- *Corresponding Author:
- Ran Gao
Yunnan Provincial Key Laboratory of Entomological Biopharmaceutical Research and Development, Dali University, Dali, Yunnan Province 671000, China
E-mail: yndllyo@126.com
| Date of Received | 09 November 2021 |
| Date of Revision | 25 December 2022 |
| Date of Acceptance | 06 September 2023 |
| Indian J Pharm Sci 2023;85(5):1281-1290 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Periplaneta americana is an insect belonging to the Insectoptera family Blattella. It is one of the oldest and most fertile insect groups. In China, dried extract of Periplaneta americana has been used to treat blood stasis, acne and abdominal mass for a long time. In this study, we will explore the effect of Periplaneta americana extract on the improvement of hematopoiesis damage during cyclophosphamide treatment and its potential mechanism. Firstly, we established H22 tumor-bearing mice, gave different doses of CII-3 then quantified the count of bone marrow nucleated cell in femur and peripheral blood cells. Furthermore, we measured the thymus index and bone marrow cell cycle of the mice, and pathological analysis of femur was performed by hematoxylin and eosin staining. Finally, using real-time polymerase chain reaction to detect the messenger ribonucleic acid expression of intercellular cell adhesion molecule-1 and transforming growth factor-beta1. Periplaneta americana extract notably increase the number of bone marrow nucleated cell and peripheral blood cells, increase level of hemoglobin, raise the thymus index, induce bone marrow cells to reenter the proliferation cycle, increase the proliferation index and the messenger ribonucleic acid expression of intercellular cell adhesion molecule-1 and decrease the messenger ribonucleic acid expression of transforming growth factorbeta 1. This study found that Periplaneta americana extract can significantly improve the hematopoietic impairment induced by cyclophosphamide in tumor-bearing mice. These effects may be mediated by the increase of intercellular cell adhesion molecule-1 and decrease of transforming growth factor-beta 1.
Keywords
Liver cancer, cyclophosphamide, chemotherapy, bone marrow, hematopoietic, Periplaneta americana
Liver cancer is one of the most common malignancies with a high incidence and ranks 2nd in cancer-related deaths worldwide[1]. Surgery is the main treatment strategy of cancer. However, the prognosis is still poor because of high frequencies of postoperative recurrence and metastasis[2]. Most cases of liver cancer are diagnosed at the advanced stage, and most patients are left with palliative treatments such as chemotherapy[3]. Chemotherapy is a standard treatment for cancer. Chemotherapy can cause serious side effects, of which myelosuppression is one of the most common and serious symptoms[4]. Cyclophosphamide (CTX) is an alkylating agent belonging to the oxazaphosporine group and is widely used in cancer treatment[5]. However, CTX has some severe adverse effects, especially hematopoietic function damage. Chemotherapy for tumor can cause bone marrow damage, resulting in hematopoietic impairment and anemia, and other side effects. Thrombocytopenia can reduce the quality of life of patients and the overall effectiveness of anticancer treatments[6]. There is an urgent need to reduce the damage of hematopoiesis during chemotherapy. The treatments of modern medicine for myelosuppression include blood transfusion, platelet infusion and injection of blood cell stimulating factors. Long-term use of drugs is not only expensive, but also has many side effects. Many patients are left looking for more effective and affordable treatments[7].
In East Asia, traditional Chinese medicine is widely used to treat the myelosuppression of patients after chemotherapy. A study of Zhao et al.[8] confirmed that the traditional drug combination of Liuwei Dihuang decoction and Buzhong Yiqi decoction group can promote the proliferation of progenitor cells in myeline-suppressed mice, and promote the cell cycle from G0/G1 phase to S and G2/M phase. Another study of Zhang et al.[9] found that Fufang E'jiao Jiang could promote the proliferation rate of Hematopoietic Stem Cells (HSC) and Hematopoietic Progenitor Cells (HPC) and differentiate into different cells, thus accelerating the recovery of hematopoietic function in myelosuppressive mice. And ginsenosides have been shown to improve hematopoietic injury and immunosuppression in mice after treatment with CTX[10,11].
Periplaneta americana (P. americana) is the largest insect in the cockroach family. It is a species with a strong fecundity and ancient history[12]. In China, dried P. americana is used as a traditional Chinese medicine to treat blood stasis, acne and abdominal lumps[13]. “The Experience of Treating a Disease in Yi Ethic Medicine” recorded that P. americana is a traditional medicine of Yi people[14]. Previous studies have shown that P. americana extract CII-3 could improve cisplatin-induced myelosuppression in tumor therapy. It is demonstrated that small molecular peptides are the main active components of CII-3, and it's polypeptide content has been determined[15]. Zhou et al.[16] has isolated and identified ten compounds from CII-3, all of which are small amino acid molecules. These compounds are N-acetyl phenylalanine, (2-hydroxy propionyl) phenylalanine, N-acetyl-L-leucine, N-acetyl-Lthreonine, L-valine, L-phenylalanine, L-threonine, L-alanine, leucine and isoleucine. In this study, we will explore the effect of CII-3 on the improvement of hematopoiesis damage during CTX treatment and its potential mechanism.
