- *Corresponding Author:
- Salamaa Salama
Department of Zoology, Faculty of Science, Damanhur University, Damanhur, Egypt
E-mail: sasalama@jazanu.edu.sa
| Date of Received | 06 September 2025 |
| Date of Revision | 09 September 2025 |
| Date of Accepted | 10 October 2025 |
| Indian J Pharm Sci 2025;87(5):187-196 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The Culex tritaeniorhynchus mosquito is the primary vector of Japanese encephalitis virus which can cause encephalitis in human and mammals. This mosquito also can transmit other diseases such as filariasis and West Nile virus. It has a wide global distribution. Therefore, it was a necessary to eliminate this insect to reduce the infections. Through this study, we evaluated the effect of methanolic and ethyl acetate extracts of Tarchonanthus camphoratus L leaves on different stages of Culex tritaeniorhynchus mosquito using five concentrations ranging from 200 ppm to 1000 ppm. Both methanol and ethyl acetate extracts completely eliminated larva by 100 % at the concentration of 1000 ppm. At the concentration of 750 ppm, methanolic extract eliminated larva by 73 %±3.00 %, pupa by 80 %±1.00 % and adult by 80 %±3.00 % whereas ethyl acetate extract eliminated larva by 72 %±3.00 %, pupa by70 %±5.00 % and adult by 62 %±4.00 %. The LC50 value of methanol and ethyl acetate extracts against larva mortality was 519.95 (ppm) and 454.94 (ppm) respectively. The results showed that both extracts had significant lethal toxic effects on the life cycle of Culex tritaeniorhynchus. However, the methanol leaves extract had more effective than ethyl acetate leaves extract. In this study, we suggested that Tarchonanthus camphoratus plant could be used as a natural insecticide for combat and control mosquitoes compared to harmful chemical insecticides.
Keywords
Methanol, Ethyl acetate, Tarchonanthus camphoratus leaves, larvae, pupae, adult emergence, Culex tritaeniorhynchus mosquito
The mosquito, Culex tritaeniorhynchus (C. tritaeniorhynchus) is one genera of the Culicidae family which includes various types of medical and environmental significance. It is considered as the primary vector of Japanese Encephalitis Virus (JEV)[1-3]. This insect has been spread over the world and is the source of many diseases that affect human and animal. C. tritaeniorhynchus is detected in at least 41 countries, across 5 different continents[4]. It can transmit many diseases such as West Nile virus which cause dangerous neurological disease, Japanese encephalitis disease which affects the central nervous system and lymphatic filariasis disease[5,6] which cause tissue enlargement due to Wuchereria bancrofti parasite[7]. Thus, there is a critical necessity to combat and control this mosquito to prevent diseases outbreak. The use of insecticides and the nonstop use this harmful materials for mosquitoes control can affect the environment and human health. These chemicals can disrupts natural adversaries and outbreaks of some species of insect[8]. Also the high cost and development of resistance of mosquito species to chemical insecticides led to exploiting a biological control using plants and their derivatives against insects[9]. More than 2000 species of plants have toxic effects to several insect species without harmful to environment[10]. A number of plant derivatives are found to be effective against mosquitoes with safe manner. Tarchonanthus camphoratus (T. camphoratus) is a rare shrub has more than 6 meters in height with grey color and distributes in a number of habitats including Saudi Arabia, East and South Africa[11]. The scented tree, T. camphoratus has various medicinal applications in traditional healing by smoking of burned leaves and drinking infusion or decoctions. The infusions and tinctures of its leaves used for many diseases such as asthma, inflammation, rheumatism, bronchitis, heartburn, stomach trouble, toothache, headache, cough, indigestion, cerebral hemorrhage and paralysis[12-15]. This plant has powerful insect repellent action[16]. The rub of T. camphoratus leaves by wild animal used to rid them from mosquitoes and flies. The study of the environmental interactions between insects and plants is a vital area for understanding the ecosystems and developing sustainable strategies to address environmental and health challenges. The current study aimed to explore the relationship between the C. tritaeniorhynchus mosquito and T. camphoratus L tree with an emphasis on the chemical properties of plant and its impact on the behaviour of mosquito. Our study investigated the effect of methanolic and ethyl acetate extracts of T. camphoratus leaves against various stages of C. tritaeniorhynchus. We found that these extracts had inhibition effects on larva, pupa, adult emergence and adult of C. tritaeniorhynchus mosquito. This manuscript provided a scientific insight into the possibility to using the T. camphoratus plant extract as effective and safe way to control and combat the breeding of mosquitoes which can contributes to reducing transmission of diseases and achieving environmental sustainability.
