 10 X tissue lysate solution at 1:10, P protease inhibitor solution at 1:50 and 100 mM PMSF solution at 1:100.
Preparation of rat middle cerebral artery occlusion model (MCAO):
Longa thread embolization method was used to simulate cerebral ischemia-reperfusion injury in rat MCAO model. Rats were anesthetized with isoflurane. Mechanical and endotracheal intubation was used for ventilation. After the preparation, the rats were fixed on the operating table in supine position. A transverse incision was made in the middle of the neck. The skin was cut about 2 cm with a scalpel to open the neck. The left common carotid artery, external carotid artery and internal stiff artery were exposed. The superior thyroid artery was clamped with a coagulator and the distal end of the external carotid artery was ligated. The left common carotid artery and the internal rigid artery were temporarily closed with clips. The scalpel was used to cut an incision in the external carotid artery and a silicone head thread bolt was inserted into the internal rigid artery. The timing began as soon as the silica gel head line bolt reached the beginning of the middle cerebral artery (MCA) along the external carotid artery. Once the rats were fully awake, the silica gel thread plug was fixed, the clamp was removed, the suture incision of the left common carotid artery was opened and the tracheal intubation was removed. After 1.5 h of ischemia, the silicone head thread was completely removed from the anesthetized rat brain and the rats were fed back to their original position after awakening. The experimental procedure of the sham-operated group is similar to that in MCAO, but there is no need to insert a silicone head thread bolt.
Treadmill training:
In this experiment, the rats were trained on an electric treadmill rehabilitation exercise. This kind of treadmill was a completely closed speed controllable electric treadmill with horizontal and backward continuous transmission of electrical track. At the tail of the electric track, a row of electric fences was set up to shock the tail of the rat so that the rat could actively crawl at the set speed on the electric track. The closed electric treadmill training was designed to imitate human running. Like the treadmill, the time and speed of movement in time could be adjusted and quantitative data was recorded. In the process of sports training, attention should always be paid to the physical condition and performance of rats, so as to avoid the occurrence of overwork. According to the previous experimental results, the intensity of treadmill training for rats could be increased. Three days before MCAO, the rats were trained for 5-10 m/min, 5 min a day, and the welladapted rats were put into the group for operation. The training steps of the rat treadmill were as follows, First, one day after MCAO, the rats in the early care training group were trained on the treadmill. The training was conducted at the same time and in the same location. Animals in the control group were grabbed similarly, but did not undergo treadmill training.
Neurological deficit score:
The neurobehavioral score of all rats could be used to analyse the degree of neurological impairment in rats. In this experiment, the Rogers method was used to evaluate the neurobehavioral score of rats. The scoring criteria are listed in Table 1.
| Score | Behavioural manifestations in rats |
|---|---|
| 0 | The rat's forelimbs extend to the ground. The behaviour and limb condition of the rats are observed The rats show normal behaviour without any other neurological characteristics |
| 1 | The wrist and elbow of the forelimb and shoulder joints of the rats show continuous bending and internal rotation of the shoulder joints |
| 2 | Rats with mild neurological impairment can resist external thrust, while rats with severe abnormal neurological impairment can significantly reduce the resistance thrust |
| 3 | The rats moving on the ground are pulled to the tail and they rotate to the paralysed side |
| 4 | Rats rotate to the paralysed side when they move on the ground without applying external force |
| 5 | Rats lie on the ground and walk when stimulated externally |
| 6 | Rats are unconscious, incapacitated and unresponsive to any stimulus |
| 7 | Rats died |
Table 1: Neurological deficit score criteria
Tissue frozen section:
SD rats were anesthetized by intraperitoneal injection of 12 % chloral hydrate (0.4 ml/100 g) and 0.8 % of saline (220 ml) was perfused from the left ventricle. The saline should be sterilized prior to perfusion. Then, the left ventricle should be continuously perfused with 5 % polyformaldehyde solution (120 ml). The rats were fixed in supine position on the operating table and the brain tissue was removed with a scalpel. The brain tissue was immersed in a mixture of 5 % paraformaldehyde and 15 % sucrose. Next, rat brain tissue was taken out, cleaned and re-immersed in a 0.1 M phosphate buffer containing 35 % sucrose. After the rat brain tissue was deposited on the bottom of the container, it was removed from the solution. The dehydrated brain tissue was then cut into coronal sections with a thickness of about 35 μm using a freezing microtome. The brain tissue sections were immersed in the in situ hybridization protection solution and preserved at room temperature of -20°±2.