Materials and Methods
Preparation of drugs:
CII-3 was provided by Associate Professor He Zhengchun of Dali University (Lot: 20200814). CTX for injection was obtained from Baxter Oncology GmbH (Lot: 9L352A). Sodium chloride for injection was obtained from Guizhou Tiandi Pharmaceutical Co., Ltd. (Lot: C20072503). And rhG-CSF injection was purchased from Xiamen Tebao Bioengineering Co., Ltd. (Lot: S20033047).
Reagents:
Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Lot: 2126369); Fetal bovine serum (Gibco, Lot: 1515306); cell cycle and apoptosis analysis kit (Beyotime Biotechnology, CAT: C1052); TRIzol reagent (Tiangen Biochemical Technology Co., Ltd, Lot: S8022); diethyl pyrocarbonate (Solarbio, Cat: D8210); 5× HiScript II qRT super mix for qPCR (Nanging Vazyme Biotech Co., Ltd., Cat: R223-01); Green Taq Mix (Nanging Vazyme Biotech Co., Ltd., Cat: P131) and 2× ChamQ SYBR qPCR master mix (Nanging Vazyme Biotech Co., Ltd., Cat: Q311-02).
Cell culture:
The H22 cells were donated by the Pharmacology Laboratory of the Dali University. The cells were incubated in a constant temperature incubator at 37°, 5 % Carbon dioxide (CO2) and saturated humidity.
Animal study:
120 Bagg Albino (BALB/c) mice old 6-8 w (half male and half female; 20±2 g; Hunan Slake Jingda Laboratory Animal Co., Ltd). (SCXK (Xiang) 2019-0004). The animal experiment was approved by the Animal Care Committee of Dali University of Chinese Medicine. The 0.2 ml volume of H22 cell suspension (2×106/ml) was injected into the enterocoelia of mice under sterile conditions for 7 d-10 d. The mice were sacrificed by cervical dislocation, and the ascites were extracted under aseptic conditions and diluted into 2×106/ml with normal saline. 0.2 ml cell suspension was injected into the right armpit of BALB/c mice to establish a H22 liver cancer mice model.
Grouping and administration of H22 tumor-bearing model:
The mice were acclimated in an environmentally controlled breeding room (temperature 22°-24°, relative humidity 50 %-60 %, 12 h light/dark cycle) for 3 d of pretreatment. Mice inoculated with tumor cells were randomly divided into 7 groups with 10 mice in each group (Table 1). After 15 d, all indexes were detected.
| Group | Route of administration | Dosage (mg/kg) |
|---|---|---|
| Normal | Intragastric administration | - |
| Tumor | Intragastric administration | - |
| L-CTX | Intraperitoneal injection | 20 |
| H-CTX | Intraperitoneal injection | 80 |
| CII-3 | Intragastric administration | 100 |
| L-CTX+CII-3 | Intraperitoneal injection+intragastric administration | 20+100 |
| H-CTX+CII-3 | Intraperitoneal injection+intragastric administration | 80+100 |
| L-CTX+rhG-CSF | Intraperitoneal injection | 20+0.012 |
Table 1: Grouping and Administration of H22 Tumor-Bearing Mice (X?±S, N=10)
Determination of nucleated cells in the bone marrow:
After the mice were sacrificed, the right thigh bone was cleaned, and the bone marrow cavity was washed with normal saline. In the end, bone marrow nucleated cells were counted.
Characteristic of peripheral hemogram:
20 μl of orbital venous plexus blood was collected from each mouse 24 h after the last administration. The number of peripheral White Blood Cells (WBCs), Red Blood Cells (RBCs), Platelets (PLT) and Haemoglobin (HGB) were measured by an automatic whole blood analyzer for veterinary use (Nanjing Pulang Medical Equipment Co., Ltd., Jiangsu, China).
Determination of thymus index:
The thymus tissue of the mice was separated and moistened with normal saline. The residual normal saline was sucked up with filter paper. The weight of thymus tissue was determined accurately, and the thymus index was calculated. The calculation formula is as follows
Thymus index=thymus mass (mg)/mouse mass (g)
Bone marrow cell proliferation activity:
Both femurs were removed from sacrificed mice 24 h after final administration. The bone marrow in the femur was rinsed with normal saline and nucleated cells were collected. The sample was centrifuged at 1000 r/min for 5 min, and the precipitated cells were retained. Then, 1 ml blood red cell lysing reagent (Solarbio, Cat: R1010) was added, mixed and allowed to wait for 5 min, and centrifuged (5 min, 1000 r/min); add 1 ml of cold Phosphate Buffer Saline (PBS), centrifuged (5 min ,1000 r/min) and leave cell precipitate. The process was repeated once to make the cell suspension. The cells were fixed in ethanol for 48 h, washed with PBS, then added with 100 μl PBS, and mixed well. Finally, the cells were stained with the 50 g/ ml concentration of propyl iodide dye (Beyotime Biotechnology, CAT: C1052-2), the reaction was performed for 30 min at 4° in darkness. The mouse bone marrow nucleated cell cycle was measured by a FACS Calibur flow cytometer (Becton, Dickinson and Company, New Jersey, United States of America (USA)). The cell proliferation index is calculated by the following formula
PI=[(S+G2/M)/(G0/G1+S+G2/M)]×100
Histopathologic examination of femur tissue:
The mice were sacrificed 24 h after the last dose. The femur was removed from the muscle and connective tissue. The femur was immersed in 10 % concentrated nitric acid for decalcification and then transferred to 10 % formaldehyde solution. The femur was wrapped in paraffin wax and cut into 5-micron pieces, stained with hematoxylin and eosin, and the bone marrow was observed under an optical microscope.