Materials and Methods
C. tritaeniorhynchus Larvae were obtained from the Vector-Borne Diseases Centre in Jazan. They were bred in the lab. For six generations in Department of Biology, Faculty of Sciences, Jazan University under controlled conditions of (27°±2°), RH (70 %-80 %) and (12-12) light-dark. Adult mosquitoes were kept in (30×30×30 cm) wooden cages. After emergence, the adult was daily provided with cotton pieces soaked in 10.0 % sucrose solution for 3 d. Then, the female mosquitoes were allowed to take a blood meal from a pigeon host, which is necessary for laying the eggs. To facilitate eggs laying, a plastic cup oviposition (15×15 cm) containing dechlorinated tap water was placed in the cage. After that, the egg clusters were picked up from the plastic cup and transferred into plastic pans (25×30×15 cm) has 3 l of tap water and leftover for 24 h. Daily the emerged larvae were supplied.
Plant collection and preparation of plant extract: In the blooming season (April 2024), leaves of T. camphoratus L (Family: Asteraceae) (Fig. 1) were collected at Al-Hashr Mountain in the Jazan region of Saudi Arabia (17°26′34.80′′ N, 43°2′34.79′′ E). The plant was recognized at Biology Department, faculty of Sciences, Jazan University, Saudi Arabia, and added in the herbarium collection according to the guidelines of Tackholm (1974) and Boulos (1995)[17,18]. The collected leaves were washed and dried in the shade at room temperature (27°-31°) for 10 d up to become a brittle texture. Then they were grounded to a powder using a hammer mill. For extraction, two solvents used, methanol and ethyl acetate. A 100 g of leaves powder were extracted with methanol and ethyl acetate separately three times with 300 ml at room temperature. After 24 h of extraction, the supernatant was aspirated and filtered through Whatman filter paper No. 5. Then the filtered supernatant was concentrated in a rotary evaporator at 40°. Finally we got 12.9 g and 9.1 g of semi-solid crude leaves extracts. Finally, the prepared extracts were stored in a deep freezer
Larval toxicity test: To investigate extracts toxicity, two drops of Tween 80 was added to the extracted crude to make an emulsifier. Different concentrations of methanol and ethyl acetate leaves extracts were prepared (1000 ppm, 750 ppm, 500 ppm, 400 ppm, and 200 ppm). A 30 late 3rd instar larvae were used and put in 300 ml plastic cups containing 200 ml of dechlorinated tap water and they maintained until the emergence of adults optimized conditions of 27°±2°, 70 %±10 % RH, and 12-12 LD. To control larvae, two drops of Tween80 were added in 100 ml water. Every day, mortality was recorded, and the dead larvae and pupae were collected. All experiments were done in triplicates.
Experimental bioassay: Larval mortality was indicated by a failure to respond to mechanical stimulation[19]. Larval mortality percent was estimated by using the following equation[20]: Larval mortality %=A- B/A×100 (Where: A=number of tested larvae. B=number of resulted pupa). Percentage of pupation was estimated by using the following equation: Pupation %=A/B×100 (Where: A=number of resultant pupae. B=number of tested larvae).Pupal mortality was observed by a failure to developing to adult stage. The pupal mortality percent was determined using the following equation: Pupal mortality %=A/B×100 (Where: A=number of dead pupae. B=number of resulted pupae. For adult emergence: The males and females of adults emergence had counted and the adult emergence percent was calculated using this equation: Adult emergence %=A/B×100 (Where: A=number of emerged adults. B=number of resulted pupae).
LC50 and LC90 determination: The LC50 value which represents the lethal concentration for 50 % larval mortality and the LC90 value which exhibits the lethal concentration for 90 % larval mortality were calculated through linear regression analysis using Microsoft Excel.
Statistical analysis: Data are represented as mean±Standard Deviation (SD). Statistical analysis of the data was carried out using One-way analysis of variance Analysis of Variance (ANOVA) was applied to find the differences between the activity of tested extracts using Tucky’s test at 0.005 probability level, where means with p>0.05 are not statistically significant. Statistical analysis was carried out using SPSS software.
