- *Corresponding Author:
- K. R. Iyer
Department of Pharmaceutical Chemistry, Bombay College of Pharmacy, Kalina, Mumbai-400 098, India
E-mail: krishnaiye@gmail.com
| Date of Submission | 21 May 2015 |
| Date of Revision | 06 January 2015 |
| Date of Acceptance | 03-Sep-2012 |
| Indian J Pharm Sci 2015;77(3):283-289 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Organic solvents used for solubilization of the substrates/NCEs are known to affect the activity of cytochrome P450 enzymes. Further, this effect varies with the solvents used, the substrates and CYP450 isoforms in question. In the present study, we have investigated the effect of ten commonly used water miscible organic solvents (methanol, ethanol, 1-propanol, 2-propanol, acetonitrile, acetone, dimethyl sulphoxide, N,N-dimethyl formamide, dioxane and polyethylene glycol 400) on p-nitrophenol hydroxylase activity at 0, 0.1, 0.25, 0.5, 0.75 and 1% v/v concentration in rat liver microsomes. All the solvents studied showed concentration dependent inhibition of the p-nitrophenol hydroxylase activity except acetonitrile which showed activation of the activity at concentration range studied. Out of ten solvents studied, dioxane was found to be the most inhibitory solvent (inhibition >90% at 0.25% v/v concentration). Overall, solvents like dimethyl sulphoxide, dimethyl formamide and dioxane appeared to be unsuitable for characterizing p-nitrophenol hydroxylase (CYP2E1-mediated) reactions due to a high degree of inhibition. On the other hand, methanol and acetonitrile at concentrations <0.5% v/v appeared to be appropriate solvents for substrate solubilization while evaluating CYP2E1-mediated catalysis. The results of this study imply that caution should be exercised while choosing solvents for dissolution of substrate during enzyme studies in liver microsomes.
Keywords
CYP2E1, p-nitrophenol, p-nitrocatechol, RLM and HPLC
The cytochrome P450 (CYP450) isoforms are predominant family of enzymes which are involved in the metabolism of clinically used drugs and other xenobiotics. In particular, CYP1, CYP2 and CYP3 family are primarily involved in phase 1 metabolism. These enzymes are found in many tissues but highest levels are observed in the liver.
In vitro drug metabolism studies involve incubation of NCEs with in vitro study models such as subcellular fractions (liver microsomes, liver S9 fractions, or cDNA expressed microsomes), whole cells (hepatocytes) and/or liver slices [1]. In vitro studies/incubations are performed in aqueous physiological buffers and require NCEs to be in solubilized form. However, a significant number of the substrates and NCEs are lipophilic in nature [2]. This leads to a difficulty in evaluating reactions characterized by a high apparent Km or low Vmax [3]. Therefore, it is common practice to dissolve compounds in organic solvents (methanol- MeOH, ethanol- EtOH, acetonitrile- ACN or dimethyl sulphoxide- DMSO) to aid their solubility while performing incubations at high substrate concentration. It has been previously reported that some organic solvents affect the activity of enzymes involved in the biotransformations of xenobiotic compounds [3-11]. Most of these authors have used lipophilic probe substrates to evaluate the effect of organic solvents on CYP450 activities and since these substrates are practically insoluble in water, the establishment of a true control (solvent free) sample has been an issue. In these reports [3-11], substrate was either dissolved in minimal organic solvent [7,9] to obtain a control incubation or the substrate was first dissolved in organic solvent which was evaporated and residue redissolved in microsomes to set up a control (without organic solvent) incubation [3,6]. In the first case, solvent free incubation (control) to evaluate the effect of solvents was missing while in the latter case complete re-solubilization of substrate was assumed.
In the present study, we chose to use a substrate (p-nitrophenol) with sufficient solubility in aqueous and organic solvents. This allowed us to obtain a solvent free control to truly evaluate the effect solvents on CYP450 activities. We report the effect of MeOH, EtOH, n-propanol (nPA), 2-propanol (IPA), acetone (ACE), ACN, DMSO, N,N-dimethyl formamide (DMF), dioxane (Diox) and polyethylene glycol 400 (PEG400) on p-nitrophenol hydroxylase activity [11] in rat liver microsomes, using p-nitrophenol (PNP) which has sufficiently high solubility in water and which does not precipitate in presence of organic solvents.