Reverse Transcription- quantitative Polymerase Chain Reaction (RT-qRCR) analysis:
Total RNA was extracted from the femurs with TRIzol reagent. 1 μg total RNA was obtained and qPCR was performed using 5× iScript™ qRT supermix with a volume of 10 μl. 1 μl of complementary Deoxyribonucleic Acid (cDNA) was amplified with specific primers and quantified on a CFX96™ Real-Time system. Using 2× ChamQ SYBR qPCR Master Mix, with normalization to Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH). Table 2 lists the nucleotide sequences of primers used for PCR. The cycling program were 95° for 15 min, followed by 45 cycles of 95° for 10 s, and 62° for 32 s. The data were calculated using the 2−ΔΔCt method.
| Target gene | Sequence of forward and reverse primers (5' to 3') | Bases | |
|---|---|---|---|
| GAPDH | Forward | AGGTCGGTGTGAACGGATTTG | 21 |
| Reverse | GGGGTCGTTGATGGCAACA | 19 | |
| ICAM-1 | Forward | TGTCAGCCACCATGCCTTAG | 20 |
| Reverse | CAGCTTGCACGACCCTTCTA | 20 | |
| TGF-β1 | Forward | ACTGGAGTTGTACGGCAGTG | 20 |
| Reverse | GGGGCTGATCCCGTTGATTT | 20 | |
Table 2: Primer Sequences
Statistical analysis:
Experimental results are calculated as the mean±Standard Deviation (SD). One-way Analysis of Variance (ANOVA) was used to compare different treatment groups. Values of p<0.05 indicates that the data is meaningful.
Results and Discussion
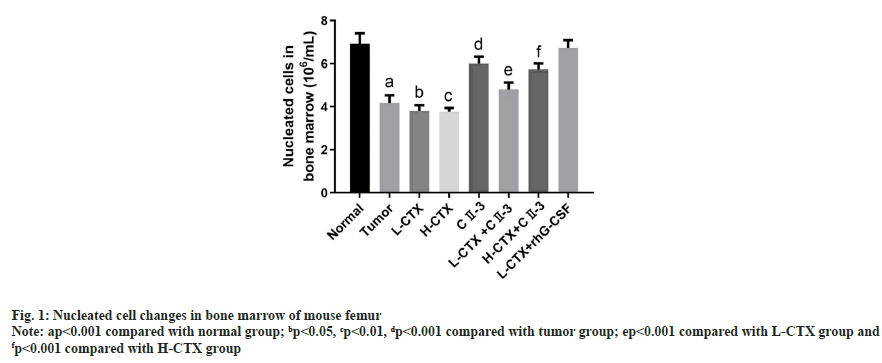
Compared with the tumor group, the number of bone marrow nucleated cells in L-CTX and H-CTX groups was significantly decreased (p<0.001). And the number of bone marrow nucleated cells increased markedly in L-CTX+CII-3 and L-CTX+human Granulocyte Colony Stimulating Factor (rhG-CSF) groups, compared with L-CTX group (p<0.001). Moreover, the number of bone marrow nucleated cells in H-CTX+CII-3 group was significantly higher than H-CTX group (p<0.001) as shown in fig. 1.