Results and Discussion
The biological activity of methanolic and ethyl acetate leaves extracts of Trachnoanthus Camphoratus at various concentrations (1000 ppm, 750 ppm, 500 ppm, 400 ppm, 200 ppm) had toxic effects against different stages of C. tritaeniorhynchus mosquito. From Table 1 showed that the methanolic extract had a maximum larvicidal effect on larva stage at a concentration of 1000 ppm where 100 % of mortality was observed (Table 1 and Fig. 2).
| Concs. ppm | Larval mortality % | Larval duration/days | Pupation % | Pupal mortality % | Adult emergence % | Adult mortality % |
|---|---|---|---|---|---|---|
| Methanol leaves extract | ||||||
| 1000 | 100±0.00 | 3.33±1.00 | ------ | ------ | ----- | ----- |
| 750 | 73±3.00 | 4.33±1.00 | 27±2.00 | 80±1.00 | 20±1.00 | 80±3.00 |
| 500 | 53±1.00 | 5.33±1.00 | 47±2.00 | 57±2.00 | 43±3.00 | 67±2.00 |
| 400 | 37±1.00 | 5.67±1.00 | 63±4.00 | 19±1.00 | 81±1.00 | 21±1.00 |
| 200 | 10±1.00 | 5.67±1.00 | 90±3.00 | 12±2.00 | 88±2.00 | 18±1.00 |
| Control | 0 | 5.00±1.00 | 100±0.00 | 0 | 100±0.00 | 0 |
| Ethyl acetate leaves extract | ||||||
| 1000 | 100±0.00 | 4.33±1.00 | ------ | ----- | ------ | ------ |
| 750 | 72±3.00 | 5.33±1.00 | 28±3.00 | 70±5.00 | 30±5.00 | 62±4.00 |
| 500 | 58±3.00 | 5.33±1.00 | 42±3.00 | 48±3.00 | 52±5.00 | 40±5.00 |
| 400 | 49±2.00 | 5.67±1.00 | 51±3.00 | 28±4.00 | 72±7.00 | 27±6.00 |
| 200 | 32±3.00 | 5.67±1.00 | 68±3.00 | 16±1.00 | 84±5.00 | 11±2.00 |
| Control | 0 | 5.00±0.00 | 100±0.00 | 0 | 100±0.00 | 0 |
Table 1: The Biological Effect of Methanolic and Ethyl Acetate Leaves Extract of t. Camphoratus Leaves on Different Stages of Culex Tritaeniorhynchus Mosquito
At 750 ppm, 500 ppm, and 400 ppm, the percent of larval mortality reached 73±3.00 %, 53±1.00 %, 37±2.00 % respectively (Table 1 and Fig. 2). While at low concentration (200 ppm) the larva mortality was 10±1.00 % compared to control (Table 1 and Fig. 2).
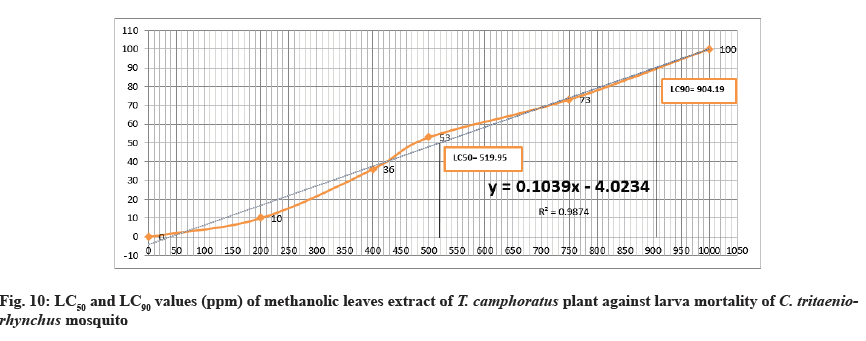
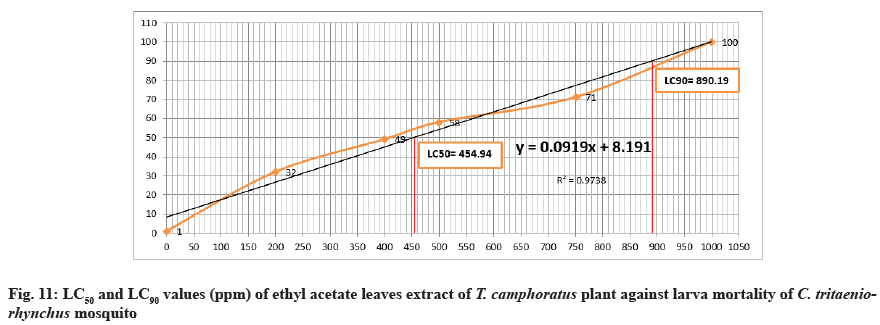
The LC50 value against larva mortality was 519.95 (ppm) while LC90 was 904.19 (ppm) (Table 1 and Fig. 2-Fig. 12) this represented a significant toxicity of methanol extract. Duration of larva was 3.33±1.00 d at concentration of 1000 ppm while at concentration 750 ppm was 4.33±1.00 d compared to control (5.00±1.00 d) (Table 1). At the concentration of 750 ppm, the pupation percent was 27±2.00 %.