Materials and Methods
p-Nitrocatechol was obtained from Lancaster Chemicals, Mumbai. Salicylamide and NADPH were obtained from SRL Chemicals Ltd., Mumbai. Tris-HCl was obtained from Sigma Chemicals Co., USA. Sucrose AR, p-nitrophenol, glycerol, ethylenediaminetetraacetic acid AR, dipotassium hydrogen phosphate AR, Triton X-100, sodium dithionite purified, ethanol AR, coomassie brilliant blue G-250, phosphoric acid (85% w/v), conc. HCl, acetone, dioxane, n-propanol and PEG 400 were obtained from S. D. Fine-Chem. Ltd. Mumbai. Calcium chloride AR was obtained from Thomas Baker. Carbon monoxide gas was obtained from Alchemi Gases and Chemicals Pvt. Ltd. Mumbai. Bovine serum albumin was obtained as a gift sample from Piramal Life Sciences Ltd. ACN, MeOH (HPLC grade), diethyl ether LR was obtained from Qualigens Ltd., Mumbai, DMF and DMSO were obtained from Merck India Ltd. All other chemicals were of analytical grade.
Isolation and characterization of rat liver microsomes
Livers used in this study were obtained from rats sacrificed as a part of other experiments approved by the Institutional Ethical Committee from Department of Pharmacology, Bombay College of Pharmacy, Kalina. Rat liver microsomes (RLM) were prepared in-house by calcium chloride aggregation method [12], The entire microsomal isolation protocol was carried out at 0-4°, in an ice bath. Briefly, finely chopped pooled liver from up to six rats (10 g) were mixed with four volumes (40 ml) of ice cold 10 mM Tris-HCl buffer, containing 0.25 M sucrose, pH 7.4, and then homogenized in a potter glass homogenizer equipped with Teflon pestle. The homogenate was centrifuged at 13 000×g for 10 min at 4°, and the precipitate was discarded. To the supernatant, CaCl2 was added to yield a final concentration of 10 mM. The solution was stirred for 15-20 min and then centrifuged at 25 000×g for 10 min at 4°. The firmly packed pellets of microsomes were resuspended by homogenization in 100 mM Tris-HCl buffer containing 20% w/v glycerol and 10 mM EDTA, pH 7.4. The microsomes were stored at -70° until use. Further, RLM were characterized for spectral CYP450 content by the method of Omura and Sato [13] and for protein content by Bradford method [14].
Effect of organic solvents on p-nitrophenol hydroxylase activity
The effect of ten different water miscible organic solvents (MeOH, EtOH, nPA, IPA, ACE, ACN, DMF, DMSO, Diox and PEG400) on p-nitrophenol hydroxylase activity was studied at varying concentration (0.1, 0.25, 0.5, 0.75 and 1% v/v). Initially, a stock solution 5 mM PNP was prepared in distilled water then, 500 μl of this substock was further diluted with respective organic solvent-distilled water mixture to get 2.5 mM of PNP and 5, 12.5, 25, 37.5 and 50% v/v of organic content as given in Table 1. Further, 10 μl of 2.5 mM PNP (final concentration 50 μM) substocks containing 5, 12.5, 25, 37.5 and 50% v/v of respective organic solvents were incubated with 40 μl of RLM. The total volume of reaction (500 μl) was adjusted with phosphate buffer to obtain 0.1, 0.25, 0.5, 0.75 and 1% v/v final concentration of organic solvents, respectively. The reaction was initiated by addition of 50 μl of 6 mM NADPH and maintained at 37° on water bath shaker. The reaction was then terminated by addition of 250 μl perchloric acid (0.6 M). Then, 50 μl of 350 μg/ml salicylamide was added to each tube and stored at - 70° until analysis. PNP substock prepared in distilled water was considered as control. Each experiment was conducted in triplicate.
