- *Corresponding Author:
- G. A. Kurian
Vascular Biology Lab, School of Chemical and Biotechnology, SASTRA University, Thanjavur-613 401, India
E-mail: ginokurian@hotmail.com
| Date of Submission | 03 June 2016 |
| Date of Revision | 15 September 2016 |
| Date of Acceptance | 28 September 2016 |
| Indian J Pharm Sci 2016;78(5):641-650 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Mitochondria are the key players of cardiac function and their dysfunction due to oxidative stress is implicated in myocardial ischemia reperfusion injury, one of the important mediators of cardiac mortality. The present study evaluates the mitochondrial protective effect of sodium thiosulfate on isolated cardiac mitochondria (interfibrillar mitochondria and subsarcolemmal mitochondria) subjected to oxidative stress by ischemia and reperfusion. Briefly, interfibrillar mitochondria and subsarcolemmal mitochondria were treated with cobalt chloride (500 µM) for 20 min to induce oxidative stress by chemical method and nitrogen gas purging for 20 min in an ischemic buffer was used for the physiological method. The mitoprotective effect of sodium thiosulfate was evaluated with different mode of sodium thiosulfate incubation (pretreatment, cotreatment and post treatment) with the mitochondria. The mitochondria led to an increased oxidative stress (measured by malondialdehyde, reduced glutathione, superoxide dismutase and glutathione peroxidase) and decreased mitochondrial enzyme activities (measured by succinate dehydrogenase, malate dehydrogenase and NAD+ dehydrogenase) with both the models and sodium thiosulfate treated groups showed significant improvement in the above alterations. By comparing the efficiency among the different modes of sodium thiosulfate treatment, we found that pretreatment renders significant (P<0.05) mitochondrial protection against ischemia reperfusion injury by nitrogen gas. However, no such differences were observed among the treatments in cobalt chloride mediated mitochondrial dysfunction. In summary, we found that sodium thiosulfate can modulate and preserve cardiac mitochondria for the treatment of myocardial ischemia reperfusion injury.
Keywords
Interfibrillar mitochondria (IFM), subsarcolemmal mitochondria (SSM), oxidative stress, sodium thiosulfate, mitochondrial dysfunction
Mitochondrial dysfunction leads to a wide range of cardiac pathologies including myocardial ischemia reperfusion, because of its primary role in heart metabolism with respect to energy production, metabolism and homeostasis. Incidentally, the majority of intracellular reactive oxygen species (ROS, superoxide, hydroxide and hydrogen peroxide) that mediate the oxidative stress, are derived from mitochondria via electron transport chain (ETC), especially complex I and complex III redox sites [1-3]. Ischemia reperfusion (I/R) injury, a major contributor to the damage encountered during myocardial infarction is experimentally studied by performing microsurgery on rodents; isolated heart perfusion with Langendorff setup and by mimicking it in cell lines utilizing anaerobic chamber. All these approaches have their own benefits with certain limitations and thus the translation of the preclinical results from these models is not very encouraging. Considering the significant role of mitochondria in determining the pathology of I/R injury and importance in the cardiac cell as a “small miniature factory” that sustains the viability of myocytes, we propose to utilize mitochondria as an initial experimental model to study reperfusion injury and its associated changes.
According to the earlier reports, ROS mediate mitochondrial damage through a series of redox reactions that lead to mitochondrial enzyme damage, mutation in mitochondrial DNA, alteration in transmembrane potential leading to loss of membrane integrity [4]. In the cardiac tissue, Palmer et al. have showed the presence of morphologically and functionally distinct heterogeneous mitochondria in heart, namely, interfibrillar mitochondria (IFM) and subsarcolemmal mitochondria (SSM) [5], that show distinct response to I/R injury.
Studies have shown that hypoxic conditions can activate various transcriptional factors that are involved in cellular homeostasis including cell death through mitochondrial ROS [6]. Cobalt chloride (CoCl2) can be used to induce I/R in cardiac cell line as it can mimic hypoxia/ischemic conditions by up regulating hypoxia inducible transcription factor (HIF-1) and by generating ROS [7]. Similarly, nitrogen gas infusion can induce hypoxic injury by reducing oxygen level [8] and by inhibiting F0F1 ATPase enzyme.
Owing to the importance of mitochondria in heart and in regulating redox cellular signaling, various compounds have been explored for their effect on the mitochondria, whose status is used to provide a mechanistic explanation for the compound’s toxicological/pharmacological effects [9]. It is well established that drug-induced mitochondrial dysfunction is an important contributor for the drug-associated heart failure and thus the need to screen the drug for mitochondrial protection, becomes a prerequisite for the development of cardiovascular drugs. The most accurate prediction of toxic vs. beneficial effects of the drug on mitochondrial functional activity is one of the key components of drug development by the pharmaceutical industry.
There are several potential drugs that can lead to toxicity at the mitochondrial level, but only few were demonstrated in the clinical scenario. It is a general belief that mitochondrial toxicity depends on the production of free radicals. In the present study, we evaluate the mitochondrial protective effect of sodium thiosulfate (STS), a FDA approved drug for cyanide toxicity [10], calciphylaxis [11], found to be cardioprotective [12], reduce free radical release and could act as a calcium ion chelator [13].