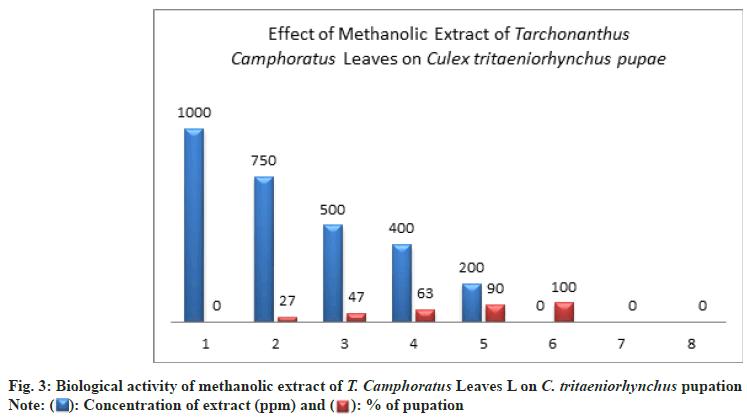
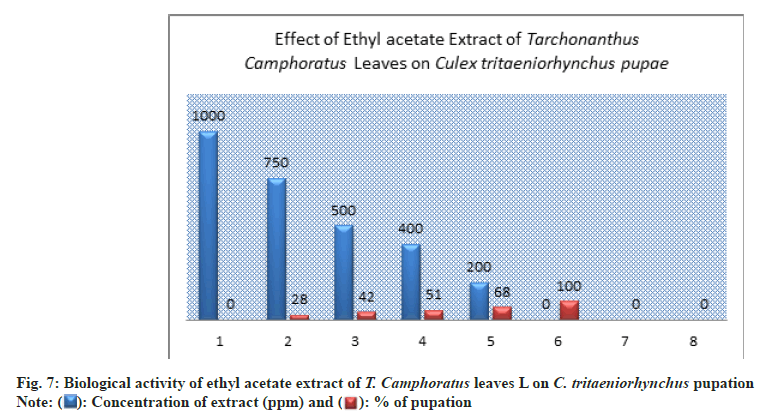
The higher percentage of pupation showed at low concentration of 200 ppm with 90±3.00 % compared to the control (100±0.00 %.) (Table 1 and Fig. 3).
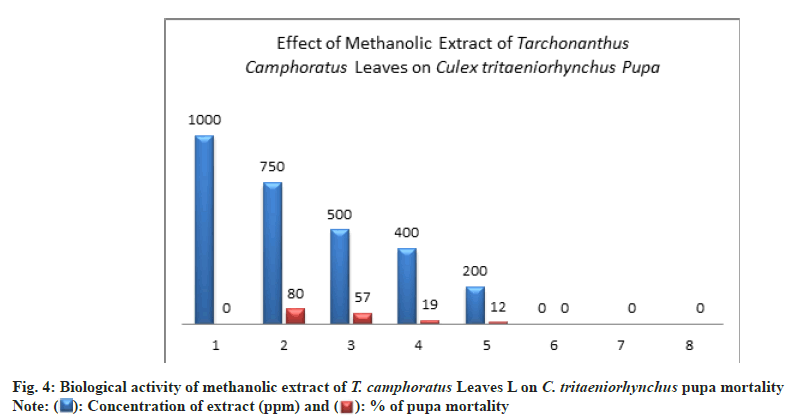
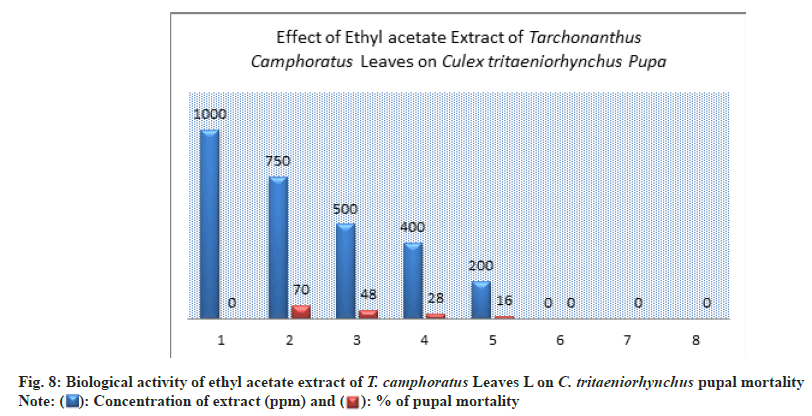
Methanol extract had a toxic effect on pupae life where the pupal mortality was 80±0.00 % at concentration of 750 ppm compared to untreated pupae (Table 1 and Fig. 4).
