- *Corresponding Author:
- Zhuoni Zhang
Department of Oncological Surgery, China
E-mail: Zhangzn7976@tzzxyy.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “277-283” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To provide reference basis for the apoptosis of breast cancer cells and the carcinogenesis of breast cancer, the effects of 7,12-dimethylbenz(a)anthracene on apoptosis regulators Fas ligand and B-cell lymphoma-2 were investigated. In this study, 62 female C57BL/6 mice aged from 4 w to 6 w were randomly divided into control group and test group, with 31 mice in each group. The test group was given 7,12-dimethylbenz(a) anthracene solution by gavage at a dose of 50 mg/kg and the control group was given normal saline of equal volume. On the 2nd d after the experiment, all the mice were killed by cervical dislocation. The results showed that after hematoxylin-eosin staining, the tumor cells in the test group were stacked up to form a substantial structure. The expression level of Fas ligand protein in the control group was greatly lower than that in the test group and the positive rate was 20.25 %, which was greatly lower than that of 89.65 % in the test group (p<0.01). The expression level of B-cell lymphoma-2 protein in the mammary gland tissues of mice in the test group was greatly higher than that of the control group and its positive rate was 87.96 %, which was greatly higher than that of 31.48 % in the control group (p<0.01). The expression levels of Fas ligand messenger ribonucleic acid in the mammary gland tissues of mice in the test group and control group were 5.82±4.37 and 1.27±0.12, respectively and there was a statistically obvious difference (p<0.05). The messenger ribonucleic acid expression levels of B-cell lymphoma-2 in the test group and the control group were 18.97±2.65 and 2.02±0.54, respectively, and there was an extremely obvious difference (p<0.01). The apoptosis rate of mammary gland cells in the test group was (19.79±3.53) % and that in the control group was (2.93±0.28) % and there was an extremely obvious difference (p<0.01). It indicated that 7,12-dimethylbenz(a)anthracene inhibited the apoptosis of breast cancer cells by regulating the upregulation of Fas ligand and B-cell lymphoma-2 expression.
Keywords
7,12-dimethylbenz(a)anthracene, Fas ligand gene, B-cell lymphoma-2 gene, breast cancer cell, apoptosis
Breast Cancer (BC) is one of the malignant tumors that harm female health in recent years and is with the highest incidence among female malignant tumors[1]. According to statistics, nearly 1 million BC patients are diagnosed every year, 60 % of whom die from it[2]. The carcinogenesis of tumor results in abnormal cell proliferation and death. Current studies show that the inhibition of apoptosis has a significant correlation with the carcinogenesis and progression of malignant tumors[3]. Apoptosis is not only important in maintaining normal morphology and functions of tissues and organs, but also is related to physiological processes such as growth, development, reproductive aging, etc. And it is related to the carcinogenesis of malignant tumors, carcinogenesis of proliferative diseases and other pathological phenomena[4].
With the continuous development of cell molecular technology, researchers have found that the genes of the B-cell lymphoma-2 (Bcl-2) family are involved in the regulation of apoptosis[5], which is the central regulator of programmed cell death and one of the key factors in the regulation of apoptosis. Bcl-2 gene is a representative apoptotic inhibiting factor in the Bcl-2 family[6], which not only delays tumor proliferation, but is positively correlated with physiological behaviors such as low cell proliferation rate and less cell necrosis[7]. Many studies have found that the expression of Bcl-2 gene is related to the development of gastric cancer, pancreatic cancer, BC and other tumors[8]. Bcl-2 can resist cell death in a variety of ways and is recognized as an anti-apoptotic factor currently[9]. Fas/Fas Ligand (FasL) is a pair of proteins mediating apoptosis. FasL also known as death factor, is a transmembrane protein in the Tumor Necrosis Factor (TNF) family[10], whose mechanism is mainly to regulate apoptosis by activating the expression of T lymphocytes and Natural Killer (NK) cells. Studies have found that when FasL binds to the receptor Fas, a variety of proteases or endogenous endonuclease are activated, causing apoptosis of cells that express Fas, transmitting the signal to inner cell membrane and eventually leading to programmed cell death[11]. FasL expression varies greatly among different types of tumors, whose expression is higher in lymphatic system-related cancers and greatly high in BC, liver cancer and other non-lymphatic system tumors[12].
7,12-Dimethylbenz(a)anthracene (DMBA) is a kind of polycyclic aromatic hydrocarbon, which is a lipophilic carcinogen. It can induce multiple tumors such as BC and leukemia, ovarian cancer and leukemia, etc. Many researchers often use DMBA to establish animal models of BC or leukemia through gavage, local application or subcutaneous injection. Studies have found that DMBA dose of 15 mg can induce 100 % breast tumor in rats[13]. Other studies have found that there are many similarities between DMBA induced BC and human BC in tumor tissue morphology, tumor origin and hormone dependent of tumor[14]. But the pathogenesis of BC is still unclear. Although DMBA is often used as a modeling drug for animal BC research, yet there are few studies on the effect of DMBA on FasL gene and Bcl-2 gene expression and the relationship between DMBA and apoptosis of BC cells.