Materials and Methods
All chemicals used were of analytical grade purchased from HiMedia, Mumbai, India. In accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines, all animal experiments were conducted and approved by the Institutional Animal Ethics Committee (IAEC No.214/SASTRA/IAEC). Eight-week old male Wistar rats, weighing 180-200 g, were obtained from Central Animal Facility, SASTRA University. Animals were kept in polycarbonate cages under controlled temperature of 25±3°; 60±10% relative humidity was maintained with a 12 h dark/light cycle. Rats were acclimatized with standard laboratory diet and tap drinking water ad libitum.
Rats (n=6) were injected with heparinized saline (500 IU/kg b.w.) intraperitoneally, 30 min before terminal sacrifice (sodium thiopentone, 80 mg/kg b.w. i.p.). The heart was excised, mounted on a Langendorff apparatus (AD instruments, Australia) and perfused retrogradely for 120 min, via the aorta with Krebs Henseleit (KH) buffer composed of NaCl (118.5 mM), KCl (5.8 mM), NaHCO3 (25 mM), KH2PO4 (1.2 mM), MgSO4 (1.2 mM), glucose (11 mM) and CaCl2 (2.5 mM) with pH 7.4, maintained at 37°; bubbled with 5% CO2 and 95% O2, at a constant flow rate of 8 ml/min as described by Chevion et al. [14].
Mitochondria subpopulation isolation
The differential centrifugation technique (in 0.25 M sucrose) was used to isolate rat heart mitochondrial subpopulations, namely IFM and SSM, essentially according to the method described elsewhere Kurian et al. [15]. IFM and SSM were purified using a 60% percoll gradient and western blot analysis of HSP 60, the marker protein against β-actin was used to determine the purity. IFM and SSM were isolated from normal rat hearts and their physiology was assessed by the respiratory control ratio and P/O before the start of experiments. Protein content was determined spectrophotometrically at 595 nm using Bio-Rad reagent (HiMedia Laboratory, Mumbai, Bradford 1976) with BSA as a standard.
The isolated mitochondrial fractions were divided into the following groups depending upon the treatments. Group 1 (normal control) with isolated IFM and SSM resuspended in respiration buffer; incubated at 37° for 30 min and stored at -80° until use. Group 2a (CoCl2 control) with IFM and SSM resuspended in respiration buffer containing 500 μM CoCl2; incubated at 37° for 20 min and centrifuged and pellet was resuspended in respiration buffer and stored at –80° until use. Group 2b (STS pretreated) with isolated mitochondria incubated with STS (10 μM) for 10 min (37°) and then subjected to centrifugation. To the pellet, 500 μM of CoCl2 was added and incubated for 20 min. Treated samples were centrifuged and pellet was stored at -80°. Group 2c (STS co-treated) with IFM and SSM incubated with 500 μM CoCl2 and STS (10 μM) for 20 min at 37°, the samples were centrifuged and pellet was stored at –80°. Group 2d (STS post-treated) - mitochondria were first treated with 500 μM cobalt chloride (20 min; 37°); centrifuged. Then the pellet was incubated with 10 μM STS (10 min), after incubation, centrifuged; stored the pellet at –80°. Group 3a nitrogen gas (N2)-induced I/R control - IFM and SSM were resuspended in respiration buffer and purged with nitrogen gas for 20 min (37°); centrifuged and pellet was resuspended in respiration buffer and stored at –80° until use. Group 3b (STS-pretreated) - isolated mitochondria were incubated with STS (10 μM) for 10 min (37°) and then subjected to centrifugation. To the pellet, nitrogen gas was purged for 20 min and treated samples were centrifuged and pellet was stored at –80°. Group 3c (STS co-treated) - in this group, IFM and SSM were purged with nitrogen gas for 20 min followed by incubation with STS (10 μM) for 20 min at 37°, the samples were centrifuged and pellet was stored at –80°. Group 3d (STS post-treated) -In this, mitochondria were first purged with nitrogen gas for 20 min (37°); centrifuged, the pellet was incubated with 10 μM STS (10 min). After incubation, centrifuged; stored the pellet at –80°. In all the above mentioned groups, centrifugation was carried out at 9000×G for 10 min (at 4°) until otherwise stated.
Lipid peroxidation and antioxidant enzymes
The stored treated samples from all the above mentioned groups were analyzed for its oxidative stress status as follows. The incubated samples (IFM and SSM) were mixed with thiobarbituric acid-trichloro acetic acid (TBA-TCA) reagent. The assay mixture was heated for 45 min at 100°. The absorbance was read at 532 nm. Thiobarbituric acid reactive substances (TBARS) assay is performed to determine the level of lipid peroxides in the incubated samples (IFM and SSM). The results were expressed in μM MDA/mg protein. The concentration of TBARS in the heart mitochondrial fraction was estimated by the method of Fraga et al.
The activity of glutathione peroxidase (GPx) in the heart mitochondrial fraction was assayed by the method of Rotruck et al. The assay mixture contained 0.4 M sodium phosphate buffer (pH 7.4), 10 mM sodium azide, 4 mM reduced glutathione, 2.5 mM H2O2 and an aliquot of incubated samples. The samples were then incubated at room temperature for 10 min. To the reaction mixture, 10% TCA was added and centrifuged at 5000×g for 3 min. The pellet was discarded and to the supernatant 0.04% DTNB in 1% sodium citrate was added. The absorbance was read at 412 nm. The results were expressed in U/mg of protein.
For the activity of reduced glutathione (GSH), incubated samples were precipitated with 5% TCA and centrifuged at 3500×g for 30 min. To the supernatant, 20 mM phosphate buffer and 0.04% DTNB (5,5’-dithiobis(2-nitrobenzoic acid)) in 1% sodium citrate were added and read the absorbance at 412 nm. The results were expressed in μM of GSH/ mg protein. The level of glutathione (GSH) in the heart mitochondrial fraction was estimated by the method of Ellman.