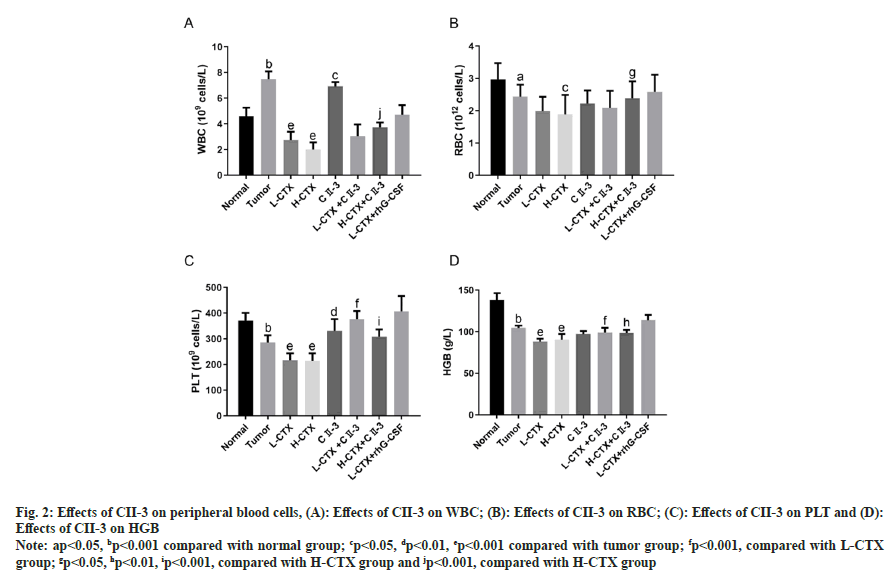
As shown in fig. 2, compared with the number in tumor group, the number of peripheral WBC, RBC and PLT in CTX groups was decreased (p<0.05 or p<0.001). And the number of PLT in L-CTX+CII-3 group was markedly increased compared with L-CTX group (p<0.001). And the number of peripheral WBC, RBC and PLT in H-CTX+CII-3 group was significantly higher than the number in H-CTX group (p<0.001 or p<0.05, fig. 2A-fig. 2C). The HGB content in CTX groups was significantly lower than that in tumor group (p<0.001), and the level of HGB in L-CTX+CII-3 group was higher than that in L-CTX group (p<0.001, fig. 2D).
Fig. 2: Effects of CII-3 on peripheral blood cells, (A): Effects of CII-3 on WBC; (B): Effects of CII-3 on RBC; (C): Effects of CII-3 on PLT and (D): Effects of CII-3 on HGB
Note: ap<0.05, bp<0.001 compared with normal group; cp<0.05, dp<0.01, ep<0.001 compared with tumor group; fp<0.001, compared with L-CTX group; gp<0.05, hp<0.01, ip<0.001, compared with H-CTX group and jp<0.001, compared with H-CTX group
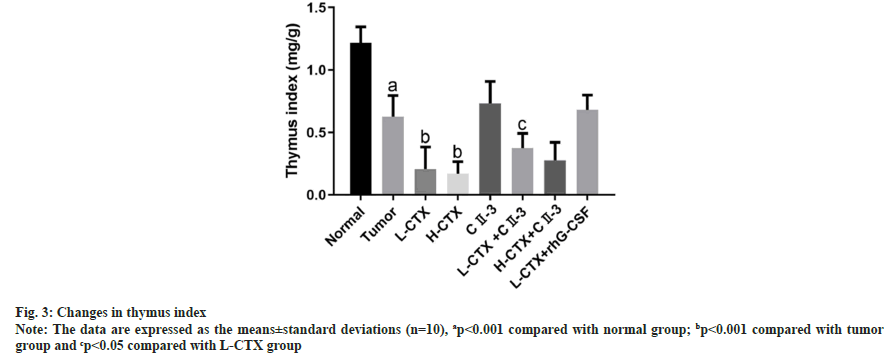
As shown in fig. 3, compared with tumor group, the thymus index of CTX groups was significantly decreased (p<0.001). However, after the administration of CII-3, the thymus index was increased in L-CTX+ CII-3 (p<0.05).
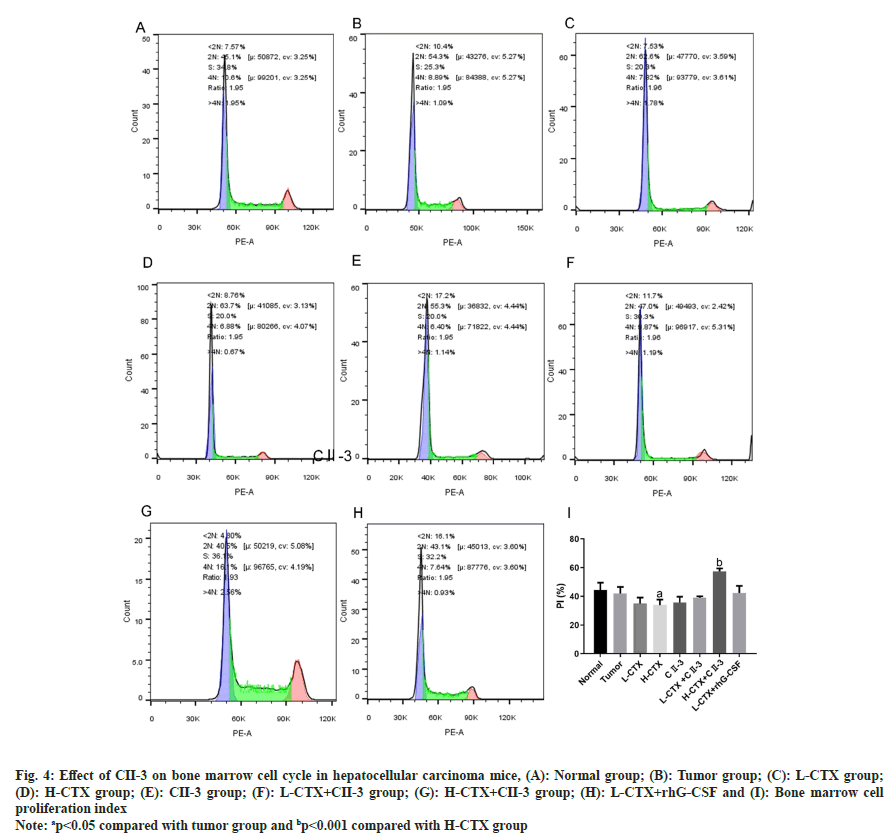
In the H-CTX group, compared with the tumor group, the percentage of S-phase cells in the bone marrow nucleated cells increased, the proportion of G1 phase cells decreased significantly, and the percentage of G2/M phase cells decreased, suggesting that the bone marrow cells were trapped in the S phase. The proliferation rate of bone marrow cells decreased after CTX administration (p<0.05, fig. 4A-fig. 4I). After the administration of C23, compared with H-CTX group, the proportion of G1 phase cells in bone marrow nucleated cells was increased, the percentage of S phase cells was decreased, the percentage of cells in G2/M phase was increased, and the proliferation index was increased. The above results indicated that CII-3 can induce bone marrow cells to enter G2/M phase from S phase and restart the cell cycle, and thus improve the proliferation rate of bone marrow cells (p<0.001, fig. 4I).