Total protein extraction:
The SD rats were anesthetized by intraperitoneal injection of 12 % chloral hydrate (0.4 ml/100 g body weight). After deep anaesthesia, the rats were decapitated and killed. The rats were quickly fixed on ice in supine position. The ischemic ipsilateral cortex was quickly separated with a scalpel. Then, the cerebral cortex was immersed in tissue lysate (450 μg/450 μl) and homogenized every 10 s with an electric homogenizer for a total of 2. The fully homogenized homogenate was placed on the ice for 12 min and poured into the prepared EP tube. The EP tube was weighed and the brain slices were shattered with an ultrasound crusher. Next, the brain slices were decomposed every 12 and 10 s for a total of 3 times. The EP tube was placed in an ice box for 10 min and transferred into a refrigerator of –4°±2 and frozen for 30 min. After centrifugation at 12 000 rpm for 20 min in a cryogenic high-speed centrifuge, the total protein was absorbed from the supernatant. At that point, the protein concentration was determined and the total protein was stored in a refrigerator of –78° after sub-packing.
Statistical analysis:
All data were processed by SPSS19.0 statistical analysis, and the data were expressed as mean±standard deviation (x±s). The data were analyzed by factor variance analysis and compared by post hoc Schaffe tests, and p<0.05 was statistically significant.
Results and Discussion
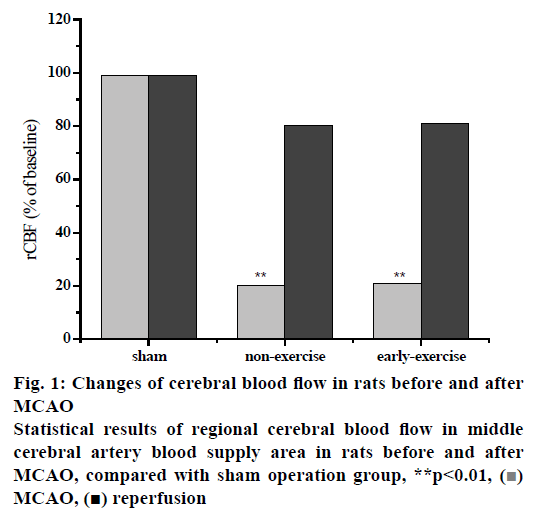
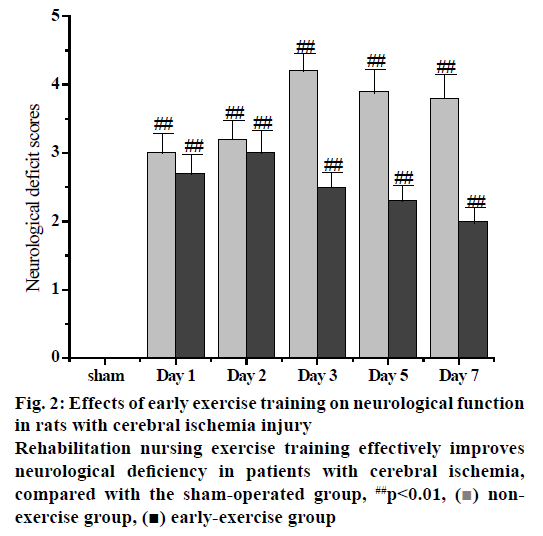
The PH, PaCO2, PaO2 and blood pressure were measured before, during and after MCAO prior to the formal experiment. The results showed that there was no significant difference in PH value, PaCO2, PaO2, blood pressure and other physiological indexes between sham operation group, ischemic control group and early nursing training group during the whole operation period. There was no obvious fluctuation of regional cerebral blood flow in the MCA of rats after the insertion of silica gel cephalic thread bolt into the external carotid artery. As the silicone cephalic thread was inserted into the origin of the MCA, the regional cerebral blood flow in the rat’s blood supply area of the MCA decreased significantly to 22 % of the baseline value before operation, and remained at a low level until the silicone cephalic thread was pulled out. After removal of the silica gel head line embolus, the regional cerebral blood flow in the MCA blood supply area of the rats began to rise gradually to approximately 78 % of the preoperative baseline value. Compared with the sham-operated group, the regional cerebral blood flow of the MCA was significantly different in the nonexercise group. On the other hand, the regional cerebral blood flow of the MCA in the early-exercise group was significantly different (p<0.01) than the sham-operated group. It is worth to note that there was no significant change in regional cerebral blood flow in the rat MCA blood supply area compared to the non-exercise group and the early exercise group. The results showed that MCAO rats had obvious neurological deficits, while sham-operated rats had no obvious neurological deficits. There was a significant difference between MCAO rats and sham-operated rats. In the ischemic control group, the recovery rate of nerve function after ischemia injury was slow and even the nerve dysfunction was aggravated. From the observation, the most serious condition occurred on the third day but it gradually improved. The neurological function of the rats in the nursing training group was obviously lacking at the early stage. After 3 d of intervention, the neurological function of the rats in the nursing training group was significantly improved. Comparing the data of ischemic control group with that of nursing training group, the difference was statistically significant (p<0.01) until the seventh day. These results indicated that the rehabilitation nursing training could effectively restore the neurological function of rats with ischemic injury and its mechanism is to protect the brain by inhibiting the cascade reaction of ischemia in the early stage of ischemic injury (Table 2, fig. 1).