| Organic solventconcentration(% v/v) | Vol. of5 mMPNP (µl) | Vol. oforganicsolvent (µl) | Vol. ofdistilledwater (µl) | Totalvol.(µl) |
|---|---|---|---|---|
| 0 | 500 | - | 500 | 1000 |
| 5 | 500 | 50 | 450 | 1000 |
| 12.5 | 500 | 125 | 375 | 1000 |
| 25 | 500 | 250 | 250 | 1000 |
| 37.5 | 500 | 375 | 125 | 1000 |
| 50 | 500 | 500 | - | 1000 |
Table 1: Protocol For Preparation Of 2.5 Mm Pnp Containing Different Concentrations Of Organic Solvents
Sample preparation for HPLC analysis
After the incubations were terminated as stated above, 500 mg of ammonium sulfate was added to each reaction tube, and then samples were extracted using diethyl ether (4 ml). The two layers (i.e. aqueous and ether) were allowed to separate. A dry ice-acetone bath was prepared by gradual addition of dry ice to acetone. All the samples were placed in the dry iceacetone bath for 20s. After the freezing of the bottom aqueous layer, the upper ether layer was decanted and collected in separate glass centrifuge tubes. The ether layer was evaporated on a water bath at 40°. The residue was reconstituted in 200 μl of mobile phase for HPLC analysis.
HPLC analysis
A Dionex HPLC instrument equipped P680 HPLC pump, ASI 100 automated sample injector with UVD 340U PDA detector was used and chromatograms were analyzed by Chromoleon client 6.80 SP2 version software. Stationary phase used was a reverse phase Thermo Hypersil BDS, C18 (250×4.6 mm, 5 μM). Mobile phase containing 0.1% v/v formic acid and acetonitrile was used in the ratio of 65:35. The analytes were eluted at flow rate of 1 ml/min and detected at 345 nm. The retention time of salicylamide (SAL, IS), p-nitrocatechol (PNC, metabolite) and PNP was found to be about 4.99, 5.74 and 8.63 min.
Data analysis
Results are represented as a mean of triplicate incubations. The extent of inhibition or enhancement in the activity is the ratio of peak area ratios of PNC/IS in the incubations containing organic solvent over the control incubation containing no organic solvent, expressed as a percentage.
Results and Discussion
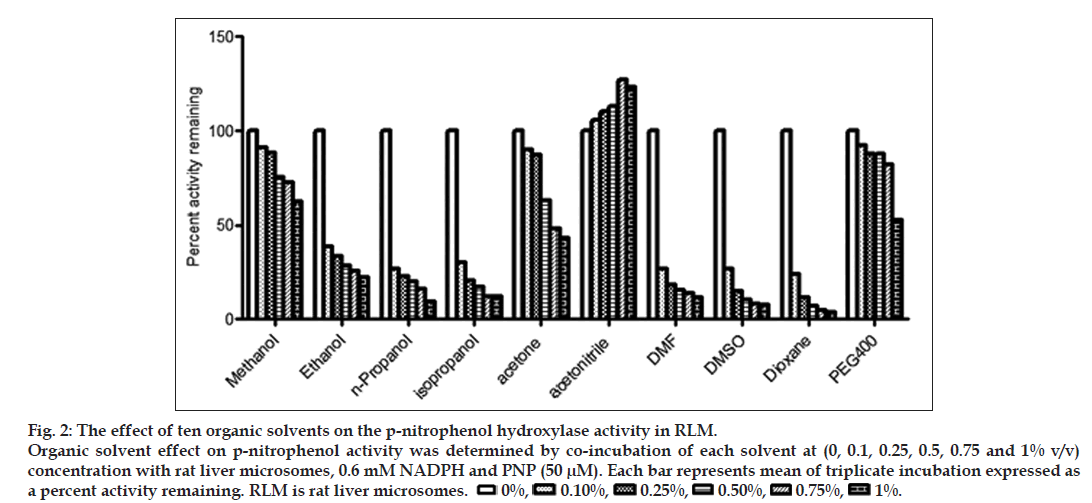
A typical chromatogram of solvent inhibition (reduction in peak area ratio of metabolite/IS formed compared to control) is shown in fig. 1. The inhibitory effect of ten different organic solvents on p-nitrophenol hydroxylase activity in RLM represented as percent activity remaining as shown in fig. 2. Overall, all the organic solvents studied except ACN, showed concentration dependent inhibition of p-nitrophenol hydroxylase activity, while, ACN showed activation (23% activation at 1% v/v) of the p-nitrophenol hydroxylase activity. Among the ten solvents evaluated, dioxane showed maximum inhibition (96% inhibition at 1% v/v) of p-nitrophenol hydroxylase activity, followed by DMSO (92% inhibition at 1% v/v) and DMF (88.1% inhibition at 1% v/v). For evaluating the best solvent out of the ten solvents studied for use to assay p-nitrophenol hydroxylase activity, the inhibition shown by solvents were rank ordered at each concentration level, for example, solvent showing least inhibition at 0.1% v/v concentration was assigned rank as 1 and solvent showing maximum inhibition at 0.1% v/v concentration was ranked as 10. Thus, the best solvent would score rank 5 (rank of 1 at each of the five concentration levels studies) while worst solvent would score rank 50 (rank of 10 at each of the five concentration levels studies) as given in Table 2. Although, we used these organic solvents in percent volume by volume basis, it must be noted that the molar concentration of these organic solvents used in the incubation vary substantially at same percent volume by volume units as shown in Table 3. Thus, the solvents having relatively low molecular weights were present in high molar concentration in the incubation samples.