Based on the current lack of studies on the influence of DMBA on FasL gene and Bcl-2 gene expression, DMBA was used as a modeling drug for BC to explore the influence of DMBA on FasL gene and Bcl-2 gene expression and the relationship between DMBA and apoptosis of BC cells.
Materials and Methods
Experimental animals and grouping:
62 female C57BL/6 mice aged from 4 w to 6 w, weighing 20 g-25 g, were kept in a clean laboratory animal room with room temperature of 25° and relative humidity of about 55 %. Under 12 h of light, they could drink and eat freely[15]. After 2 w of adaptive feeding, all mice were randomly divided into Control Group (CG) and Test Group (TG), with 31 mice in each group. The TG was given DMBA solution at a dose of 50 mg/kg once a week for 5 w and the CG was given normal saline of equal volume. The mice in both groups were raised in normal way. All animal experiments in this study were approved by the experimental animal management committee and the experimental methods were conducted according to the approval guidelines.
Morphological observation of mice Mammary Gland Tissue (MGT):
The morphology of mice mammary gland was observed after Hematoxylin-Eosin (HE) staining. On the 2nd d after the experiment, all mice were sacrificed by cervical dislocation and the mammary gland of the mice was completely removed. The second pair of mammary glands of the mice was taken, fixed by formalin, embedded in paraffin and sliced in 4 μm continuously. The obtained sections were dewaxed with xylene for 30 min, hydrated with different alcohol gradients for 50 min and washed with haematoxylin for 20 min. After washing, the sections were dyed with eosin for 2 min, dehydrated with different gradient alcohol for 30 min and xylene was used for transparency for 20 min. Neutral gum was used to seal the sections and the pathomorphological diagnostic criteria referred to the conventional criteria[16].
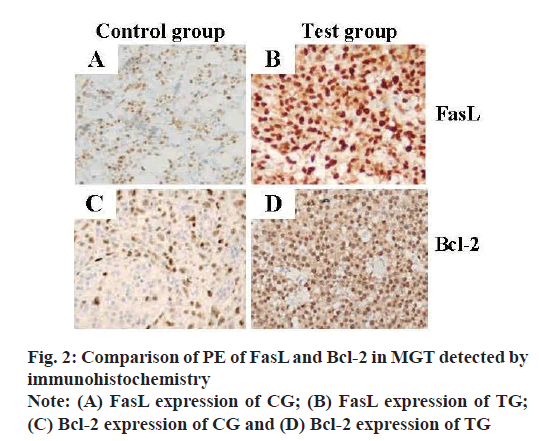
Protein Expression (PE) of FasL and Bcl-2 detected by immunohistochemistry:
10 mammary tissue specimens were taken from each group and the PE of FasL and Bcl-2 was detected by immunohistochemical Streptavidin-Peroxidase (SP) method. Anti-Fasl and anti-Bcl-2 antibodies were used as primary antibodies. The SP method was operated according to the normal operation[17,18]. The expression of FasL and Bcl-2 protein detected by SP method was as follows. Under 400x microscopic observation, the brown yellow staining of FasL and Bcl-2 cytoplasm or cell membrane was positive and the average proportion of positive cells was the Positive Rate (PR).
Messenger Ribonucleic Acid (mRNA) expression of FasL and Bcl-2 detected by quantitative Polymerase Chain Reaction (qPCR):
Total RNA was extracted from mice BC cells. Trizol was used for RNA extraction and the specific operation methods were as follows. After the BC cells were ground with liquid nitrogen, 1 ml of Trizol was added and the cells were centrifuged for 5 min at 12 000 r/ min; the supernatant was taken and mixed with 200 μl of chloroform for oscillation for 15 s; after being placed at room temperature for 10 min, 600 μl of isopropyl alcohol was added after centrifugation; after centrifugation, the supernatant was discarded, washed with 75 % ethanol, dried and dissolved in Diethyl Pyrocarbonate (DEPC). The Hifair® III 1st Strand cDNA Synthesis SuperMix for qPCR reverse transcription kit (Takara) was used. The quantitative primer of FasL was synthesized, the sequence was Fas-F: GATGACCGTCTTGGCTGTCC; Fas-R: CATCGCTGAACGCTACTGGG; and the quantitative primer of FasL was synthesized, the sequence was Bcl-2-F: TGGCTGTCTCTGAAGACGCT; Bcl-2-R: CTGCTGACCTCACTTGTGGC. The GAPDH gene of mice was selected as internal reference. The primer of Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) gene for amplification were Fm: AGTTCAACGGCACAGTCAAGG and Rm: CAGCCTTCTCCATGGTGGTG. Each sample was amplificated with the three pairs of primers and each reaction was repeated for 3 times. The annealing temperature was 60° and the annealing time was 30 s, with 30 cycles. The 2-ΔΔct method was used to calculate the relative expression quantity[19].