At the concentrations of 500 ppm, the pupal mortality percent was exceed 50 % by 57±2.00 % this indicated to a significant toxicity of extract (Table 1 and Fig. 4).
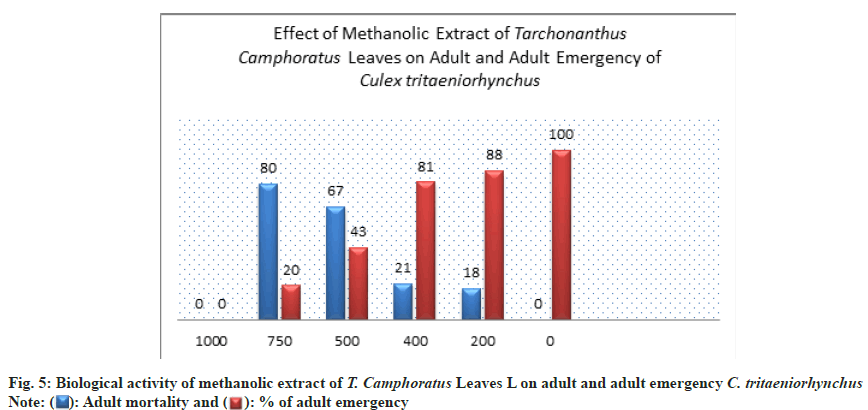
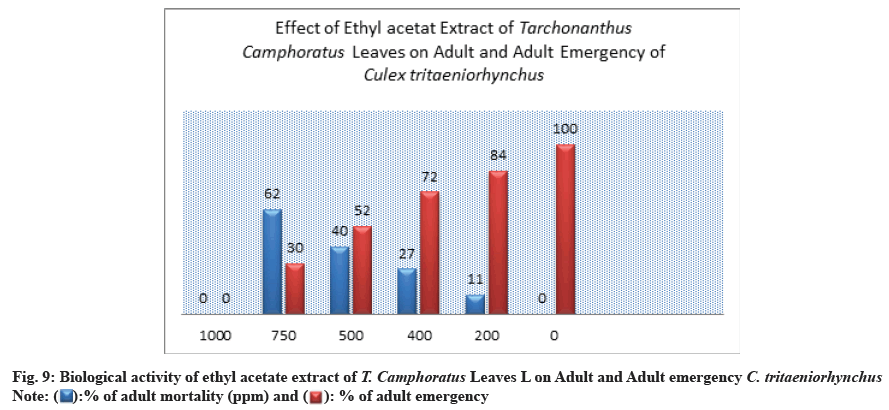
The effect of methanolic extract on adult emergence and adult mortality was recorded, where at 750 ppm the percent of adult emergence was 20±1.00 % whereas at low concentration 200 ppm, the percentage of adult emergence increased to 88±2.00 % (Table 1 and Fig. 5).
The highest mortality percent of adult mosquito was showed at concentration of 750 ppm by 80±0.00 % (Table 1 and Fig. 5). A 50 % of adult mortality was exceed at concentration of 500 ppm by 67% this appeared a significant toxicity of methanol extract (Table 1 and Fig. 5).