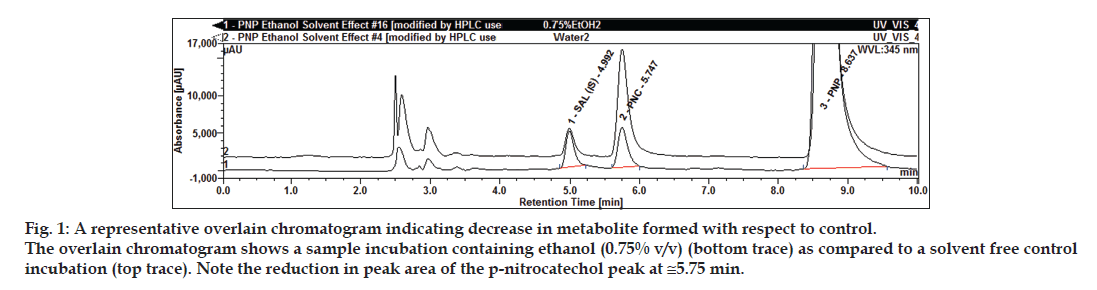
Fig. 1: A representative overlain chromatogram indicating decrease in metabolite formed with respect to control. The overlain chromatogram shows a sample incubation containing ethanol (0.75% v/v) (bottom trace) as compared to a solvent free control incubation (top trace). Note the reduction in peak area of the p-nitrocatechol peak at ≅5.75 min.
Fig. 2: The effect of ten organic solvents on the p-nitrophenol hydroxylase activity in RLM. Organic solvent effect on p-nitrophenol activity was determined by co-incubation of each solvent at (0, 0.1, 0.25, 0.5, 0.75 and 1% v/v) concentration with rat liver microsomes, 0.6 mM NADPH and PNP (50 μM). Each bar represents mean of triplicate incubation expressed as a percent activity remaining. RLM is rat liver microsomes.  0%,
0%,  0.10%,
0.10%,  0.25%,
0.25%,  0.50%,
0.50%,  0.75%,
0.75%,  1%.
1%.
| Solvents | Percent solvent (v/v) | Total out of 50 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.10% | 0.25% | 0.50% | 0.75% | 1% | ||||||||||||||||
| Avg. % inhibition | Rank | Avg. % inhibition | Rank | Avg. % inhibition | Rank | Avg. % inhibition | Rank | Avg. % inhibition | Rank | |||||||||||
| ACN | -5.7 | 1 | -10.4 | 1 | -13 | 1 | -27 | 1 | -23 | 1 | 5 | |||||||||
| PEG400 | 7.9 | 2 | 12.2 | 3 | 12.4 | 2 | 18.1 | 2 | 20.8 | 2 | 11 | |||||||||
| MeOH | 8.9 | 3 | 11.5 | 2 | 24.5 | 3 | 27.7 | 3 | 37.7 | 3 | 14 | |||||||||
| ACE | 10.2 | 4 | 12.8 | 4 | 37.3 | 4 | 51.9 | 4 | 57 | 4 | 20 | |||||||||
| EtOH | 61.5 | 5 | 66.6 | 5 | 71.2 | 5 | 74.2 | 5 | 77.5 | 5 | 25 | |||||||||
| nPA | 72.8 | 7 | 77 | 6 | 80.2 | 6 | 83.9 | 6 | 86.1 | 6 | 31 | |||||||||
| IPA | 69.6 | 6 | 79.4 | 7 | 82.7 | 7 | 87.6 | 8 | 87.6 | 7 | 35 | |||||||||
| DMF | 73.2 | 9 | 81.5 | 8 | 84.3 | 8 | 86 | 7 | 88.2 | 8 | 40 | |||||||||
| DMSO | 73 | 8 | 85.3 | 9 | 89.6 | 9 | 91.5 | 9 | 92.4 | 9 | 44 | |||||||||
| Diox | 76.2 | 10 | 88.6 | 10 | 93 | 10 | 95 | 10 | 96.3 | 10 | 50 | |||||||||
Table 2: Rank Order For Each Solvent Based On Inhibition On P-Nitrophenol Hydroxylase Activity In Rlm At Different Concentration
| Solvents | Mol. Wt. | Percent Solvent (v/v) | ||||
|---|---|---|---|---|---|---|
| 0.10% | 0.25% | 0.50% | 0.75% | 1% | ||
| Concentration in mM | ||||||
| MeOH | 32.04 | 24.7 | 61.9 | 123.7 | 185.6 | 247.4 |
| EtOH | 46.06 | 17.2 | 42.9 | 85.8 | 128.6 | 171.5 |
| nPA | 60.09 | 13.4 | 33.5 | 67.0 | 100.4 | 133.9 |
| IPA | 60.09 | 13.1 | 32.8 | 65.5 | 98.3 | 131.0 |
| ACN | 41.05 | 19.0 | 47.4 | 94.8 | 142.1 | 189.5 |
| ACE | 58.07 | 13.7 | 34.2 | 68.3 | 102.5 | 136.6 |
| DMF | 73.09 | 12.9 | 32.3 | 64.7 | 97.0 | 129.3 |
| DMSO | 78.13 | 14.1 | 35.3 | 70.5 | 105.8 | 141.1 |
| Diox | 88.11 | 11.7 | 29.4 | 58.7 | 88.0 | 117.4 |
Table 3: The Concentration Of Each Solvent Present In Incubation Expressed In mM