Fig. 4: Effect of CII-3 on bone marrow cell cycle in hepatocellular carcinoma mice, (A): Normal group; (B): Tumor group; (C): L-CTX group; (D): H-CTX group; (E): CII-3 group; (F): L-CTX+CII-3 group; (G): H-CTX+CII-3 group; (H): L-CTX+rhG-CSF and (I): Bone marrow cell proliferation index
Note: ap<0.05 compared with tumor group and bp<0.001 compared with H-CTX group
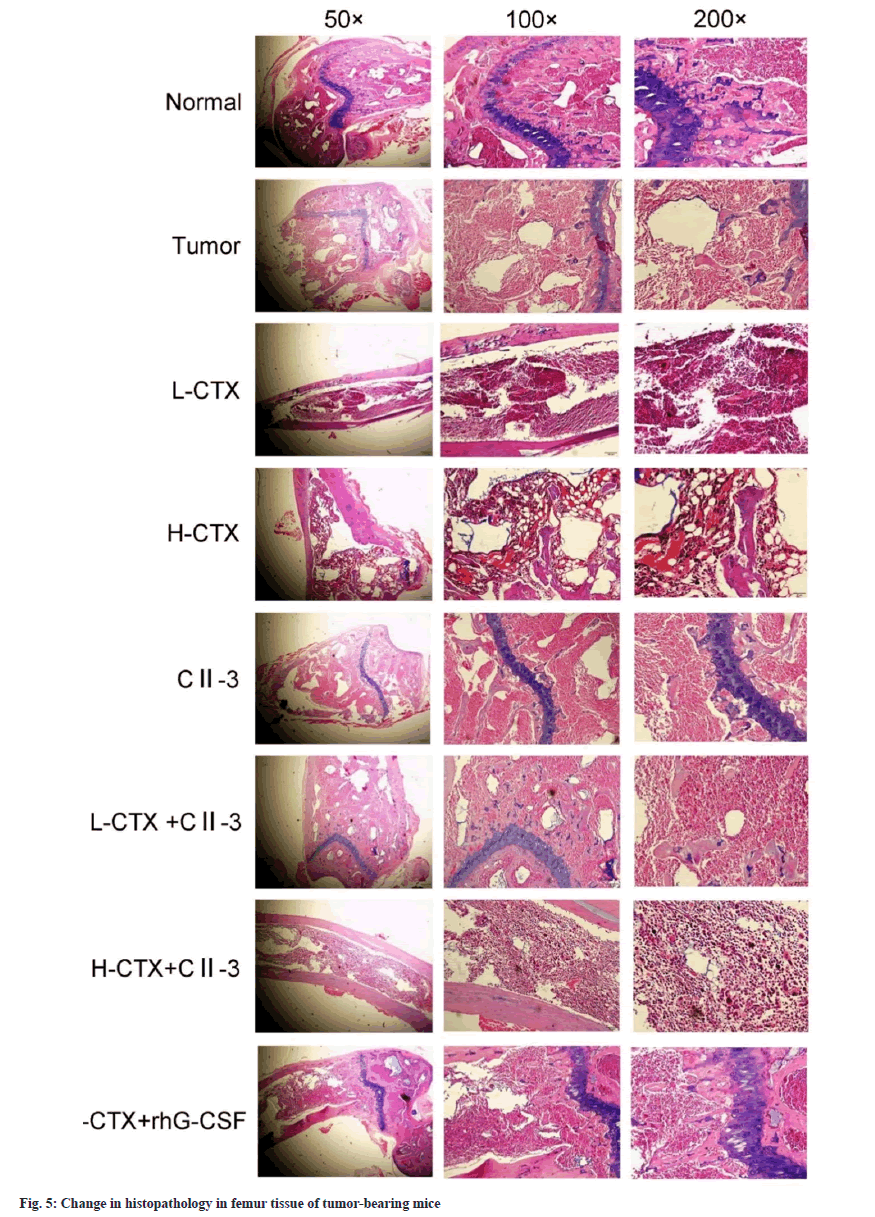
Pathological examination of the femur showed that the femur tissue structure of the normal group and the tumor group remained intact, the bone marrow cells were evenly distributed, the cells were regular, the blood sinuses were clear, and the nuclear staining was different. In CTX groups, the structure of bone marrow tissue was destroyed, the distribution of bone marrow cells was disordered, the cell density was reduced, the number of fat vacuoles was increased, the bone marrow was obviously adipose, the nucleolus of nucleus was increased, and the staining was deepened. In the L-CTX+rhG-CSF and L-CTX+CII-3 groups, the above situation was obviously improved. Compared with H-CTX group, the count of bone marrow nucleated cells increased after C23 administration. The results of the staining experiment showed that CII-3 could improve and recover the bone marrow pathological changes of CTX induced myelosuppression mice as shown in fig. 5.
