- *Corresponding Author:
- K. N. Shah
Department of Toxicology, Jai Research Foundation, Valvada, Gujarat 396105, India
E-mail: kunjan.shah@jrfonline.com
| Date of Received | 15 December 2021 |
| Date of Revision | 20 March 2024 |
| Date of Acceptance | 23 May 2024 |
| Indian J Pharm Sci 2024;86(3):980-990 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The effects of vehicles play a pivotal role in defining the conclusion of any regulatory and pre-clinical toxicology study. The functional observational battery is one of the vital parameters to study the toxicological effect of compounds. This study investigates the impact of oral dosing with reverse osmosis water, 0.5 % w/v carboxymethyl cellulose and corn oil on functional observational battery parameters (neurobehavioural observations, motor activity, sensory reactivity measurements, grip strength and foot splay) in Wistar rats when administered for 90 d through oral gavage. Functional observational battery was performed during the 12th w of treatment. No mortality, morbidity or clinical sign of toxicity was observed. Body weight decreased marginally in carboxymethyl cellulose treated group during the 12th w. Motor activity was increased significantly in reverse osmosis water and carboxymethyl cellulose treated groups. The decrease in foot splay was also noted in all vehicles treated groups. The variation observed in grip strength by vehicles was not definitive or conclusive. No abnormality was observed in qualitative parameters of functional observation battery (sensory reactivity and neurobehavioral observations) and gross examination. From these results, it is evident that all vehicles have some notable influence on functional observation battery performed at the 12th w of treatment. This type of variation is critical to figure out sought conclusion for the compound of interest in regulatory studies. The recommendation is given to use strong and reliable historical control data to optimise or fix these variations and to avoid false conclusion regarding the compound where these vehicles are used as a control in regulatory toxicological studies.
Keywords
Functional observational battery, reverse osmosis water, corn oil, carboxymethyl cellulose, oral gavage, Wistar rat, historical control data
Vehicle selection is an important aspect for the formulation to be used in various pre-clinical efficacy and regulatory toxicology studies. Lack of formulation acceptance data results in excessive animal use, unplanned delays in the evaluation, development and registration of drugs and vehicle-dependent results[1]. While evaluating the non-clinical safety potential of new molecules, the material of interest is to be formulated in a manner that allows adequate administration of the test material[2]. Selection of a vehicle in regulatory toxicology studies is dependent upon many criteria including the complexity of formulation with the test material, the route of administration in the test system, the analytical approach such as stability, precision and accuracy, the safety and toxicity profile, tolerability and volume limitations in species and the ease of formulation with a high dose of the test material. Additionally, the duration of toxicological study marks another important parameter for the vehicle selection where multiple options are available for short-term studies without many obligations as alleviation of toxicity can be seen with a short duration of the study. However, for long-term toxicological studies, the selection criteria become stringent where the focus needs to be laid on selecting an acceptable vehicle with limited strength for first-in-human use clinical study[3]. The vehicle selection window ranges from aqueous to non-aqueous and organic solvents for getting a true solution. Aqueous solvents are the most widely preferred approach for any regulatory studies due to their homogeneity and uniformity as a solution, where Reverse Osmosis Water (ROW) has its own dominant position amongst all. Other preferred aqueous solution or suspension is normal saline and 0.5 or 1 % w/v Carboxymethyl Cellulose (CMC). Non-aqueous solvents include oils like Corn Oil (CO), vegetable oil, olive oil, etc., which are useful as a vehicle for hydrophobic compounds. Organic and other solvents are generally not preferred due to their toxicity, but in some cases with limited volume, dimethyl sulfoxide, Tween 80, Polyethylene glycol 400 and other agents may be used with a condition of availability of their safety data on species and strain to be used in the study. While discussing formulations, solutions and suspensions are the best and most preferred choices for any Good Laboratory Practice (GLP) toxicological studies[3]. Organization for Economic Cooperation and Development (OECD) and other regulatory guidelines also suggest an aqueous solution/suspension first, followed by considering a solution/suspension in oil and then by possible solution in other vehicles[4]. Hence, most of the regulatory toxicology studies performed using water, CMC and CO as a vehicle for the formulation depending upon the solubility of the test material. Several scientific studies provide insight into the safety and toxicity data of these three vehicles with short-term and long-term exposure and considered as fervently safe to use as a vehicle in terms of its systemic toxicity[1,5-9]. All such studies focus on general safety without prioritising the standard neurotoxicology or Functional Observational Battery (FOB) used in the routine repeated dose toxicity studies driven by regulatory guidelines. This study evaluates the possible hindrance of these three standard vehicles on FOB performed at 12th w of sub-chronic toxicity study. The FOB is a systematic assessment of nervous system function in the rat encompassing various parameters across autonomic, neuromuscular, sensorimotor and behavioural domains[10]. The FOB is a pivotal contributor in sub-chronic toxicity studies to derive the no observed adverse effect level of the test material. Scientists and regulators need prudent evaluation while sketching the FOB oriented result and conclusion to avoid any unwanted effect which has no relation with the test material. Behavioural evaluation in rodents is a sensitive parameter that can be easily affected by factors like environmental conditions, handling procedures during various study activities, housing conditions, and other experimental barriers. In the current study, we have tried to extrapolate the possibility of influence of the vehicle selected for the study upon these FOB parameters despite maintaining other parameters constant. The study was performed to evaluate such unusual or unnecessary variations of dosing with ROW, 0.5 % CMC and CO on FOB parameters performed during the 12th w in Wistar rat when administered for 90 d through oral gavage.