Superoxide dismutase (SOD) was estimated by the following method. The assay mixture contained 45 mM Tris base, 1 mM EDTA (pH: 8.5), 2.5 mM pyrogallol and sample. The absorbance was read at 420 nm. The results were expressed in U/mg protien. SOD activity was measured by the method of Teare et al. with little modification (measured in presence of sodium cyanide, an inhibitor of cytosolic SOD).
For catalase, the incubated samples were mixed with 0.3% H2O2, 0.0067 M phosphate buffer, 5 N H2SO4, 0.005 M KMnO4. The absorbance was read at 515 nm as described by Baudhin et al.
Estimation of mitochondrial enzymes
NADH dehydrogenase activity was determined according to Minakami et al. [16]. In brief, the reaction mixture contained 100 mM phosphate buffer of pH 8, 0.03 M potassium ferrocyanide was prepared. The temperature of the reaction mixture was brought down to 25° and 0.1% NADH was added and the fluorescent intensity was read at 340/470 nm. The activity of NADH dehydrogenase was expressed as U/mg protein.
Malate dehydrogenase activity was measured according to the method described Mehler et al. [17]. The reaction mixture contained 100 mM of phosphate buffer of pH 7.4 with 0.5 mM of oxalic acid. The reaction was initiated by the addition of 0.15 mM of NADH and the absorbance was measured at 340 nm for 10 min. The activity of malate dehydrogenase was expressed as U/ mg protein.