This is the comprehensive study of the effect of water miscible organic solvents at a concentration range of 0.1 to 1% v/v on p-nitrophenol hydroxylase (a representative of CYP2E activity) activity in RLM. In the present study, the solvent effect observed on the CYP2E activity in presence of organic solvents represents the true solvent effect since the control incubation was truly solvent free and further no reconstitution of the residue of substrate with buffer was performed. Further, the concentration of PNP (50 μM) used in this study was close to that of the Km value (110 μM) in RLM.
In this study, we have selected all possible water miscible organic solvents which can be used for dissolving NCEs/substrates while performing in vitro incubations. These includes some alcohols (MeOH, EtOH, nPA and IPA), ketone (ACE), ether (Diox), nitrile (ACN), amide (DMF), sulphoxide (DMSO) and PEG400. It was found that although these solvents have different functional groups, they all affected p-nitrophenol hydroxylase activity and except for ACN, all solvents showed inhibitory effect in concentration dependent manner (0.1 to 1% v/v) see fig. 2. This data is suggestive of the fact that solvents might have in general affected the phospholipid membrane in which the CYP450 enzymes are embedded thereby affecting the structural stability of CYP450 enzymes. In contrast, ACN was found to show activation (23% activation at 1% v/v) of the p-nitrophenol hydroxylase activity. ACN has been previously found to show the activation (139% of control) of tolbutamide hydroxylase CYP2C8/2C9 activity at 5% v/v concentration [6] in human liver microsomes. In another report, ACE, ACN and IPA were reported to show activation of caffeine N3-demethylation (CYP1A2) activity in human liver microsomes [9]. We observed that DMSO showed maximum inhibition of the p-nitrophenol hydroxylase activity while significantly less effect was observed on the imipramine metabolism (unpublished data). This was in agreement with the findings of Chauret et al. [6] Hickman et al. [9] and Li et al. [10] that DMSO affects CYP2E activity to a great extent than CYP1A activity. These evidences indicate that in addition to effects on lipid bilayer, solvents do interact with CYP450 enzymes, either by affecting the binding of substrate with the active site or through enzyme inactivation thus reducing the amount of enzyme present for catalysis. This is also evident from the findings of Kumar et al. [15] who have shown that the binding of nelfinavir to CYP3A4 enzyme improves in presence of EtOH. Kumar et al. reported that the spectral dissociation constant of nelfinavir decreases from 0.227 to 0.041 μM in presence of 20 mM EtOH [15]. Similarly, Backes and Canady [16] found that binding of ethylbenzene to the rat liver microsomes varies with the organic solvents (apparent binding constant of ethylbenzene was found to be 28, 23, 16, and 25 mM in presence of methanol, ethanol, acetone and propanol, respectively) [16] thus indicating that solvents seem to affect the first step of catalysis i.e. binding of the substrate to the active site of CYP450.