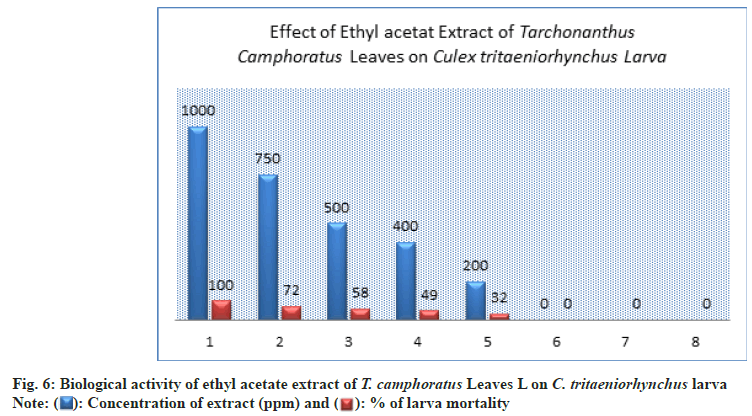
The prepared concentrations showed variations toxic activity against different stages of C. tritaeniorhynchus. The maximum larva mortality was found at the concentration of 1000 ppm by 100±0.00 % with duration of 4.33±1.00 d (Table 1 and fig. 6). At 200 ppm the larva mortality reached to 32±3.00 % with duration of 5.67±1.00 d. At 750 ppm the percent of larva mortality was 72±2.00 %. At the concentration of 500 ppm a 50% of larva mortality was exceed by 58±3.00% this appeared a significant toxicity of extract (Table 1 and Figure 6). The value of LC50 against larva mortality was 454.94 (ppm) whereas the value of LC90 was 890.19 (ppm) (Table 1 and fig. 11-fig. 12B). The higher percent of pupation was observed at low concentration with 68±3.00 % compared to control (100±0.00%) (Table 1 and fig. 7). The ethyl acetate extract also represented a lethal toxicity on pupae life, where the highest percent of pupal mortality was showed at 750 ppm with 70±3.00 % (Table 1 and fig. 8). At 500 ppm the pupal mortality had 48±3.00 % did not exceed the 50 % this indicted that there is not significant toxicity found at this concentration against pupae life (Table 1 and fig. 8). The Effect activity of ethyl acetate extract on adult emergency and adult mortality also was reported. A 84±5.00 % of adult emergency was showed at low concentration of 200 ppm compared to control (100±0.00 %) but at high concentration, 750 ppm the adult emergency percent reduced to 30±3.00 % (Table 1 and fig. 9). A 62±4.00 % of adult mortality was observed at high concentration of 750 ppm and at 500 ppm, the adult mortality was 40±5.00 % this value did not exceed the 50 %, this indicated that ethyl extract at this concentration had not significant toxicity against adult mosquito (Table 1 and fig. 9).
Many diseases that infect humans and animals can be transmitted by mosquitoes. C. tritaeniorhynchus mosquito is one type of mosquitoes which can transmits several diseases such as West Nile fever, Japanese encephalitis, and Filariasis[5-7]. These diseases can affect humans health and cause morbidity and mortality. To prevent transmission of such diseases, many chemical insecticides used to control breeding of mosquitoes around the word. But these insecticides can affect environment, animal and humans and create undesirable side effects by continuous using. In recent years, the researchers tend to find a new alternatives of insecticides are harmless and safe to humans and environment. During the past three decades, the manners for controlling insects have been directed to use insecticides from plant origin because they are safe and friendly to environment, human, and animals. A number of plants had been effected against mosquitoes with safe manner. T. camphoratus is one type of medicinal plant has various medicinal applications in traditional healing. It used for many diseases such as asthma, inflammation, rheumatism, bronchitis, heartburn, stomach trouble, toothache, headache, cough, indigestion, cerebral hemorrhage and paralysis[12-15]. Roark (1947) described around 1200 plant species listed and discussed 344 plant species that exhibited mosquitocidal activity[21]. T. camphoratus exhibits powerful insect repellent action. The life cycle of mosquito starts with laying of its eggs in the water, the eggs hatch and develop to larvae, then to pupae and adult emergence and finally become adult mosquito after that fly out to air. In this study, both the methanol and ethyl acetate extracts of T. camphoratus leaves against multiple stages of C. tritaeniorhynchus had lethal toxic efficacy. Both the extracts had highest mortality rate against larvae at concentration of 1000 ppm with 100 % (Table 1 and fig. 2 and fig. 6). At 1000 ppm concentration, the pupae, adult emergency and adult were disappeared as a result of complete death of larva. At the concentration of 750 ppm, the methanol extract was more effective against larvae, pupae and adult of mosquito than ethyl acetate extract by percentages of 73±3.00, 80±1.00, and 80±3.00 respectively (Table 1 and fig. 2-fig. 5). At 750 ppm of methanol extract, the adult emergency was reduced to 20±1.00% (Table 1 and fig. 5) compared to ethyl acetate extract effect (Table 1 and fig. 9). The time duration of larva life at concentration of 1000 ppm and 750 ppm of methanol extract had short time compared to ethyl extract (Table 1). At 500 ppm of methanol extract effect, percent of mortality against larva, pupa and adult was exceed 50% (Table 1 and fig. 2 and fig. 4, 5) than ethyl acetate extract effect (Table 1 and fig. 6 and fig. 8, 9). The rate of pupation was increased gradually at the concentrations, 750 ppm, 500 ppm, 400 ppm, and 200 ppm due to decreasing in mortality percent of larvae in both effects of methanol and ethyl acetate extracts (Table 1 and fig. 3 and fig. 7). The values of LC50 and LC90 for both extracts against larva mortality were approximately similar in effect (Table 2 and fig. 10-fig. 12B). Lethal concentration (LC) 50 % of methanolic extract for kill 50 % of larva is needed at concentration of 519.95 (ppm) and Lethal concentration (LC) 90% for kill 90% of larva is needed at 904.19 (ppm) (Table 2 and fig. 11-fig. 12B).