| Operative period | pH | PaCO2 (mm Hg) | PaO2 (mm Hg) | MABP (mm Hg) | |
|---|---|---|---|---|---|
| Sham operation group | 7.23±005 | 42.6±1.3 | 97.5±2.9 | 96.3±4.9 | |
| Non-exercise group | Before MCAO operation | 7.32±0.05 | 40.42±3.8 | 89.4±2.6 | 94.12±4.6 |
| During MCAO operation | 7.41±0.05 | 47.95±6.9 | 83.1±4.1 | 88.3±7.8 | |
| After MCAO operation | 7.38±0.04 | 39.47±3.7 | 88.2±7.8 | 89.2±2.3 | |
| Early-exercise group | Before MCAO operation | 7.42±0.04 | 45.1±7.8 | 90.5±3.4 | 93.6±5.5 |
| During MCAO operation | 7.28±0.07 | 48.3±8.6 | 82.7±4.1 | 85.6±6.3 | |
| After MCAO operation | 7.29±0.07 | 40.1±9.6 | 87.3±6.9 | 89.5±10.1 |
Table 2: Monitoring results of physiological indexes in peri-ischemic period
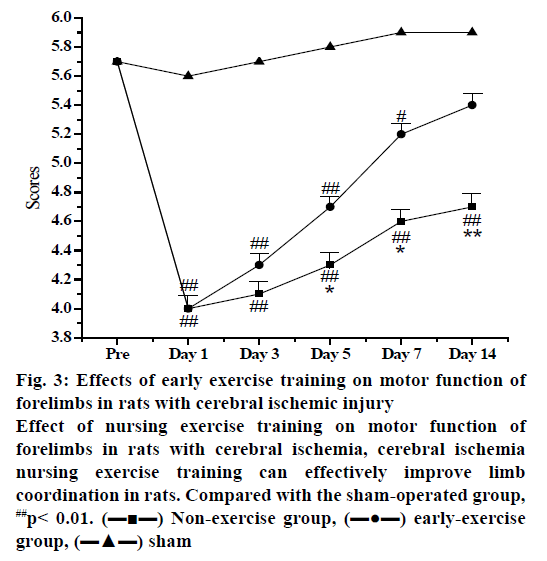
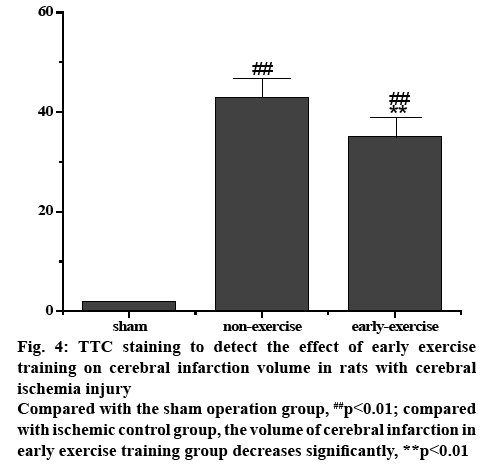
The score of nerve function could clearly indicate whether there is a nerve defect in each group of rats. After the intervention of nursing exercise training, the rats in each group were scored in the staggered step experiment, so that the degree of nerve defect in each group could be further analysed. The forelimb of the sham operation group had neither obvious neurological deficit nor motor dysfunction, so the score was close to perfect score. After ischemic injury of the left MCA in MCAO rats, the right forelimb was obviously hemiplegia. The difference in the degree of nerve function defect was statistically significant compared with the sham operation group (p<0.01). After 3 d of treadmill training, the rats in the nursing exercise training group recovered their nerve function and improved their coordination of limb movement. After 5 d of treadmill training, the coordination of right forelimb in the nursing exercise training group was significantly better than that of the ischemic control group. It can be seen from the results that nursing exercise training could accelerate the recovery of neurological function in rats with ischemic stroke. Studying the brain protection mechanism through exercise training within 3 d after cerebral ischemia injury is of great significance to its functional prognosis. To observe the effect of nursing exercise training on cerebral infarction volume in rats with ischemic stroke, TTC staining was used to detect the volume of cerebral infarction 3 d after exercise training. The results revealed that the brain tissue of rats in sham-operated group was intact and the infarction focus of rats in MCAO group was significant (p<0.01). Compared with the ischemic control group, the volume of cerebral infarction in the early exercise training group was significantly reduced and the difference was statistically significant (p<0.01, figs. 2-4).