CYP2E enzyme is also known to metabolize low molecular weight compounds [17,18] like ACE, EtOH, DMSO and DMF. Acetone has been reported to be metabolized to acetol [18,19], EtOH to acetaldehyde [18,20], DMF to N-(hydroxymethyl)-N-methylformamide [21] and DMSO to dimethyl sulphone [22]. Thus, the inhibition of the p-nitrophenol hydroxylase activity in presence of these solvents may additionally be due to competition of solvents with p-nitrophenol and the effects of the metabolite of the solvent itself. One might argue that these effects can be minimized by holding the volume of solvents used in the incubation to less 1% v/v. However, if we transform these apparently low % v/v values to molar concentration scale, it can be seen that at 1% v/v concentrations, the molar concentration of DMF ACE, DMSO and EtOH are in the range of 136.64 to 171.52 mM, respectively (Table 3) and if only 10-20% conversion of these solvents were to be assumed then the respective metabolite concentrations can potentially rise to 25 to 35 mM (note that p-nitrophenol concentration in the incubation was 50 μM). Thus, the metabolites of the solvents may also play a major role in the inhibition of CYP450 activity, In this respect, Tolando et al. [21], have reported that DMF and dimethyl acetamide (DMAc) caused metabolism dependent loss of CYP450 activity in RLM. They found that the incubation of 10 mM of DMF or DMAc with RLM for 15 min resulted in 30% and 24.4% loss of total CYP450 activity, respectively, while in presence of non specific CYP450 inhibitor carbon monoxide there was little effect on the total CYP450 activity on preincubation suggesting that the loss of CYP450 activity is through generation of reactive intermediate/s during their catalysis. Further, they found that haem inactivation due to the attack of DMF and DMAc reactive metabolite on the prosthetic group of the enzyme was the probable cause for CYP450 activity loss.
Another interesting aspect of solvent effects on CYP450 activity is that, the effect of solvents varies with substrate/enzyme pair, Tang et al. [3], have reported that ACN shows substrate dependent effect on CYP2C9 activity in human liver microsomes. They reported that ACN increased diclofenac hydroxylation and tolbutamide hydroxylation activity but decreased celecoxib hydroxylation activity and that hydroxylation of phenytoin was found to be relatively resistant to its effect. Therefore, study of the potential influence of substrate dependent solvent effect on CYP450 activities also needs to be considered.
Overall, the effect of organic solvents on CYP450 activity probably represents a combined effect on the phospholipid bilayer housing the CYP450 system, the binding of substrate with the active site of enzyme and enzyme inactivation, substrate/ enzyme pair, also in some cases competitive metabolism and metabolism dependent inhibition. However the exact mechanism and the relative contribution of above stated facts on the activity of the CYP450 enzyme is not well understood. Such data would be valuable for interpretation of in vitro studies in which organic solvents are essential for effective solubilization of the substrates. Clearly, if one were to perform drug metabolism studies, the selection of appropriate organic solvent and its concentration is the critical step and can have substantial effect on the results obtained. For instance, in order to select appropriate solvent and its concentration, we have rank ordered our solvent inhibition data of p-nitrophenol hydroxylase activity from 1 to 10 at each level of concentration. The solvent showing minimum inhibition ranked 1, solvent showing maximum inhibition was ranked 10 at 0.1% v/v concentration and so on. Thus, a least inhibitory solvent would score a total of 5 and most inhibitory solvent would score a maximum 50 as shown in Table 2. It was observed that ACN has the least inhibitory effect (sum of the ranks is 5) and dioxane has the maximum inhibitory effect (sum of the rank is 50) amongst the ten solvents on p-nitrophenol hydroxylase activity. Such a table is of utility in selection of overall appropriateness of the solvent.