Succinate dehydrogenase (SDH) activity was estimated according to the method of Slater et al [18]. The reaction mixture contained 0.3 M phosphate buffer of pH 8, 0.03 M EDTA, 10 mM sodium succinate, 5 mM potassium ferricyanide, BSA (1 mg/ml) and sodium azide (3 mM). Absorbance was measured at 455 nm for 10 min and the activity of SDH was expressed as U/mg protein.
Mitochondrial swelling
Ca2+-induced swelling was used to assess the opening of the mitochondrial permeability transition pore [19]. Mitochondria were incubated at 30° in buffer containing 125 mM sucrose, 50 mM KCl, 5 mM 2- [4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), 2 mM KH2PO4 and 1 mM MgCl2 to assess non-energized swelling. The absorbance was recorded at 540 nm and reported after 8 min interval.
Statistical analysis
Each experiment (n=6) was carried out in triplicate and all the values were represented as mean±SD. One way ANOVA was used to compare between groups, at various time points during the experiment followed by multiple comparison tests (Dunnet’s test) using Graph Pad Prism software version 5.0. A probability value of P<0.05 was considered to be statistically significant.
Results and Discussion
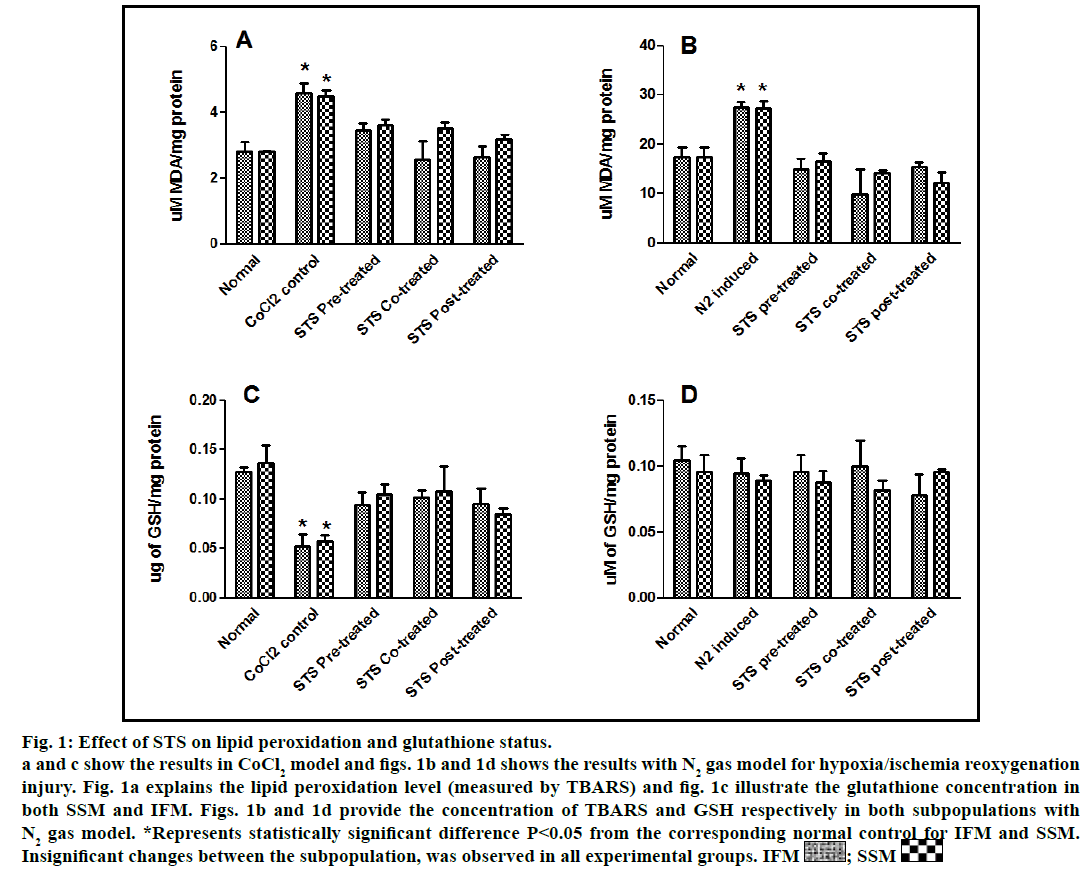
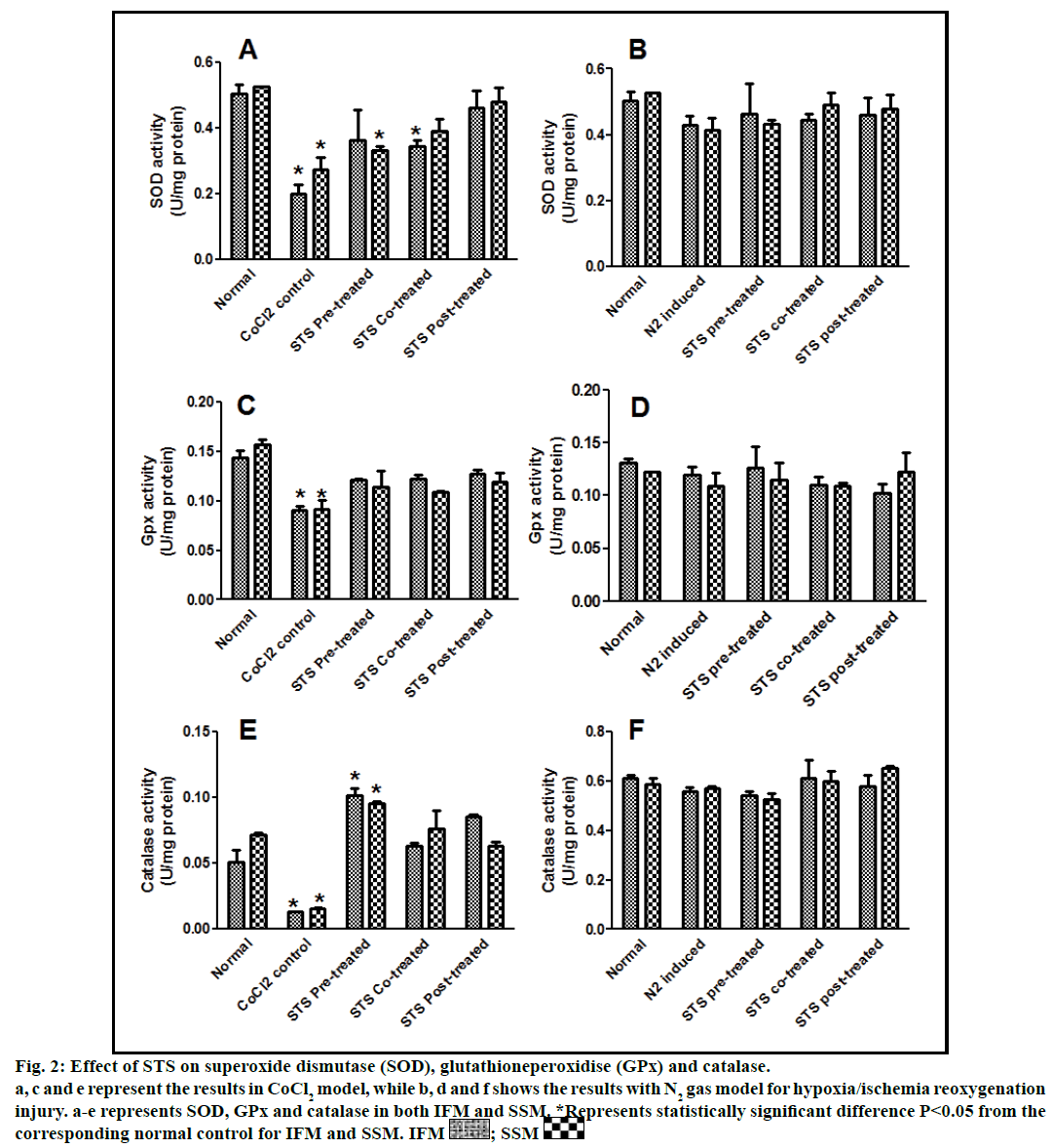
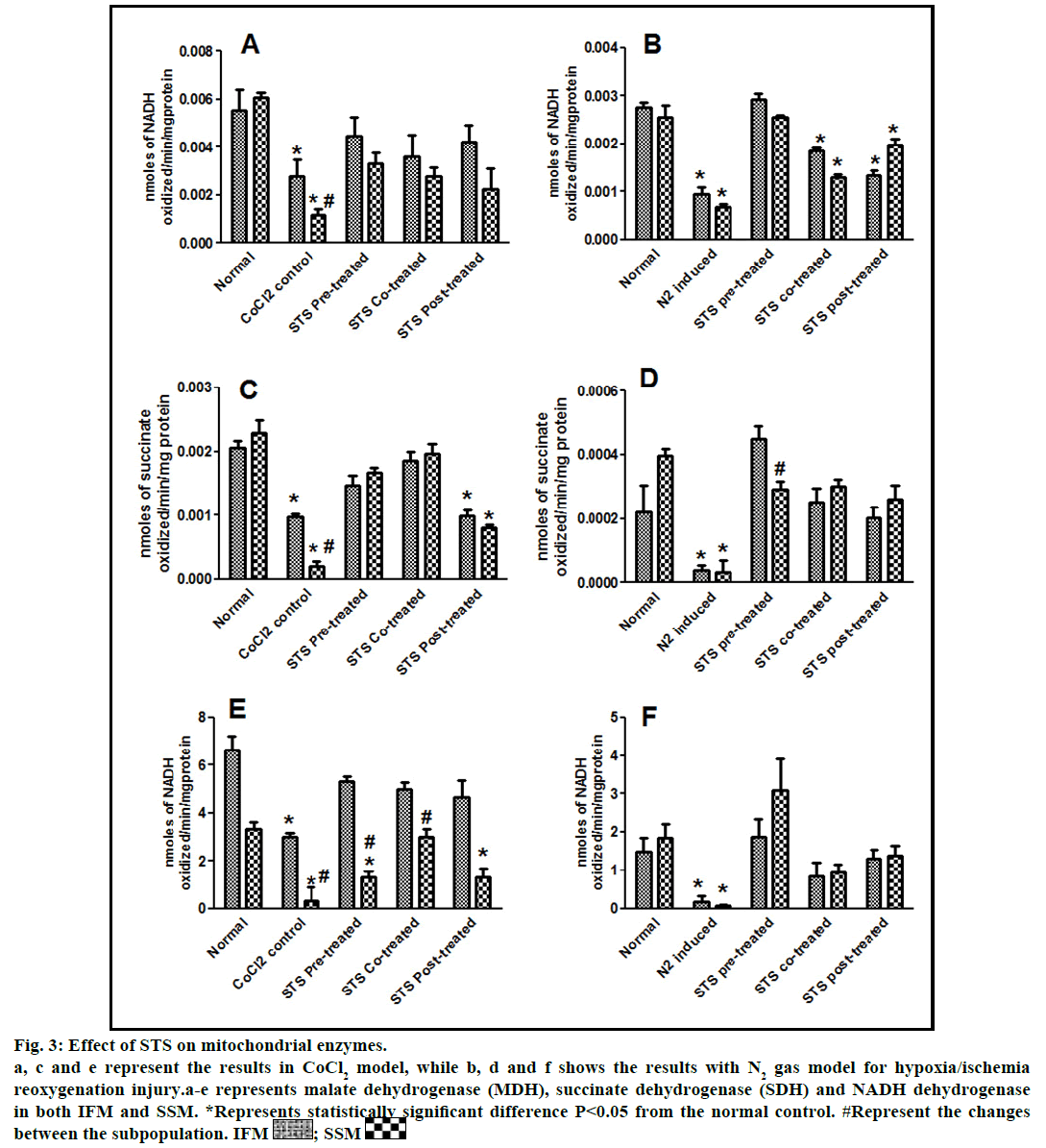
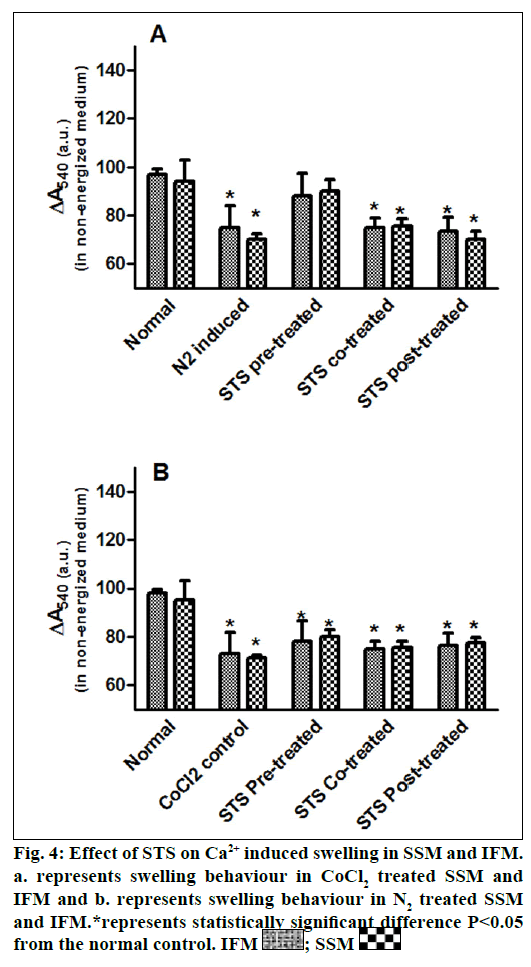
In the present study, we made a comparative assessment between the two hypoxia/ischemiareoxygenation models (CoCl2 and N2 gas) in isolated cardiac mitochondria to induce the dysfunction, one of main characteristic features