Materials and Methods
Animals:
Rat is selected as a test system because it is recommended in the test guidelines of OECD as a suitable model for toxicity assessment studies. Wistar strain has been chosen because of its widespread use as a rodent model in toxicology studies and well-established historical information available about its genetic and physiologic background. Therefore, the Wistar rat was selected as the test system to study the effect of various vehicles on FOB parameters. Healthy, young adult male and female rats (Rattus norvegicus) of Wistar (RccHan: WIST) strain of 5-7 w age was obtained from the in-house breeding facility of testing laboratory (Jai Research Foundation), India. Nulliparous and non-pregnant female rats were used for the experiment. The body weight variation among the rats was within ±20 % of the mean body weight for each gender.
Housing:
Rats were maintained in temperature (22±3°) and humidity (30 %-70 %) controlled room, with the photoperiod of 12 h light/dark cycle (light hours were 06.00-18.00 h). Light intensity was maintained between 130 and 325 LUX, and air changes were minimum of 15 per hour. Rats were housed in groups of 2 rats/cage/gender in sterile polypropylene cages with bedding material. Feed and water were provided ad libitum to rats. Environmental enrichment material i.e., wooden chew block was also provided to rats in each cage. Cages were placed on 5 tier racks and cage rotation was performed at weekly intervals to ensure almost similar environmental conditions to different groups. Adequate sanitation was maintained during the experiment considering frequently changing cages, bedding materials, cage lids, water bottles, enrichment material, floor cleaning and racks disinfecting. Rats were acclimatised for 5 d before randomisation.
Animal welfare:
The study was conducted in compliance with the guidelines of the “Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International” and “Guidelines for Laboratory Animals Facility” issued by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India to ensure utmost animal welfare. The experimental plan was conducted as per the OECD Guideline N° 408[11] and has been approved by the Institutional Animal Ethics Committee (IAEC) of Jai Research Foundation, India.
Assignment of animals:
Rats were randomised as per their body weight in such a manner that the variation between group mean body weight and dose-volume of each rat was minimal. Randomisation was performed using the in-house developed and validated computerised software program. A total 40 male and 40 female rats were assigned to different groups as mentioned in Table 1.
| Group N° | Treatment | Total N° of rats | |
|---|---|---|---|
| Male | Female | ||
| G1 | Naive (without any treatment) | 10 | 10 |
| G2 | RO water | 10 | 10 |
| G3 | CMC (0.5 % w/v) | 10 | 10 |
| G4 | CO | 10 | 10 |
Table 1: Experimental Design
Vehicle and administration:
Reverse Osmosis (RO) water was taken from in-house RO water filtration system; CMC was supplied by Merck Life Science Private Limited, India; CO was supplied by Sigma Aldrich, India. 0.5 % w/v CMC was prepared twice a week in RO water. All three vehicles were administered through oral gavage to male and female rats for a period of 90 consecutive d using an intubation cannula attached to a graduated sterile syringe. A constant dose volume of 10 ml/kg b.wt/d was used, and the individual dose was adjusted according to the most recently recorded body weight of each rat. The male and female dosing was initiated with gap of 1 d to accommodate feasible number of rats for the FOB and necropsy.
Parameters evaluated:
Clinical observations, including mortality, morbidity and other clinical signs were recorded. The body weight of rats was recorded at the beginning of the treatment (pre-treatment) and at weekly intervals after that. The FOB performed in this study was based on a standardised procedure developed and used by the laboratory. It is based on procedures published in the literature[12-14] and in the United State Environmental Protection Agency (USEPA) guideline OCSPP 870.6200 (office of chemical safety and pollution prevention)[15]. The FOB parameters were performed sequentially, as mentioned in Table 2, during the 12th w of the treatment for each rat. The male and female FOB was performed with the gap of 1 d before dosing day. The FOB was performed in the same experimental room conditions and whole procedure was completed within 4 h from the initiation. The procedure of data recording was unblinded to resemble the normal practice used for recording FOB data in regulatory 90 d toxicity studies.
| FOB | |||
|---|---|---|---|
| Parameter | Observation | Procedure | Recording |
| Home cage | Posture | Observations without disturbing the original home cage | Rank/description |
| Convulsion | |||
| Handling | Ease of removing | Observations while removing from the home cage and during handling | Rank/description |
| Handling reactivity | |||
| Palpebral closure | |||
| Lacrimation | |||
| Eye and skin examination | |||
| Salivation | |||
| Piloerection | |||
| Open field | Gait and Mobility | Observations for 3 min in a polyacrylic open arena (size: 495× 495×280 mm) with a flat surface covered with clean absorbent paper on it | Rank/description/actual number |
| Arousal level | |||
| Stereotypy behaviour | |||
| Bizarre behaviour | |||
| Respiration | |||
| Vocalisation | |||
| Rears | |||
| Urination and Defecation | |||
| Motoractivity | Fine activity | Instrument: Photobeam activity system (San Diego Instruments, USA) | Counts |
| Ambulatory activity | Software: Validated photobeam activity system software | ||
| Total activity | Observation period: 3 consecutive 10 min intervals (total 30 min) | ||
| Sensory reactivity | Approach response | Observations in a polyacrylic open arena (size: 495×495×203 mm) with a flat surface covered with clean absorbent paper using a blunt object (for approach and touch response), clicker (for click response), flashlight (for pupil response), forceps (for tail-pinch response), and dropped from a height of approximately 30 cm (for air righting reflex) | Rank/description |
| Touch response | |||
| Click response | |||
| Pupil response | |||
| Tail-pinch response | |||
| Air righting reflex | |||
| Grip strength | Forelimb | Instrument: Grip strength meter (San Diego Instruments, USA) | Average of 3 trials in kg |
| Hindlimb | Frequency: 3 times for each limb | ||
| Foot splay | Landing hindlimb | The hindlimb feet were marked with a non-permanent, non-toxic ink and dropped onto a recording sheet from a height of approximately 30 cm | Average of 3 trials in mm |
Table 2: Fob Parameters
Gross pathological examination:
At scheduled sacrifice, rats were euthanised by carbon dioxide asphyxiation. Rats were subjected to a full gross necropsy under the supervision of a veterinary pathologist. Rats were examined carefully for external abnormalities. The cranial, thoracic and abdominal cavities were cut, opened and a thorough examination of organs was carried out to detect abnormalities.