| Extract | LC50 | LC90 |
|---|---|---|
| Methanol | 519.95 (ppm) | 904.19 (ppm) |
| Ethyl acetate | 454.94 (ppm) | 890.19 (ppm) |
Table 2: LC50 and LC90 values (ppm) of methanolic and ethyl acetate extracts of T. Camphoratus leaves against larva mortality of C. Tritaeniorhynchus mosquito
However the values of LC50 and LC90 of ethyl acetate extract against larva were 454.94 (ppm) and 890.19 (ppm) respectively (Table 2 and fig. 12B and fig. 11). In current study, the obtained results indicated that methanolic extract had best efficacy compared to ethyl acetate extract. Additionally, many studies of leaves extract by methanol against mosquitoes life cycle had better effect than ethyl acetate extract[21-24] this is may be due to the nature and ability of methanol solvent for extraction. The results in this manuscript are proved that the biological activity of methanolic and ethyl acetate leaves extracts of T. camphoratus tree exhibited a significant lethal toxicity effect against different stages of C. tritaeniorhynchus mosquito. It is suggested that T. camphoratus tree could be used as an alternative natural insecticide instead of harmful chemical insecticides for combating and controlling of mosquitoes. Further studies can be conducted such as phytochemical analysis of T. camphoratus plant to find the effective derivate against mosquitoes.
The mosquitoes can transmit many diseases to humans and animals which make critical healthy problems around the world. The combating and fighting of mosquitoes became an urgent necessity to reduce prevalence of diseases. The resistant of mosquitoes to chemical insecticides and the harmful effects of these chemical martials make us to tend for create a natural insecticides for fighting mosquitoes. Here in this study, it concluded that the methanolic and ethyl acetate leaf extracts of T. camphoratus tree are proved their efficacy against the different stages of C. tritaeniorhynchus mosquito which transmits many diseases. These extracts killed the larvae, pupae and adult at various concentrations. The study demonstrates with increasingly likelihood to use this T. camphoratus plant as alternative natural insecticides instead of harmful chemical insecticides.
Acknowledgments:
The authors are grateful to Biology Department, College of Science, Human Anatomy Department, and Faculty of Medicine at Jazan University, Saudi Arabia for the facilities.
Conflict of interests:
The authors declared no conflict of interests.
References
- Buescher EL. Ecologic studies of Japanese encephalitis virus in Japan. II. Mosquito infection. Am J Trop Med Hyg 1959:8:651-64.
[Crossref] [Google Scholar] [PubMed]
- Gould DJ, Edelman R, Grossman RA, Nisalak A, Sullivan MF. Study of Japanese encephalitis virus in ChiangMai valley, Thailand IV. Vector studies. Am J Epidemiol 1974;100(1):49-56.
[Crossref] [Google Scholar] [PubMed]
- Hammon WM, Rees DM, Casals J, Meiklejohn GO. Experimental transmission of Japanese B encephalitis virus by Culex tritaeniorhynchus and Culex pipiens var. pallens, suspected natural vectors. Am J Hyg 1949;50:46-50.
[Crossref] [Google Scholar] [PubMed]
- Tong Y, Jiang H, Xu N, Wang Z, Xiong Y, Yin J, et al. Global distribution of Culex tritaeniorhynchus and impact factors. Int J Environ Res Public Health 2023;20(6):4701.
[Crossref] [Google Scholar] [PubMed]
- Subra R. Biology and control of Culex pipiens quinquefasciatus* Say, 1823 (Diptera, Culicidae) with special reference to Africa. Int J Trop Insect Sci 1981;1(4):319-38.
- Rajagopalan PK, Das PK, Kalyanasundaram M, Tyagi BK, Arunachalam N, Somachary N, et al. Bangalore Mosquito control Project master plan. 1987.
- Amaechi EC, Ariyo AA, Aderogba AA, Nwachukwu PC, Nwadike CC, Ezekiel OO, et al. Burden, knowledge and perception of lymphatic filariasis in resource-poor communities in north central Nigeria. J Parasit Dis 2024;48(4):823-30.
[Crossref] [Google Scholar] [PubMed]
- Grainge M, Ahmed S. Handbook of plants with pest-control properties John Wiley & Sons. New York; 1988. pp. 40.
- Katade SR, Pawar PV, Wakharkar RD, Deshpande NR. Sterculia guttata seeds extractives-an effective mosquito larvicide. Indian J Exp Biol 2006;44(8):662.