of I/R injury. Further, we evaluated the effect of STS in these models to identify its ability to prevent mitochondrial dysfunction. The oxidative damage of mitochondrial lipids was measured by TBARS and its elevated level (56-63%, figs. 1a and 1b) and subsequent decline in GSH levels (6-59%, figs. 1c and 1d) in both IFM and SSM, compared to the normal mitochondrial samples, emphasize the efficiency of the two existing models in inducing oxidative stress. In addition, enzymatic antioxidant defence system in the mitochondria was evaluated. Fig. 2 shows the activity of antioxidant enzymes like SOD, catalase and GPx in both IFM and SSM. The activity of these enzymes were significantly declined by 48, 78, 42% (in SSM) and 60, 74, 36% (in IFM), respectively in CoCl2-induced groups whereas in N2 gas model, SSM showed 21.4, 1.7, 10.6% decline and IFM exhibited 14.8, 9.8, 9.1% decrease in SOD, catalase, GPx activities, respectively, as compared with the normal group. In order to investigate mitochondrial function, its characteristic enzymes like NADH, SDH and MDH were measured and are represented in fig. 3. Compared to the normal mitochondria, the subpopulations subjected to CoCl2 and N2 gas treatment provides significant (P<0.05) impairment in mitochondrial function. Mitochondrial swelling, an indicator of the opening of the mitochondrial permeability transition pore, is a hallmark of mitochondrial dysfunction, was found to be elevated in both experimental models (fig. 4).