Data analysis:
All numerical data was processed to get group means and standard deviations. All parametric (body weight, grip strength, and foot splay) and nonparametric (motor activity, urination, defecation, and rearing count) data were analyzed as per the statistical tests suggested in the OECD guidance document 116. Parametric data were subjected to Bartlett's test to meet the homogeneity of variance before conducting the Analysis of Variance (ANOVA) and Dunnett's test (when ANOVA test was found significant). When the data did not meet the homogeneity of variance and non-parametric data were subjected to the Kruskal-Wallis test followed by the Dunn's test (when Kruskal-Wallis test was found significant). All statistical analysis was performed using in-house developed and validated statistical software at a 5 % confidence interval between naive and vehicle-treated groups.
Results and Discussion
There was no mortality or morbidity observed in any treatment or naive group. All rats were normal and healthy during the whole experimental period. The body weight growth of male and female rats in vehicle treated groups was normal and comparable to the naive group during the experiment. Our major focus is on how the body weight stands for 12th w, during which FOB was performed (Table 3).
| Gender | Parameter | Naive | RO Water | CMC (0.5 % w/v) | CO |
|---|---|---|---|---|---|
| Male | Body weight at 12th w | 462.31 | 456.84 | 440.12 | 447.14 |
| % change from naive | 0 | -1.2 | -4.8 | -3.3 | |
| Female | Body weight at 12th w | 260.95 | 262.89 | 253.78 | 265.74 |
| % change from naive | 0 | 0.7 | -2.8 | 1.8 |
Table 3: Comparison of body weight of treated groups with naive group at 12th W