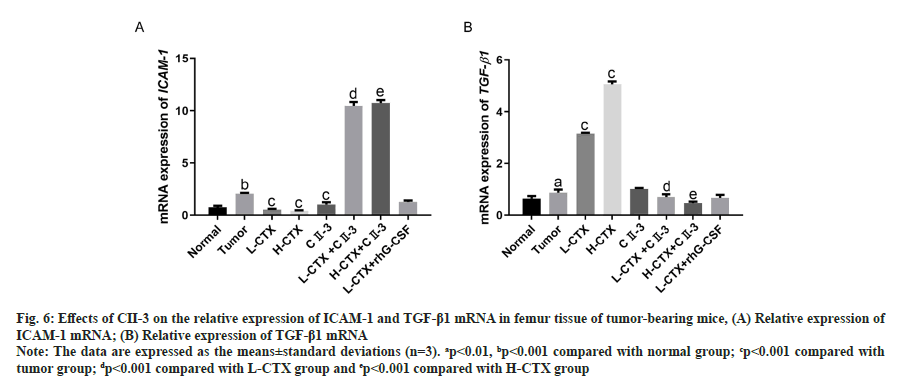
As shown in fig. 6, compared to the tumor group, the messenger Ribonucleic Acid (mRNA) level of ICAM-1 in L-CTX and H-CTX groups was significantly decreased, and the mRNA level of TGF-β1 was markedly increased (p<0.001). With the administration of CII-3, the mRNA level of ICAM-1 was significantly increased, TGF-β1 was decreased in all combined treatment groups, compared with L-CTX and H-CTX groups respectively (p<0.001).
Fig. 6: Effects of CII-3 on the relative expression of ICAM-1 and TGF-β1 mRNA in femur tissue of tumor-bearing mice, (A) Relative expression of ICAM-1 mRNA; (B) Relative expression of TGF-β1 mRNA
Note: The data are expressed as the means±standard deviations (n=3). ap<0.01, bp<0.001 compared with normal group; cp<0.001 compared with tumor group; dp<0.001 compared with L-CTX group and ep<0.001 compared with H-CTX group
At present, chemotherapy and radiotherapy are one of the main treatments for malignant tumors. But they don't just kill cancer cells, they can destroy healthy cells, cause bone marrow suppression and destroy the blood-forming system[17,18]. Drug combinations can make cancer cells more sensitive to chemotherapy drugs. An increasing number of studies have shown that traditional Chinese medicine has great potential in reducing the side effects of chemotherapeutic drugs[19]. In this study, we found P. americana extract could reduce the myelosuppression of CTX and significantly improve the hematopoietic function of mice.
The amount of bone marrow nucleated cell is an objective index of judgment of bone marrow hemopoietic function[20]. Wang et al.[21] find that different proportion of ginseng and prepared Rehmannia root could increase the number of bone marrow nucleated cell, improve CTX-induced bone marrow suppression. In this study, we found CII- 3 significantly increased the number of nucleated cells in bone marrow. And the photograph of HE staining proved that CII-3 could significantly reduce the distribution disorder and cell density of bone marrow cells induced by CTX.
Chemotherapy can destroy the precursor activity of peripheral blood cells, reduce the levels of WBCs, PLTs and HGB in peripheral blood, and shorten the life span of blood cells[22]. Because of the different cycle of hematopoietic cells, the inhibitory effect of chemotherapy drugs on hematopoietic cells in bone marrow is also different. The shorter the cell cycle, the more easily it is inhibited. WBC and PLT-l count can more accurately reflect the hematopoietic function of mice[23]. Our studies indicated that after the administration of CII-3, the peripheral blood cells (especially, the WBC and PLT) significantly increased and recovered to varying degrees.