In conclusion, it is evident that pure aqueous incubations are preferred for CYP2E activity studies. If one has to use an organic solvent then the concentration should be kept below 1% v/v. Further, since ACN and MeOH have less influence on the CYP2E activity they are the suggested solvents of choice.
Acknowledgements
PP and TS are recipients of AICTE fellowship and SK is a BMS Research Fellow, and PP, TS, SK and KI thank BMS and AICTE for their financial support.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Wrighton SA, Ring BJ, Vandenbranden M. The use of in vitrometabolism techniques in the planning and interpretation of drug safety studies. ToxicolPathol 1995;23:199-208.
- Lewis DF, Jacobs MN, Dickins M. Compound lipophilicity for substrate binding to human P450s in drug metabolism. Drug Discov Today 2004;9:530-7.
- Tang C, Shou M, Rodrigues AD. Substrate-dependent effect of acetonitrile on human liver microsomal cytochrome P450 2C9 (CYP2C9) activity. Drug MetabDispos 2000;28:567-72.
- Aasa J, Hu Y, Eklund G, Lindgren A, Baranczewski P, Malmquist J, et al. Effect of solvents on the time dependent inhibition of CYP3A4 and the biotransformation of AZD3839 in human liver microsomes and hepatocytes. Drug MetabDispos 2013;41:159-69.
- Busby WF Jr, Ackermann JM, Crespi CL. Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug MetabDispos 1999;27:246-9.
- Chauret N, Gauthier A, Nicoll-Griffith DA. Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug MetabDispos 1998;26:1-4.
- Easterbrook J, Lu C, Sakai Y, Li AP. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyltransferase, and phenol sulfotransferase in human hepatocytes. Drug MetabDispos 2001;29:141-4.
- Gonzalez-Perez V, Connolly EA, Bridges AS, Wienkers LC, Paine MF. Impact of organic solvents on cytochrome P450 probe reactions: Filling the gap with (S)-warfarin and midazolam hydroxylation. Drug MetabDispos 2012;40:2136-42.
- Hickman D, Wang JP, Wang Y, Unadkat JD. Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug MetabDispos 1998;26:207-15.
- Li D, Han Y, Meng X, Sun X, Yu Q, Li Y, et al. Effect of regularorganic solvents on cytochrome P450-mediated metabolic activities in rat liver microsomes. Drug MetabDispos 2010;38:1922-5.
- Ma B, Shou M, Schrag ML. Solvent effect on cDNA-expressed human sulfotransferase (SULT) activities in vitro. Drug MetabDispos 2003;31:1300-5.
- Walawalkar PS, Serai PS, Iyer K. Isolation and catalytic competence of different animal liver microsomal fractions prepared by calcium-aggregation method. Indian J Pharm Sci 2006;68:262.
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. J BiolChem 1964;239:2370-85.
- Walker JM. The Protein Protocols Handbook: Humana PrInc; 1996.
- Kumar S, Earla R, Jin M, Mitra AK, Kumar A. Effect of ethanol on spectral binding, inhibition, and activity of CYP3A4 with an antiretroviral drug nelfinavir. BiochemBiophys Res Commun 2010;402:163-7.
- Backes WL, Canady WJ. The interaction of hepatic cytochrome P-450 with organic solvents. The effect of organic solvents on apparent spectral binding constants for hydrocarbon substrates. J BiolChem 1981;256:7213-27.
- Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol 1991;4:168-79.
- Koop D. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J 1992;6:724-30.
- Bondoc FY, Bao Z, Hu WY, Gonzalez FJ, Wang Y, Yang CS, et al. Acetone catabolism by cytochrome P450 2E1: Studies with CYP2E1- null mice. BiochemPharmacol 1999;58:461-3.
- Koop DR, Coon MJ. Role of alcohol P -450-oxygenase (APO) in microsomal ethanol oxidation. Alcohol 1985;2:23-6.
- Tolando R, Zanovello A, Ferrara R, Iley JN, Manno M. Inactivation of rat liver cytochrome P450 (P450) by N, N-dimethylformamide and N, N-dimethylacetamide. ToxicolLett 2001;124:101-11.
- Hucker H, Ahmad P, Miller E, Brobyn R. Metabolism of dimethyl sulphoxide to dimethyl sulphone in the rat and man. J Occup Environ Med 1966;8:556-7. As per PubMed Site Journal name is Nature 1966;209:619-20.