In the home cage, rats from different vehicle-treated and naive groups showed normal posture viz., asleep (curled up often asleep), sitting A (sitting but with head hung down), sitting C (sitting or standing alert, watching) and rearing. Convulsions in the form of clonic and tonic movements were absent in the home cage during Neurobehavioral Observation (NBO).
Handling observations indicated rats showed normal behaviour during removal (very easy-rats sit quietly) and handling (easy-alert, limbs put against the body). Eyelids were wide open in all rats. No rat showed lacrimation, salivation and piloerection. Eye and skin examination did not reveal any abnormality in any rat.
Open field observations indicates in the open field, rats from vehicle-treated and naive groups showed normal gait, mobility and respiration for a 3 min observation period. Vocalisation, stereotypic behaviour and bizarre behaviour were absent in this assessment. The arousal level rears count and the number of urination and defecation pool of male and female rats from vehicle-treated groups were comparable with the naive group.
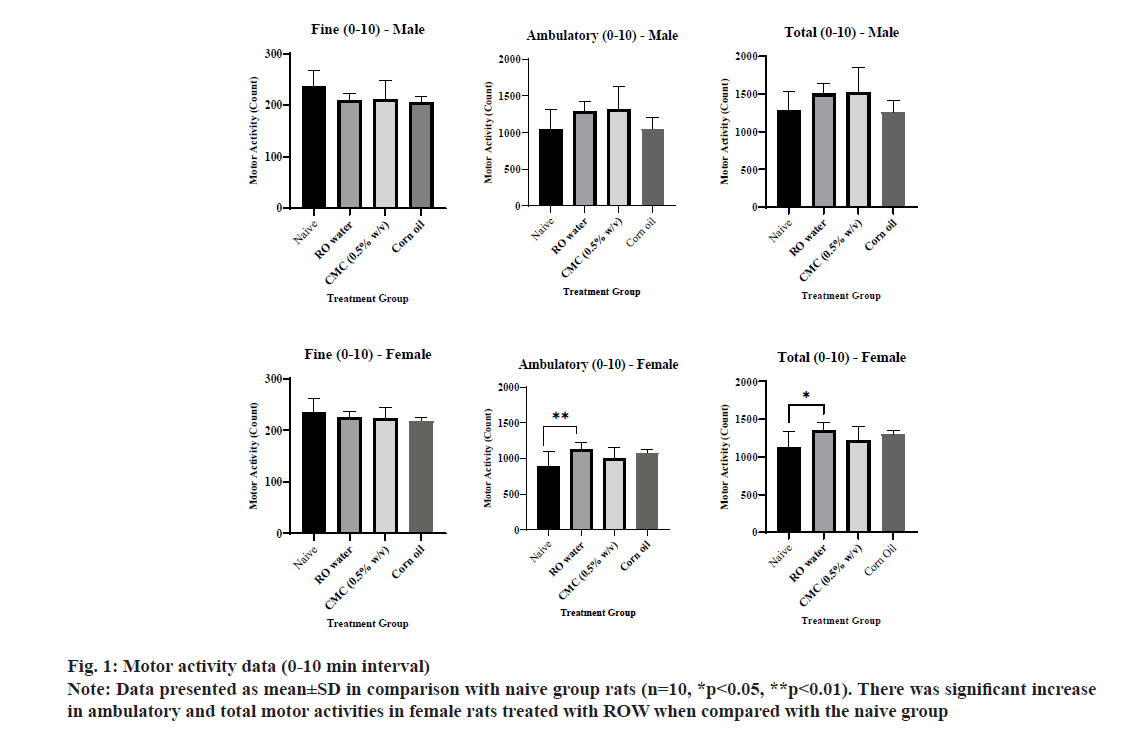
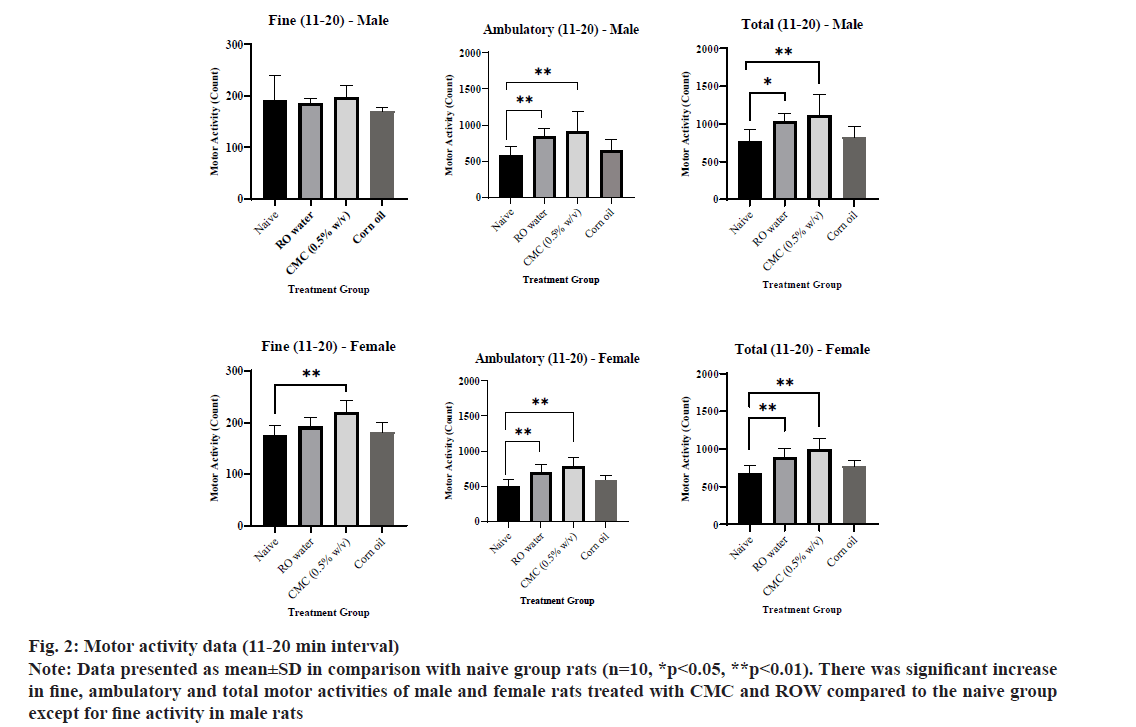
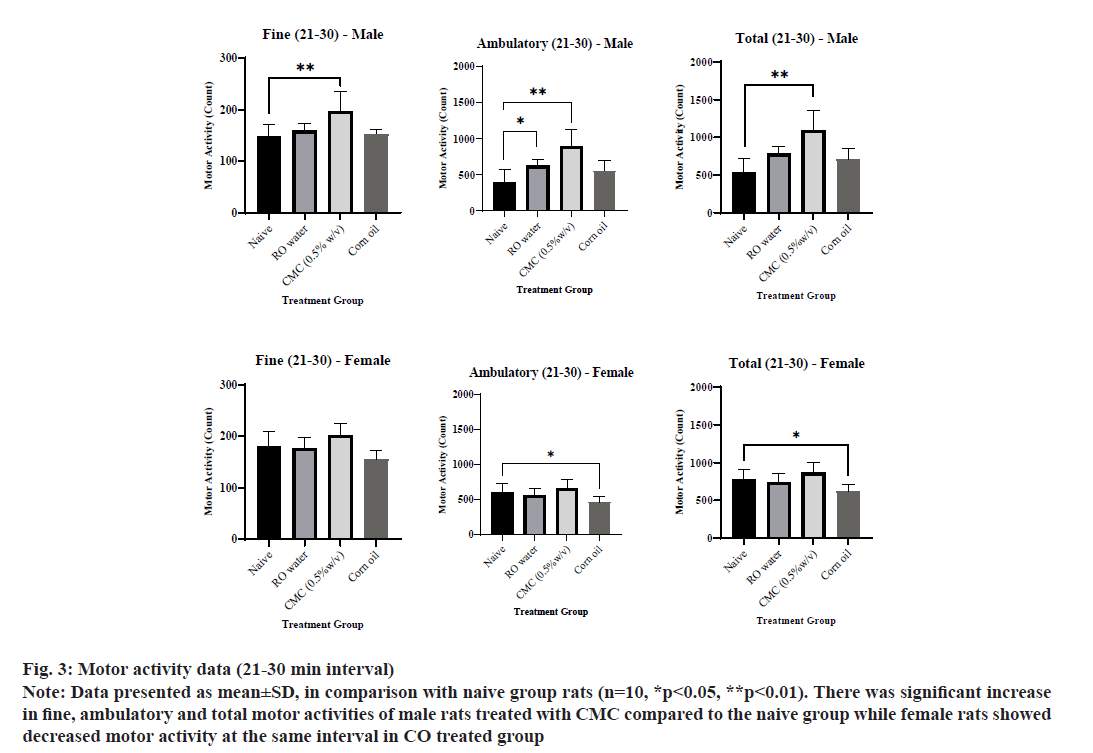
The motor activity data of individual group animals were presented in Table 4. The ambulatory and total activities of female rats for 0-10 min interval in the ROW treated group was increased significantly compared to the naive group (fig. 1). The fine, ambulatory and total activity of male and female rats for 11-20 min interval in CMC and ROW treated groups was increased significantly compared to the naive group except for fine activity in male rats (fig. 2). The fine, ambulatory and total activities of male rats for 21-30 min interval in CMC and ROW treated groups was increased significantly compared to the naive group, while female rats showed decreased motor activity at the same interval in CO treated group (fig. 3).