Fig. 1: Effect of STS on lipid peroxidation and glutathione status.
a and c show the results in CoCl2 model and figs. 1b and 1d shows the results with N2 gas model for hypoxia/ischemia reoxygenation
injury. Fig. 1a explains the lipid peroxidation level (measured by TBARS) and fig. 1c illustrate the glutathione concentration in
both SSM and IFM. Figs. 1b and 1d provide the concentration of TBARS and GSH respectively in both subpopulations with
N2 gas model. *Represents statistically significant difference P<0.05 from the corresponding normal control for IFM and SSM.
Insignificant changes between the subpopulation, was observed in all experimental groups. IFM  ; SSM
; SSM 
Fig. 2: Effect of STS on superoxide dismutase (SOD), glutathioneperoxidise (GPx) and catalase.
a, c and e represent the results in CoCl2 model, while b, d and f shows the results with N2 gas model for hypoxia/ischemia reoxygenation
injury. a-e represents SOD, GPx and catalase in both IFM and SSM. *Represents statistically significant difference P<0.05 from the
corresponding normal control for IFM and SSM. IFM  ; SSM
; SSM 
Fig. 3: Effect of STS on mitochondrial enzymes.
a, c and e represent the results in CoCl2 model, while b, d and f shows the results with N2 gas model for hypoxia/ischemia
reoxygenation injury.a-e represents malate dehydrogenase (MDH), succinate dehydrogenase (SDH) and NADH dehydrogenase
in both IFM and SSM. *Represents statistically significant difference P<0.05 from the normal control. #Represent the changes
between the subpopulation. IFM  ; SSM
; SSM 
STS is considered to be readily accessible source of hydrogen sulfide [20] in the mitochondria (the site for its metabolism), where the latter reported to preserve the activities of SSM and IFM during (I/R) injury [21]. By utilizing both experimental models, preconditioning effect of STS on the mitochondria were studied and the results are depicted in figs. 1 and 2. On comparison with CoCl2 induced group, STS pretreatment significantly (P<0.05) improved the antioxidant enzymes (SOD: IFM 45%, SSM 18%, GPx: IFM 25%, SSM 20% and catalase: IFM 87%, SSM 84%, fig. 1) and decreased the lipid peroxidation (TBARS: IFM 22%, SSM 19%, fig. 2), whereas in N2 gas model, no significant changes were observed. Indeed, STS significantly (P<0.05) recovered the mitochondrial enzymes activity in N2 gas model (MDH: IFM 69%, SSM 76% and SDH: IFM 91%, SSM 89%) as well as in CoCl2 model (MDH: IFM 39%, SSM 67% and SDH: IFM 36%, SSM 87%, fig. 3) and swelling behavior in N2 gas model, but not with CoCl2 model (fig. 4).
STS cotreatment significantly improved the impaired antioxidant enzyme activities (fig. 2) in both the models.
In fact, the improvement in antioxidant enzymes was prominent in CoCl2 induced groups than N2 gas model. However, with respect to mitochondrial enzymes, both models showed similar pattern of changes, but major recovery was observed with N2 gas model. In addition, mitochondrial enzymes (fig. 3) were also recovered by around 9 fold (NADH), 9.3 fold (SDH) in SSM and 1.6 fold (NADH), 1.8 fold (SDH) in IFM, as compared to N2 gas induced control with no significant change in CoCl2 treated groups.
STS treatment given after the induction of mitochondrial dysfunction in IFM and SSM by both experimental models was used to assess the efficacy of the drug as post-conditioning agent. Post hypoxia/ischemiareoxygenation STS treatment exhibits improvement in antioxidants as well as mitochondrial enzyme activities (SOD 40%, catalase 75%, MDH 35%) in CoCl2 model and (SOD 6%, catalase 4%, MDH 27%) in N2 gas model (figs. 2 and 3).
Mitochondrial dysfunction is the key player in the pathogenesis of myocardial ischemia reperfusion injury that is implicated in different coronary interventions like coronary artery bypass grafting (CABG), percutaneous transluminal coronary angioplasty (PTCA) [22]. Recent advancements in cardiovascular research underlines the importance of mitochondria as a therapeutic targets, due to the fact that it remains as the major site for various cardioprotective converging signaling pathways. Hence, the toxic evaluation of cardioprotective drugs with respect to mitochondria is becoming a necessity to prevent mitochondriabased pathologies. In this regards, we isolate cardiac mitochondria, which comprises of IFM and SSM, subjected them to hypoxia/ischemia- reoxygenation by two methods namely, by cobalt chloride induction and by purging N2. Our results suggest that both models are efficient in inducing oxidative stress in mitochondria, despite of having distinct mechanism of action. But mitochondrial functional and morphological changes were prominent with N2 gas model, emphasizes that significance of using the latter model for hypoxia or ischemia reperfusion studies. Furthermore, we used these models to assess the efficacy of STS as a therapeutic drug to prevent mitochondrial dysfunction associated with hypoxia/ischemia reperfusion injury and the results substantiate the protective effect of STS to preserve mitochondrial function. In fact, we could not find any significant changes between the functional activities among the subpopulations, as identified by our group [15] as well as by others [5] in isolated rat heart model.