Thymus is the main hematopoietic organs[24]. Decreased thymus index means that the body's immune function and hematopoietic function may be decreased[25]. This study indicated that CII-3 could normalize the thymus index of mice after chemotherapy, suggesting that CII-3 has protective effect on the immune system after chemotherapy and may have a potential role in promoting extramedullary hematopoiesis.
The cell cycle changes of bone marrow cells can reflect the proliferative activity of bone marrow cells and the changes of hematopoietic system. CTX can cause mouse bone marrow cells to stall in S phase and the proliferation index of bone marrow nucleated cells to decrease. CII-3 induced the increase of G1 phase cells, decrease of S phase cells, increase of G2/M phase cells and increase of cell proliferation index in myelosuppressed mouse bone marrow cells. The effects of CII-3 on promoting the recovery of bone marrow cells could be attributed to promoting bone marrow cells entering the proliferative cycle phase (G2/M).
ICAM-1 is a glycoprotein on the cell surface, is normally expressed at a low level in cells, but is upregulated in response to inflammatory stimuli[26]. Previous studies showed that ICAM-1 deficiency in the niche impaired hematopoietic stem cells quiescence and repopulation capacity[27]. Previous studies have found that TGF-β1 can affect the chemotherapydifferentiation and proliferation of hematopoietic progenitor cells in bone marrow, and can also induce the differentiation of granulocytes and macrophages through rhG-CSF[28]. TGF-β1 is also an effective immunosuppressant, which can strongly inhibit lymphocyte proliferation and suppress many functions of immune cells[29]. Our studies showed that CII-3 could increase the mRNA expression of ICAM-1, decrease the mRNA expression of TGF-β1 of bone marrow cells. This may be the underlying mechanism by which CII- 3 improve chemotherapy-induced hematopoietic damage in bone marrow.
In conclusion, our results provide strong evidence that P. americana extract could improve the hematopoiesis damage induced by CTX in mice. The results indicate that CII-3 alleviate the chemotherapy-induced reduction of the counts of bone marrow nucleated cell, peripheral blood cell and HGB. Meanwhile, they can raise the thymus index, promote bone marrow cells entering the proliferative cycle phase and increase the PI. And these effects may be mediated by ICAM-1 and TGF-β1. This study may help in the development of novel drugs and the prevention of side effects.
Funding:
We would like to thank the fundamental research general project of local universities in Yunnan Province (202101BA070001-119) for the support of this study.
Conflict of interests:
The authors declared no conflict of interests.
References
- Zhao J, Fu Y, Wu J, Li J, Huang G, Qin L. The diverse mechanisms of miRNAs and lncRNAs in the maintenance of liver cancer stem cells. Biomed Res Int 2018;2018:2018:8686027.
[Crossref] [Google Scholar] [PubMed]
- Zhu CP, Wang AQ, Zhang HH, Wan XS, Yang XB, Chen SG, et al. Research progress and prospects of markers for liver cancer stem cells. World J Gastroenterol 2015;21(42):12190.
- Wang Z, Zhou W, Zhang H, Qiao L. Combination of anti-angiogenesis agents and transarterial embolization: Is it a promising approach for the treatment of liver cancer? Discov Med 2015;20(108):51-5.
[Google Scholar] [PubMed]
- Cao W, Gu Y, Meineck M, Xu H. The combination of chemotherapy and radiotherapy towards more efficient drug delivery. Chem Asian J 2014;9(1):48-57.
[Crossref] [Google Scholar] [PubMed]
- Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother Pharmacol 2016;78:661-71.
[Crossref] [Google Scholar] [PubMed]
- Bartucci M, Dattilo R, Martinetti D, Todaro M, Zapparelli G, di Virgilio A, et al. Prevention of chemotherapy-induced anemia and thrombocytopenia by constant administration of stem cell factor. Clin Cancer Res 2011;17(19):6185-91.
[Crossref] [Google Scholar] [PubMed]
- Yang S, Che H, Xiao L, Zhao B, Liu S. Traditional Chinese medicine on treating myelosuppression after chemotherapy: A protocol for systematic review and meta-analysis. Medicine 2021;100(4):e24307.
[Crossref] [Google Scholar] [PubMed]
- Zhao AB, Bin YU, Xian-Lin WU, Cao KJ, En-Qing LI, Qing-Mei LI, et al. Protective effects on myelosuppression mice treated by three different classic Chinese medicine formulae. Pharmacogn Mag 2011;7(26):133.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Ye T, Hong Z, Gong S, Zhou X, Liu H, et al. Pharmacological and transcriptome profiling analyses of Fufang E'jiao Jiang during chemotherapy-induced myelosuppression in mice. J Ethnopharmacol 2019;238:111869.
[Crossref] [Google Scholar] [PubMed]
- Pan L, Zhang T, Cao H, Sun H, Liu G. Ginsenoside Rg3 for chemotherapy-induced myelosuppression: A meta-analysis and systematic review. Front Pharmacol 2020;11:649.