| Motor activity | Male (N=10 for each group) | Female (N=10 for each group) | ||||
|---|---|---|---|---|---|---|
| RO water | CMC (0.5 % w/v) | CO | RO water | CMC (0.5 % w/v) | CO | |
| 0-10 min interval | ||||||
| Fine | -11 | -10.4 | -13.06 | -3.8 | -4.5 | -6.62 |
| Ambulatory | 24.2 | 26.4 | 1.23 | 27.2 | 12.8 | 21.89 |
| Total | 17.8 | 19.6 | -1.4 | 20.8 | 9.2 | 15.93 |
| 11-20 min interval | ||||||
| Fine | -2.1 | 4.1 | -10.45 | 9.3 | 25.3 | 3.52 |
| Ambulatory | 44.9 | 56.9 | 11.91 | 39.4 | 55.3 | 18.04 |
| Total | 33.4 | 43.9 | 6.41 | 31.7 | 47.5 | 14.27 |
| 21-30 min interval | ||||||
| Fine | 6.8 | 32.7 | 3.03 | -1.5 | 12.7 | -13.77 |
| Ambulatory | 61.1 | 130.8 | 43.08 | -6.9 | 10 | -22.74 |
| Total | 46.2 | 103.5 | 31.94 | -5.6 | 10.6 | -20.7 |
Table 4: Percentage difference of motor activity of ROW and CMC Treated group form naive Group
Fig. 2: Motor activity data (11-20 min interval)
Note: Data presented as mean±SD in comparison with naive group rats (n=10, *p<0.05, **p<0.01). There was significant increase in fine, ambulatory and total motor activities of male and female rats treated with CMC and ROW compared to the naive group except for fine activity in male rats
Fig. 3: Motor activity data (21-30 min interval)
Note: Data presented as mean±SD, in comparison with naive group rats (n=10, *p<0.05, **p<0.01). There was significant increase in fine, ambulatory and total motor activities of male rats treated with CMC compared to the naive group while female rats showed decreased motor activity at the same interval in CO treated group
Overall, total motor activity was increased 17 %-47 % in male rats treated with the ROW compared to 20 %-32 % increase in female rats of the same group. A similar gender difference was also noted in the CMC treated group, where total motor activity was increased 19 %-104 % in male rats compared to 9 %-48 % in female rats. Sensory reactivity parameters, viz., approach response, touch response, click response, tail pinch response, pupil response and air righting reflex of male and female rats from vehicle-treated groups were comparable with the naive group.
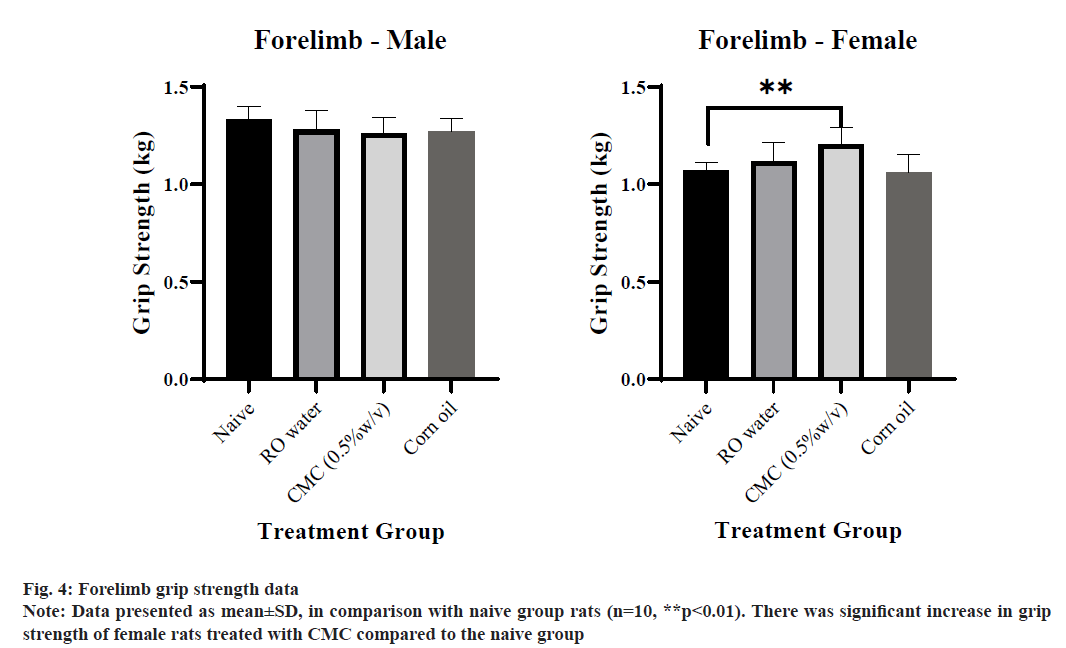
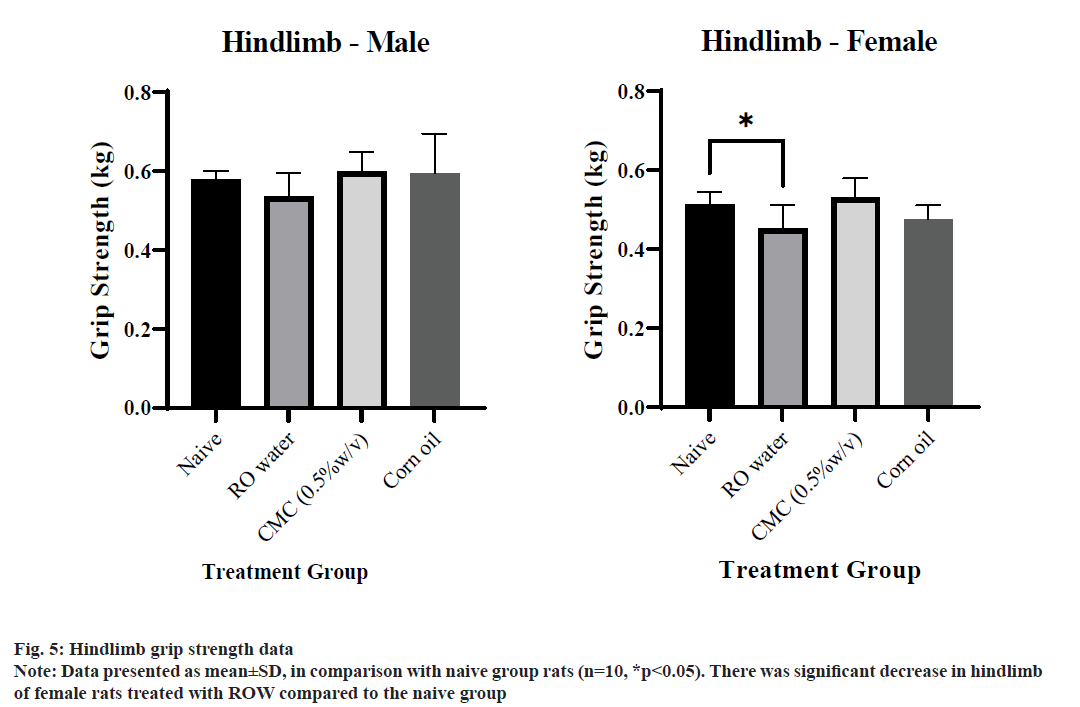
Grip strength data is represented in Table 5. Significant increase was observed in the forelimb grip strength value of female rats of the CMC treated group when compared to the naive group (fig. 4). On the other hand, a significant decrease was observed in the hindlimb grip strength value of female rats of the ROW treated group when compared to the naive group, whereas no significant difference was observed in male rats of vehicle-treated groups when compared with the naive group (fig. 5). Much gender difference was observed in forelimb grip strength of ROW and CMC treated groups compared to the CO treated group.