Figure 2: Effects of early exercise training on neurological function in rats with cerebral ischemia injury
Rehabilitation nursing exercise training effectively improves neurological deficiency in patients with cerebral ischemia, compared with the sham-operated group, ##p<0.01, ( ) nonexercise group, (
) nonexercise group, ( ) early-exercise group
) early-exercise group
Figure 3: Effects of early exercise training on motor function of forelimbs in rats with cerebral ischemic injury
Effect of nursing exercise training on motor function of forelimbs in rats with cerebral ischemia, cerebral ischemia nursing exercise training can effectively improve limb coordination in rats. Compared with the sham-operated group, ##p< 0.01. (???) Non-exercise group, (???) early-exercise group, (???) sham
Figure 4: TTC staining to detect the effect of early exercise training on cerebral infarction volume in rats with cerebral ischemia injury
Compared with the sham operation group, ##p<0.01; compared with ischemic control group, the volume of cerebral infarction in early exercise training group decreases significantly, **p<0.01
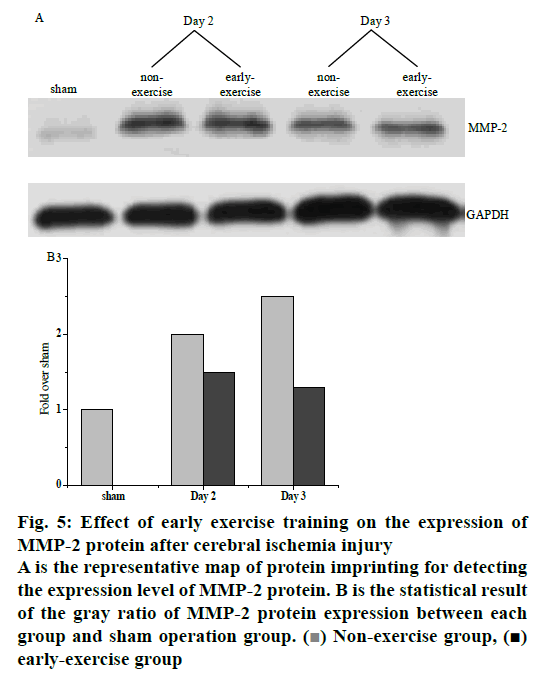
The expression of MMP-2 protein was shown in the fig. 5. After cerebral ischemia injury, the expression of MMP-2 protein began to increase gradually until the 3rd day after operation. The expression of MMP-2 in the sham-operated group remained at a low level, while the expression of MMP-2 in the ischemic control group was significantly higher than that in the nursing training group. The difference between nursing training group and sham-operated group was statistically significant (p<0.01). The difference between the nursing training group and the ischemic control group was also statistically significant (p<0.01). It was proven that in the early stage of cerebral ischemia injury, exercise training could significantly inhibit the up-regulation of MMPS protein expression and effectively protect the structure of the blood-brain barrier. Fig. 5A depicts the expression of MMP-2 at different times.