[Crossref] [Google Scholar] [PubMed]
- He M, Wang N, Zheng W, Cai X, Qi D, Zhang Y, et al. Ameliorative effects of ginsenosides on myelosuppression induced by chemotherapy or radiotherapy. J Ethnopharmacol 2021;268:113581.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Yang A, Tu P, Hu Z. Anti-tumor effects of the American cockroach, Periplaneta americana. Chin Med 2017;12(1):1-6.
- Ma X, Sun J, Ye W, Huang Y, Sun C, Tao Y, et al. Pro-apoptotic effects of Kangfuxin on human stomach cancer cells and its underlying mechanism. Oncol Lett 2018;16(1):931-9.
[Crossref] [Google Scholar] [PubMed]
- Zheng K. The experience of treating a disease in yi ethic medicine. Yunnan Science and Technology Press, Kun Ming; 2007.
- Yang Q, Shi M, Liu G, Peng F, Liu X, Guo M. Synergistic and toxicity-attenuating effects of Periplaneta americana extract CII-3 combined with cisplatin on lewis lung cancer-bearing mice. Pharmacog Mag 2020;16(72):817-29.
- Zhou J. Study on quality evaluation and active ingredient of anti-hepatoma of the bulk drug CII-3 of the Meilianjing capsule. Dali University, China; 2019.
- Wang X, Chu Q, Jiang X, Yu Y, Wang L, Cui Y, et al. Sarcodon imbricatus polysaccharides improve mouse hematopoietic function after cyclophosphamide-induced damage via G-CSF mediated JAK2/STAT3 pathway. Cell Death Dis 2018;9(6):578.
- Ito K, Ito K. Hematopoietic stem cell fate through metabolic control. Exp Hematol 2018;64:1-1.
[Crossref] [Google Scholar] [PubMed]
- Zheng X, Fu Z, Wang C, Zhang S, Dai M, Cai E, et al. Effect of Panax ginseng combined with Angelica sinensis on the dissolution of ginsenosides and in chemotherapy mice hematopoietic function. Bioorgan Med Chem 2019;27(18):4211-8.
[Crossref] [Google Scholar] [PubMed]
- Chen YZ, Lin F, Zhuang GB, Ren Y, Li PP. Protective effect of Renshen Yangrong decoction on bone marrow against radiation injury in mouse. Chin J Integr Med 2011;17(6):453-8.
[Crossref] [Google Scholar] [PubMed]
- Wang SQ, Yang X, Zhang YG, Cai EB, Zheng XM, Zhao Y, et al. Study on the changes of chemical constituents in different compatibilities of ginseng-prepared Rehmannia root and their effects on bone marrow inhibition after chemotherapy. Chem Pharm Bull 2020;68(5):428-35.
[Crossref] [Google Scholar] [PubMed]
- Han M, Jia X, Cai E, Yang L, Dai M, Sun N, et al. The effects of arctigenin-valine ester on chemotherapy-induced myelosuppression in mice. Bioorgan Med Chem 2019;27(12):2480-6.
[Crossref] [Google Scholar] [PubMed]
- Jia Y, Du H, Yao M, Cui X, Shi Q, Wang Y, et al. Chinese herbal medicine for myelosuppression induced by chemotherapy or radiotherapy: A systematic review of randomized controlled trials. Evid Based Complement Alternat Med 2015;2015:690976.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Sun H, Wang C, Zheng X, Jia X, Cai E, et al. Comparative analysis of active ingredients and effects of the combination of Panax ginseng and Ophiopogon japonicus at different proportions on chemotherapy-induced myelosuppression mouse. Food Function 2019;10(3):1563-70.
- Han J, Wang Y, Cai E, Zhang L, Zhao Y, Sun N, et al. Study of the effects and mechanisms of ginsenoside compound K on myelosuppression. J Agric Food Chem 2019;67(5):1402-8.
- Bui TM, Wiesolek HL, Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution and tumorigenesis. J Leucoc Biol 2020;108(3):787-99.
[Crossref] [Google Scholar] [PubMed]
- Liu YF, Zhang SY, Chen YY, Shi K, Zou B, Liu J, et al. ICAM-1 deficiency in the bone marrow niche impairs quiescence and repopulation of hematopoietic stem cells. Stem Cell Rep 2018;11(1):258-73.
[Crossref] [Google Scholar] [PubMed]
- Yamaguchi Y, Tsumura H, Miwa M, Inaba K. Contrasting effects of TGF-ß1 and TNF-a on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells 1997;15(2):144-53.
[Crossref] [Google Scholar] [PubMed]
- Bonham CA, Lu L, Banas RA, Fontes P, Rao AS, Starzl TE, et al. TGF-ß1 pretreatment impairs the allostimulatory function of human bone marrow-derived antigen-presenting cells for both naive and primed T cells. Transpl Immunol 1996;4(3):186-91.
[Crossref] [Google Scholar] [PubMed]