| Grip strength parameters | Male (N=10 for each group) | Female (N=10 for each group) | ||||
|---|---|---|---|---|---|---|
| RO water | CMC (0.5 % w/v) | CO | RO Water | CMC (0.5 % w/v) | CO | |
| Fore limb | -3.9 | -5.2 | -4 | 4.3 | 12.3 | -1 |
| Hind limb | -7.5 | 3.4 | 2.7 | -12 | 3.4 | -7 |
Table 5: Percentage difference of grip strength values of treated group from naive group
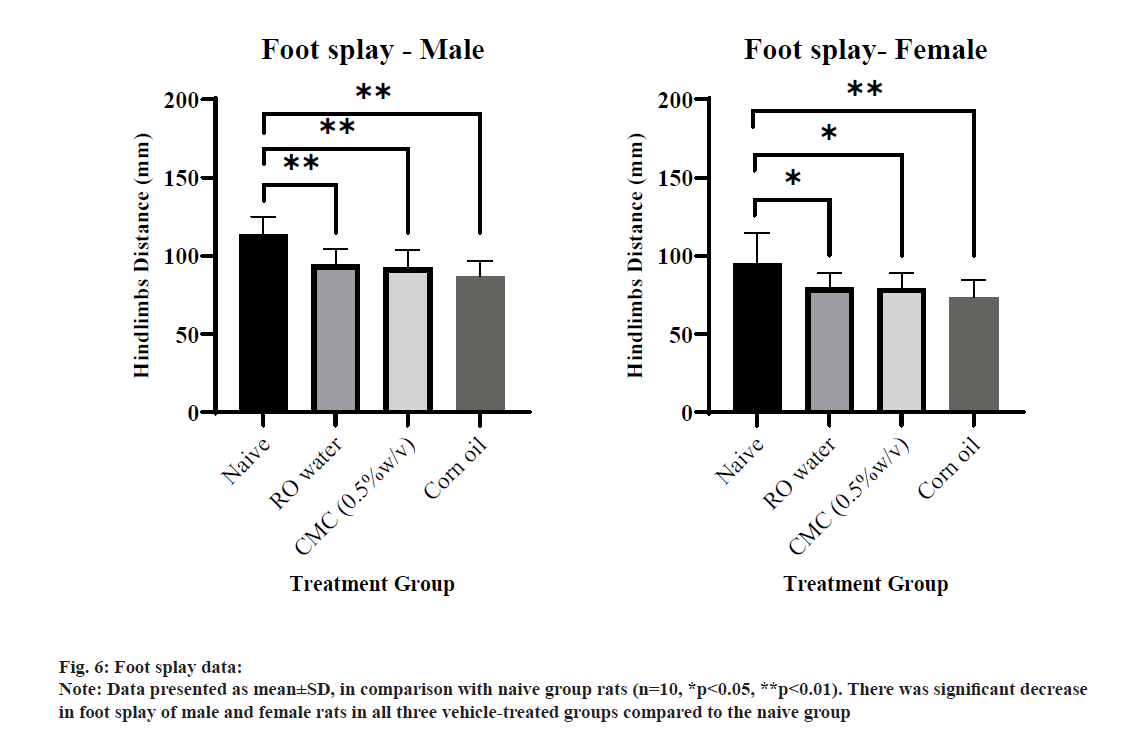
The landing hindlimb foot splay value of male and female rats in all three vehicle-treated groups was decreased significantly compared to the naive group (fig. 6). The decrease was 16 %- 24 % across the treated groups compared to the naive group without any gender difference. External and internal (visceral) examination of sacrificed rats of either gender of all groups (G1 to G4) did not reveal any abnormality.