Figure 5: Effect of early exercise training on the expression of MMP-2 protein after cerebral ischemia injury
A is the representative map of protein imprinting for detecting the expression level of MMP-2 protein. B is the statistical result of the gray ratio of MMP-2 protein expression between each group and sham operation group. ( ) Non-exercise group, (
) Non-exercise group, ( ) early-exercise group
) early-exercise group
The most scientific and effective rehabilitation nursing program is a training mode, which combines appropriate intensity and reasonable exercise mode in clinical rehabilitation nursing. This experiment proves that early intervention of safe and effective nursing exercise training after cerebral ischemia injury could significantly improve the expression of neurokines, alleviate the symptoms of cerebral ischemia injury as well as promote cerebral blood flow and cerebrovascular regeneration. In this experiment, MCAO model was constructed, and the rat MCAO model was used to simulate ischemic stroke. Starting from 24 h after cerebral ischemia, a certain intensity of exercise plan was gradually formed. It was found that the nursing exercise training group has better adaptability to treadmill exercise and nursing exercise training could reduce neuronal apoptosis after cerebral ischemia injury. On this basis, the protective effect and mechanism of nursing exercise training on brain after cerebral ischemia injury were studied by transmission electron microscopy, real-time PCR, protein imprinting, gelatin zymogram, TUNEL staining, F-J-B staining and other methods. In the study, it was found that nursing exercise training could effectively improve the permeability of blood-brain barrier, protect brain tissue and restore nerve function after cerebral ischemia injury. In summary, the following conclusions were drawn. Early nursing exercise training has a certain protective effect on brain tissue of rats with cerebral ischemia injury. Early nursing exercise training could promote the recovery of nerve function and reduce the volume of cerebral infarction in rats. Moreover, the early nursing training able to reduce the degree of cerebral ischemia injury, thereby destroying the bloodbrain barrier and reducing the permeability of the blood-brain barrier. It could also inhibit the expression and activity of MMP-2 and MMP-9 protein and reduce the degradation of ECM. In conclusion, the important mechanism of early nursing training on cerebral protection and neurological function recovery in rats with cerebral ischemia injury was to reduce the volume of cerebral infarction and inhibit neuronal apoptosis.
References
- Saulle MF, Schambra HM. Recovery and rehabilitation after intracerebral hemorrhage//Seminars in neurology. Semin Neurol 2016;36(03):306-12.
- Dawson J, Pierce D, Dixit A, Kimberley TJ, Robertson M, Tarver B, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016;47(1):143-50.
- Kimberley TJ, Pierce D, Prudente CN, Francisco GE, Yozbatiran N, Smith P, et al. Vagus Nerve Stimulation Paired with Upper Limb Rehabilitation after Chronic Stroke: A Blinded Randomized Pilot Study. Stroke 2018;49(11):2789-92.
- Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, et al. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma 2016;33(9):871-9.
- Jeffers MS, Corbett D. Synergistic effects of enriched environment and task-specific reach training on poststroke recovery of motor function. Stroke 2018;49(6):1496-503.
- Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL. 2nd, et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke 2018;49(3):710-7.
- Yagi M, Yasunaga H, Matsui H, Morita K, Fushimi K, Fujimoto M, et al. Impact of rehabilitation on outcomes in patients with ischemic stroke: a nationwide retrospective cohort study in Japan. Stroke 2017;48(3):740-6.
- Sommer CJ, Schäbitz WR. Fostering poststroke recovery: towards combination treatments. Stroke 2017;48(4):1112-9.
- Williamson MR, Dietrich K, Hackett MJ, Caine S, Nadeau CA, Aziz JR, et al. Rehabilitation augments hematoma clearance and attenuates oxidative injury and ion dyshomeostasis after brain haemorrhage. Stroke 2017;48(1):195-203.
- Belagaje SR. Stroke rehabilitation. Continuum 2017;23(1):238-53.
- Dymarek R, Ptaszkowski K, S?upska L, Halski T, Taradaj J, Rosi?czuk J. Effects of extracorporeal shock wave on upper and lower limb spasticity in post-stroke patients: a narrative review. Top Stroke Rehabil 2016;23(4):293-303.
- Balkaya MG, Trueman RC, Boltze J, Corbett D, Jolkkonen J. Behavioral outcome measures to improve experimental stroke research. Behav Brain Res 2018;352:161-71.
- Rosbergen IC, Grimley RS, Hayward KS, Walker KC, Rowley D, Campbell AM, et al. Embedding an enriched environment in an acute stroke unit increases activity in people with stroke: a controlled before–after pilot study. Clin Rehabil 2017;31(11):1516-28.
- Alawieh A, Zhao J, Feng W. Factors affecting post-stroke motor recovery: implications on neurotherapy after brain injury. Behav Brain Res 2018;340:94-101.