[Google Scholar] [PubMed]
- Wagner H, Hikino H, Farnsworth NR, editors. Economic and medicinal plant research. Academic press. London; 2012. pp. 103-144.
- Wyk BV, Oudtshoorn BV, Gericke N. Medicinal Plants of South Africa. Pretoria Briza Publications; 1997. pp. 78-9.
- Anthony CD. Consultant, July Monthly Column for Soap, Perfumery and cosmetics; 1999.
- Hedberg I, Staugard F. Traditional medicinal plants: Traditional medicine in Botswana. Ipeleng, Gaborone; 1989.
- Hutchings A, van Staden J. Plants used for stress-related ailments in traditional Zulu, Xhosa and Sotho medicine. Part 1: Plants used for headaches. J Ethnopharmacol 1994;43(2):89-124.
[Crossref] [Google Scholar] [PubMed]
- Watt JM, Breyer-Brandwijk MG. The Medicinal and Poisonous Plants of Southern and Eastern Africa (2nd ed.), Livingstone, London; 1962.
- Omolo MO, Okinyo D, Ndiege IO, Lwande W, Hassanali A. Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochemistry 2004;65(20):2797-802.
[Crossref] [Google Scholar] [PubMed]
- Täckholm V. Students’ Flora of Egypt. 2nd ed, Cairo University Publishing, Beirut; 1974. p. 888.
- Boulos L. Flora of Egypt. Checklist; Al Hadara Publishing: Cairo, Egypt; 1995.
- Williams KA, Green DW, Pascoe D, Gower DE. The acute toxicity of cadmium to different larval stages of Chironomus riparius (Diptera: Chironomidae) and its ecological significance for pollution regulation. Oecologia 1986;70(3):362-6.
[Crossref] [Google Scholar] [PubMed]
- Briggs JD. Reduction of adult house-fly emergence by the effects of Bacillus spp. on the development of immature forms. J Insect Pathol 1960;2:418-32. [Crossref]
- Roark RC. Some promising insecticidal plants. Economic Botany 1947;1(4):437-45.
- Tennyson S, Ravindran KJ, Arivoli S. Screening of twenty five plant extracts for larvicidal activity against Culex quinquefasciatus Say (Diptera: Culicidae). Asian Pacific J Trop Biomed 2012;2(2):S1130-4.
- El-Sheikh TM, Bosly HA, Shalaby N. Insecticidal and repellent activities of methanolic extract of Tribulus terrestris L.(Zygophyllaceae) against the malarial vector Anopheles arabiensis (Diptera: Culicidae). Egyptian Acad J Biol Sci A Entomol 2012;5(2):13-22.
- Areshi S, Mohamed G, Maqtan AS, Abdel Daim ZJ, Salama SA, Abdelnabi AR. Evaluation on the Efficacy of Tribulus terrestris (Zygophyllaceae) Leaf Extracts on the Different Stages of the Lymphatic Filariasis Vector, Culex quinquefasciatus (Diptera: Culicidae). J Plant Prot Pathol 2024;15(11):405-9.

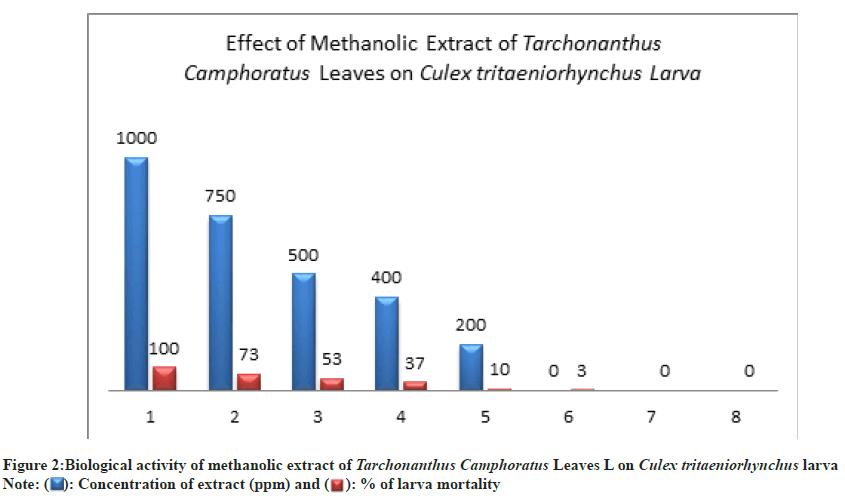
 ): Concentration of extract (ppm) and (
): Concentration of extract (ppm) and ( ): % of larva mortality
): % of larva mortality