Over many decades, ROW, CMC, and CO were used as a gold standard vehicle to prepare a test material formulation and thus evaluate the test material toxicity in regulatory toxicology studies. Many regulatory guidelines like OECD, USEPA, United States Food and Drug Administration, Japanese Ministry of agriculture, forestry and fisheries and European Commission also recommend these vehicles for toxicity studies without any doubt of the systemic toxicological effect of these vehicles. Many publications[1,8] also demonstrate that within the guideline specified limit volume, these vehicles did not produce any systemic or histological change. Accepting this consideration and no abnormality observed during gross examination of rats from treated groups, we have not evaluated histopathology of any organ in this experiment. Some of the research works demonstrated that the effect of these vehicles on body weight[16], carcinogenic potential[17], immunogenic potential[18] and organ toxicity[5] either via dietary or oral routes. None of the research works showed any effect of these vehicles on neurobehavioural, sensorimotor and musculoskeletal functions by using standard FOB parameters. These FOB parameters, which is performed in various toxicology studies can be used to achieve several goals like behavioral screening for nervous system effects, assessment of neurotoxicology, understanding the possible mechanism of neurotoxicity and selection of doses for further toxicology studies[13]. The treatment with CO and ROW did not produce any effect on body weight compared to the naive group during the 90 d study period. While CMC treated group revealed a decrease in body weight (3 %-5 % compared to the naive group) at the 12th w despite the significantly increased body weight (12 %-17 %) at the initial stage, which was in accordance with the earlier published work[18]. The results of body weight in CO treated groups are somewhat in contrast with other previously published data[19,20], where an increase in body weight was observed with gender differences. However, published works were for both dietary or gavage administration and either longer or shorter duration than 90 d.
All qualitative parameters of FOB include home cage, handling, open field and sensory reactivity observations were normal and comparable among the groups. However, quantitative parameters of FOB include motor activity, grip strength and foot splay measurements showed weighty results in some instances among the groups. Motor activity results revealed that the activity was significantly increased in rats from ROW and CMC treated groups, while rats from CO treated group did not show a significant increase in motor activity. The study observation showed contrasting results when compared to the previously reported study where CO rich diet led to a decrease in spontaneous motor activity due to its high n-6 polyunsaturated fatty acid content[19,20]. However, the study was conducted in mice for 6 w dietary administration. The ambulatory activity was affected severely rather than the fine activity which is directly related to spontaneous locomotor activity in both ROW and CMC groups. The variation was more prominent in male rats than female rats and in later 20 min than the initial 10 min interval. Motor activity results of CMC were supported by the previously published work[21] where CMC significantly increased motor endurance in male rats after 14 d treatment. With marginal differences among gender and both limbs (hindlimb and forelimb), increased or decreased grip strength was observed in rats among the various vehicles, which was considered as isolated and inconsistent findings for vehicles. All three vehicles produced decreased landing hindlimb foot splay in rats compared to the naive group without any gender differences. In terms of decrease in foot splay value, CO treated group produced a marked variation compared to ROW and CMC treated groups. Looking toward the overall results, all three vehicles have some remarkable effects on quantitative parameters of FOB at different levels of variability in terms of gender difference, affecting the number of FOB parameters and severity of the variation. The mode of action was not established for the consistent effect of CMC and ROW on motor activity and foot splay and CO on foot splay, as it was out of scope and not the primary goal for this research work. These types of influence of vehicles may synergise or subtle or overlook the effect of the compound where these vehicles were used as a control in the study. These types of variation also lead to affect the dosedependency and relevancy of the effect to other parameters measured for the compound of interest. Researchers and regulators need careful evaluation while drawing a conclusion of the study in which these types of variation are observed using these vehicles. Strong vehicle Historical Control Data (HCD) of performing laboratory and stringent control over study parameter variability may help to draw a meaningful conclusion of toxicology studies where these vehicles are used as a control.
Certain criteria sought for consideration to use HCD Mean of FOB like age, strain of species, diet and housing, type and route administration, number of years of studies to prepare HCD (3 or 5 y), and personnel expertise to handle the instrument for performing FOB. The proficiency test is one of the tools to evaluate the personnel expertise to perform FOB. It is required to perform at a certain interval to solidify the HCD by harmonise the procedural approach among the person and thus reduce the personal variability to conduct FOB. Instrument sensitivity and reliability is also one of the prime forces to obtain robust HCD. HCD adds the weight of evidence approach to address reproducibility and rigour, not to make interpretations about the toxicity. Many publications also suggest using reliable HCD in various forms of toxicity studies like bioassay, endocrine disruptor, carcinogenicity and ecotoxicity by citing references of different regulatory guidelines (EPA, FDA, European medicines agency, and OECD on using of HCD) [22-25].
The study demonstrated that the gold standard vehicles (ROW, CMC, and CO) produce a considerable variation on the quantitative parameters of FOB (motor activity, grip strength, and foot splay). However, the study did not reveal any abnormal clinical sign or any effect on qualitative parameters of FOB (NBO and sensory reactivity), which forced us to examine the effect of these vehicles on systemic toxicological findings. The marked variation of these vehicles on quantitative parameters of FOB during the 12th w of treatment needs careful attention while portraying the conclusion of regulatory toxicology study of pharmaceutical, agrochemical, industrial or food additives compounds. The recommendation is also given to use strong HCD and powerful statistical tools to justify the effect variability of vehicles in regulatory toxicology studies. Additional study is required to solidify the conclusion with careful designing to include periodic measurement on FOB, variation on associated parameters of FOB and histopathological changes on relevant organs, which was not covered in the present experiment.
Acknowledgements:
This study was conducted by Jai Research Foundation, Valvada, Gujarat, India. Kunjan Shah conducted studies, reviewed data, analysed data and contributed to manuscript writing and reviewing. Sudhakar Jadhav conducted and contributed to the live phase of studies and manuscript reviewing. Dr. Manish Patel aided resource management. Jaydip Jikadara and Nishee Modi contributed to the literature search and manuscript writing.
Conflict of interests:
The authors declare no conflict of interest.
References
- Gad SC, Spainhour CB, Shoemake C, Pallman DR, Stricker-Krongrad A, Downing PA, et al. Tolerable levels of nonclinical vehicles and formulations used in studies by multiple routes in multiple species with notes on methods to improve utility. Int J Toxicol 2016;35(2):95-178.