CoCl2 is known to increase ROS by acting on mitochondrial transition pore [23] and few other studies have shown that it can activate cell death pathway [24] that occur in mitochondria. In the present study, CoCl2 incubation with isolated IFM and SSM elevated oxidative stress and deteriorated the function of the cell organelle (fig. 3). The declined mitochondrial function in presence of CoCl2 may be due to the collapse of mitochondrial transmembrane potential (ΔΨ M) (measured by increased swelling behaviour (fig. 4), thereby leads to the generation of ROS [25]. Furthermore, CoCl2 can interact with HIF-1α, and prevents its degradation by prolyl hydroxylase, thereby mimetic hypoxic condition [26] as well. The early studies have shown that cells devoid of mitochondrial DNA (mtDNA), are unable to activate HIF-α in hypoxic conditions, emphasizes the involvement of mitochondria in HIF response [27].
The oxygen deprivation (anoxia/hypoxia) within mitochondria can induce Ca2+ overload and ROS generation that leads to mitochondrial dysfunction [28]. Further, the anoxia can exhibit profound decline in complex V (F0F1-ATPase synthase) activity in the mitochondria and can alter respiration rate and even depolarize the mitochondrial membrane potential [29]. Isolated mitochondria purged with N2 gas were used to induce mitochondrial dysfunction. According to van Deripe, mitochondria can takes up nitrogen gas, which coalesce to fill the intra mitochondrial space during hypoxia that mediates the inability of mitochondria to uptake oxygen resulting in cell death [30].
Mitochondrial dysfunction-elicited ROS production axis forms a vicious cycle in myocardial ischemia reperfusion injury and the mitochondria targeting drug development is a promising new therapeutic approach to treat ischemia reperfusion. Thiosulfate is an endogenous product from the oxidative metabolism of hydrogen sulphide in mitochondria is reported as not only a reducing and antioxidant agent involved in scavenging ROS but also can activate the gene expression of antioxidant enzymes [31]. Recently, its cardioprotective effect was documented through its ability to release hydrogen sulfide in the mitochondria [12]. CoCl2 is effective in inducing ROS via mitochondria [32] and our results with STS on this model demonstrate that the drug has the potential to scavenge ROS effectively, when it was given along with cobalt chloride. Interestingly, the absence of difference between the subpopulation in the ROS scavenging ability emphasizes the relationship between the functional difference of IFM and SSM with respect to their location. This assumption is based on the previous in vivo study report on the distinct response of IFM and SSM towards oxidative stress is due to its distinct location in the myocardium [33]. In accordance to this report, few studies describes age related alteration in antioxidant capacity of IFM [34] with marked loss in glutaredoxin and GSSG reductase activities, suggesting mitochondrial deterioration may not be uniform. In line of this findings, our results in isolated IFM and SSM suggest that the inherent antioxidant capacity of both the subpopulations are similar and the distinct effect between the subpopulations in vivo substantiate the specific role of mitochondria in an intact system. The insignificant antioxidant enzymes changes in IFM and SSM with different modes of STS treatment emphasizes the prerequisite of tissue/organ architecture for the conditioning effect of STS on mitochondria. Hence, these models may not be suitable to study the cardioprotective redox signaling pathway that converges in mitochondria.
The catabolic pathway of thiosulfate is localized in the mitochondria, where sulfide: quinine oxidoreductase, a primary enzyme in its metabolism, bound to the inner mitochondrial membrane transfers electrons to the ubiquinone pool. Further oxidative reaction by sulfur dioxygenase is also present in the mitochondrial matrix, emphasizes the probable interaction of STS with the mitochondria [35]. Importantly, thiosulfate can modulate K+ sensitive ATP channel [36] in the mitochondria either by the release of H2S or by itself, signify the involvement of STS on mitochondrial permeability transition, where the latter play a key role in cell death. The mitochondria can act as oxygen sensor that can coupled to a signal transduction system, which in turn activates the functional response [37]. Thus the N2 gas model to induce hypoxia/ischemia reperfusion will be effective in determining the mitochondrial functional response. According to our results, mitochondria conditioned with STS prior to ischemia/hypoxia insult impart significant mitochondrial functional modulation that reflects in maintaining the mitochondrial enzyme activities and swelling behavior as compared to the normal control than conditioning (co-treatment or post-treatment) modality of drug (fig. 4). Considering the multifaceted signaling pathways involved in both pre- and post-treatment of STS, the major limitation of this model is the non-involvement of mitochondria targeted nuclear control.
The present study concludes that both CoCl2 and N2 gas purging are effective in inducing oxidative stress associated with hypoxia/ischemiareoxygenation. In fact, N2 gas purging model not only induces oxidative stress but also alters mitochondrial respiratory enzymes, that leads to mitochondrial dysfunction, is more reliable method to study hypoxia/ischemia-reoxygenation in isolated mitochondria. STS, oxidative metabolite of hydrogen sulfide in mitochondria is effective in recovering mitochondrial dysfunction associated with reperfusion injury.