[Crossref] [Google Scholar] [PubMed]
- Gad SC, Cassidy CD, Aubert N, Spainhour B, Robbe H. Nonclinical vehicle use in studies by multiple routes in multiple species. Int J Toxicol 2006;25(6):499-521.
[Crossref] [Google Scholar] [PubMed]
- Lee S. The importance of formulation design in oral GLP toxicology studies. Pharm Outsourcing 2018:1-5.
- Organization for Economic Co-operation and Development (OECD). Repeated dose 28-day oral toxicity study in rodents. OECD Guideline for the Testing of Chemicals. Section 4: Health Effects. 2008.
- Bampidis V, Azimonti G, Bastos MD, Christensen H, Dusemund B, Durjava KM, et al. Safety and efficacy of methyl cellulose for all animal species. Efsa J 2020;18(7):e06212.
[Crossref] [Google Scholar] [PubMed]
- Weaver ML, Grossi AB, Schutzsack J, Parish J, Logsted J, Bogh IB, et al. Vehicle systems and excipients used in minipig drug development studies. Toxicol Pathol 2016;44(3):367-72.
[Crossref] [Google Scholar] [PubMed]
- Thackaberry EA. Vehicle selection for nonclinical oral safety studies. Expert Opin Drug Metab Toxicol 2013;9(12):1635-46.
[Crossref] [Google Scholar] [PubMed]
- National Toxicology Program. NTP comparative toxicology studies of corn oil, safflower oil, and tricaprylin (CAS Nos. 8001-30-7, 8001-23-8, and 538-23-8) in male F344/N rats as vehicles for gavage. Natl Toxicol Program Tech Rep Ser 1994;426:1-314.
[Google Scholar] [PubMed]
- Dupont J, White PJ, Carpenter MP, Schaefer EJ, Meydani SN, Elson CE, et al. Food uses and health effects of corn oil. J Am Coll Nutr 1990;9(5):438-70.
[Crossref] [Google Scholar] [PubMed]
- Tukaram SA, Smith AA, Muthu AK, Chugh Y, Manavalan R. Systemic safety evaluation of central nervous system function in the rat by oral administration of linezolid. Pharmacol 2007;2:90-102.
- OECD, 2018: OECD N° 408, "Repeated dose 90-day oral toxicity study in rodents. The Organization for Economic Cooperation and Development (OECD) guidelines for the testing of chemicals, adopted by the council on 2018.
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 1968;13:222-57.
[Crossref] [Google Scholar] [PubMed]
- Mathiasen JR, Moser VC. The Irwin test and Functional Observational Battery (FOB) for assessing the effects of compounds on behavior, physiology, and safety pharmacology in rodents. Curr Protoc Pharmacol 2018;83(1):e43.
[Crossref] [Google Scholar] [PubMed]
- Moser VC, Cheek BM, MacPhail RC. A multidisciplinary approach to toxicological screening: III. Neurobehavioral toxicity. J Toxicol Environ Health 1995;45(2):173-210.
[Crossref] [Google Scholar] [PubMed]
- US Environmental Protection Agency. Health Effects Test Guidelines: OPPTS 870.6200: Neurotoxicity Screening Battery. 1998.
- Rao GN, Haseman JK. Influence of corn oil and diet on body weight, survival, and tumor incidences in F344/N rats. Nutr Cancer 1999;19(1):21-30.
[Crossref] [Google Scholar] [PubMed]
- Wu B, Iwakiri R, Ootani A, Tsunada S, Fujise T, Sakata Y, et al. Dietary corn oil promotes colon cancer by inhibiting mitochondria-dependent apoptosis in azoxymethane-treated rats. Exp Biol Med (Maywood) 2004;229(10):1017-25.
[Crossref] [Google Scholar] [PubMed]
- Geng XC, Li B, Zhang L, Song Y, Lin Z, Zhang YQ, et al. Corn oil as a vehicle in drug development exerts a dose-dependent effect on gene expression profiles in rat thymus. J Appl Toxicol 2012;32(10):850-7.
[Crossref] [Google Scholar] [PubMed]
- Bär A, Til HP, Timonen M. Subchronic oral toxicity study with regular and enzymatically depolymerized sodium carboxymethylcellulose in rats. Food Chem Toxicol 1995;33(11):909-17.
[Crossref] [Google Scholar] [PubMed]
- Wong CK, Botta A, Pither J, Dai C, Gibson WT, Ghosh S. A high-fat diet rich in corn oil reduces spontaneous locomotor activity and induces insulin resistance in mice. J Nutr Biochem 2015;26(4):319-26.
[Crossref] [Google Scholar] [PubMed]
- Isa AS, Muhammad MS, Hudu AA, Jamba BI, Choji ES, Isah HO, et al. Assessment of cognitive and motor endurance activities in male wistar rats administered carboxymethyl cellulose. Afr J Biochem Res 2019;22(2):195-9.
- Vandenberg LN, Prins GS, Patisaul HB, Zoeller RT. The use and misuse of historical controls in regulatory toxicology: Lessons from the CLARITY-BPA study. Endocrinology 2020;161(5):14.
[Crossref] [Google Scholar] [PubMed]
- Keenan C, Elmore S, Francke-Carroll S, Kemp R, Kerlin R, Peddada S, et al. Best practices for use of historical control data of proliferative rodent lesions. Toxicol Pathol 2009;37(5):679-93.
[Crossref] [Google Scholar] [PubMed]
- Brooks AC, Foudoulakis M, Schuster HS, Wheeler JR. Historical control data for the interpretation of ecotoxicity data: Are we missing a trick? Ecotoxicology 2019;28(10):1198-209.
[Crossref] [Google Scholar] [PubMed]
- Kluxen FM, Weber K, Strupp C, Jensen SM, Hothorn LA, Garcin JC, et al. Using historical control data in bioassays for regulatory toxicology. Regul Toxicol Pharmacol 2021;125:105024.
[Crossref] [Google Scholar] [PubMed]