Acknowledgements
The authors sincerely thank the Vice Chancellor, SASTRA University. Also, the authors would like to thank Dr. David Raj for his assistance during animal surgical experiments.
Financial support and sponsorship:
This study was supported by grant from the Department of Science and Technology, New Delhi, India (No. SR/S0/HS-0255/2012), Indian Council of Medical Research, New Delhi (No. 5/4/1-24/2012-NCD-II).
Conflicts of interest:
There are no conflicts of interest.
References
- Tompkins AJ, Burwell LS, Digerness SB, Zaragoza C, Holman WL, Brookes PS. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta 2006;1762:223-31.
- Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 2014;114:524-37.
- Pala FS, Gurkan H. The role of free radicals in ethiopathogenesis of diseases. Adv Mol Biol 2008;1:1-9.
- Lagouge M, Larsson NG. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Inter Med 2013;273:529-43.
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 1977;252:8731-9.
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 2006;91:807-19.
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature 1998;392:405-8.
- Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Vis Exp 2011;54:2899.
- Pereira GC, Silva AM, Diogo CV, Carvalho FS, Monteiro P, Oliveira PJ. Drug-induced cardiac mitochondrial toxicity and protection: from doxorubicin to carvedilol. Curr Pharm Des 2011;17:2113-29.
- Hamel J. A review of acute cyanide poisoning with a treatment update. Crit Care Nurse 2011;31:72-81.
- Yu Z, Gu L, Pang H, Fang Y, Yan H, Fang W. Sodium thiosulfate: an emerging treatment for calciphylaxis in dialysis patients. Case Rep Nephrol Dial 2015;5:77-82.
- Sen U, Vacek TP, Hughes WM, Kumar M, Moshal KS, Tyagi N, et al. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology 2008;82:201-13.
- O’Neill WC, Hardcastle KI. The chemistry of thiosulfate and vascular calcification. Nephrol Dial Transplant 2012;27:521-26.
- Chevion M, Jiang Y, Har-El R, Berenshtein E, Uretzky G, Kitrossky N. Copper and iron are mobilized following myocardial ischemia: possible predictive criteria for tissue injury. Proc Natl Acad Sci USA 1993;90:1102-6.
- Kurian GA, Berenshtein E, Saada A, Chevion M. Rat cardiac mitochondrial sub-population show distinct features of oxidative phosphorylation during ischemia, reperfusion and ischemic preconditioning. Cell Physiol Biochem 2012;30:83-94.
- Minakami S, Ringle RL, Singer TP. Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. J Biol Chem 1962;237:569-76.
- Mehler AH, Kornberg A, Grisolia S, Ochoa S. The enzymatic mechanism of oxidation reductions between malate or isocitrate and pyruvate. J Biol Chem 1948;174:961-77.
- Slater EC, Bonner WD. Effect of fluoride on the succinate oxidase system. Biol Chem 1952;52:185-96.
- Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 1979;195:460-7.
- Kaji A, McElroy WD. Mechanism of hydrogen sulfide formation from thiosulfate. J Bacteriol 1959;77:630-7.
- Ansari SB, Kurian GA. Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chem Biol Interact 2016;252:28-35.
- Montaigne D, Marechal X, Lefebvre P, Modine T, Fayad G, Dehondt H, et al. Mitochondrial dysfunction as an arrhythmogenic substrate: a translational proof-of-concept study in patients with metabolic syndrome in whom post-operative atrial fibrillation develops. J Am Coll Cardiol 2013;62:1466-73
- Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, et al. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 1999;76:725-34.
- Stenger C, Naves T, Verdier M, Ratinaud MH. The cell death response to the ROS inducer, cobalt chloride, in neuroblastoma cell lines according to p53 status. Int J Oncol 2011;39:601-9.
- Suski JM, Lebiedzinkska M, Bonora M, Printon P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potentialand ROS formation. Methods Mol Biol 2012;810:183-205.
- Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1α and AP-1 and iNOS signaling. Am J Physiol Heart Circ Physiol 2004;287:H2369-75.
- Bell EL, Kinova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GRS, et al. The Q0 site of the mitochondrial complex III is required for the transduction of hypoxic signalling via reactive oxygen species production. J Cell Biol 2007;177:1029-36.
- Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 2007;130:548-62.
- Delcamp TJ, Dales C, Ralenkotter L, Cole PS, Hadley RW. Intramitochondrial [Ca2+] and membrane potential in ventricular myocytes exposed to anoxia-reoxygenation. Am J Physiol 1998;275:H484-94.
- VanDeripe DR. The swelling of mitochondria from nitrogen gas; a possible cause of reperfusion damage. Med Hypotheses 2004;62:294-6.
- Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol 2005;4:4.
- Zeno S, Zaaroor M, Leshiner S, Veenman L,Gavish M. CoCl2 induces apoptosis via the 18k Datranslocator protein in U118MG human glioblastoma cells. Biochemistry 2009;48:4652-61.
- Kurian GA, Berenshtein E, Kakhlon O, Chevion M. Energy status determines the distinct biochemical and physiological behavior of interfibrillar and sub-sarcolemmal mitochondria. Biochem Biophys Res Commun 2012;428:376-82.
- Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 2005;19:419-21.
- Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 2008;275:3352-61.
- Baldev N, Sriram R, Prabu PC, Kurian GA. Effect of mitochondrial potassium channel on the renal protection mediated by sodium thiosulfate against ethylene glycol induced nephrolithiasis in rat model. Int Braz J Urol 2015;41:1116-25.
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 1998;95:11715-20.