Culture method of mice BC cells:
Mouse BC cells 4T1 were purchased from the cell bank of Shanghai institute of cell biology, Chinese academy of sciences. The medium was Roswell Park Memorial Institute (RPMI)-1640 culture medium, penicillin and streptomycin were added as double antibodies and 10 % fetal calf serum was added at the same time. The cells were cultured in a constant temperature incubator at 37° and 5 % CO2 for 3 to 4 d for subculture.
Detection of apoptosis in BC cells:
Terminal deoxynucleotidyl Transferase (TdT) dUTP Nick-End Labeling (TUNEL) method was used to detect the apoptosis of BC cells in mice[20]. The specific operation methods were as follows. The embedded tissue sections were washed with xylene twice, 5 min for each time; 95 % and 75 % ethanol were used for washing for 2 twice each, 5 min for each time; after washing with Phosphate Buffered Saline (PBS), protease K was added to remove the histones; the sections were rinsed with distilled water and added to PBS solution containing 2 % Hydrogen Peroxide, (H2O2) for reaction for 5 min; after the sections were washed with PBS, TdT enzyme buffer was added, after 3 min of reaction, the TdT enzyme reaction solution was added for reaction at 37° for 1 h and the termination solution was added to terminate the reaction. After washing with PBS, anti-digoxin antibody labeled by peroxidase was added for 30 min of reaction, then the sections were washed with PBS, 0.05 % Diaminobenzidine (DAB) color reagent was added; after washing with distilled water, methyl green was added for re-dyeing[21]. After the sections were cleaned with distilled water, xylene was used for dehydration, sealed and dried, and fluorescence was observed under fluorescence microscope. The apoptosis rate was the proportion of apoptotic cells and the calculation method was Apoptotic Index (AI)=(number of apoptotic cells/total number of tumor cells)×100 %.
Statistical method:
The data statistics was analyzed using Statistical Package for the Social Sciences (SPSS) 19.0. The mean±standard deviation (x̄±s) was used to express measurement data and the enumeration data were expressed as percentage. The measurement data conforming to normal distribution were tested by t test and those not conforming to normal distribution were tested by Wilcoxon test. p<0.05 indicated that there was a statistically significant difference.
Results and Discussion
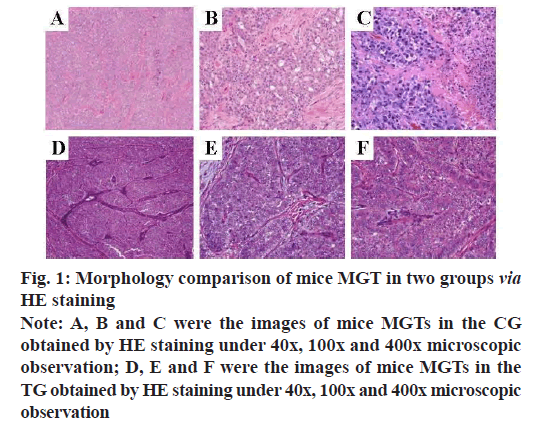
After the two groups of mice were sacrificed by cervical dislocation, their MGTs were taken and observed under a microscope after HE staining. The results were shown in fig. 1. Under the 40x microscopic observation, compared with the CG, the tumor cells in the TG were arranged into a substantial structure, separated by multiple fibers. Under 100x microscopic observation, the tumor cells formed microcapsules and microtubules, the nuclei were smaller than those of the CG, the nucleoli were clear and the cytoplasm secretions were stained slightly. Under 400x microscopic observation, there are abundant eosinophilic and basophilic secretions within the tumor cells and between the microcapsule spaces.
Fig. 1: Morphology comparison of mice MGT in two groups via HE staining
Note: A, B and C were the images of mice MGTs in the CG
obtained by HE staining under 40x, 100x and 400x microscopic
observation; D, E and F were the images of mice MGTs in the
TG obtained by HE staining under 40x, 100x and 400x microscopic
observation
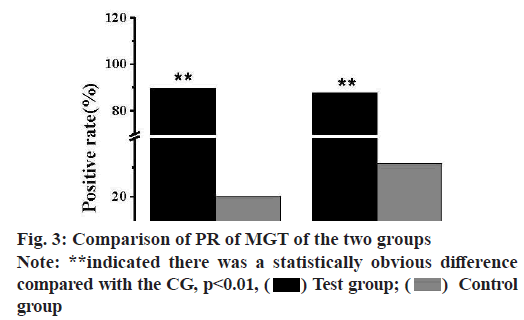
FasL and Bcl-2 were both located in the cell membrane and cytoplasm in the immunohistochemical staining of mice BC tissues, as shown in fig. 2. The expression level of FasL protein in the MGTs of the CG was greatly lower than that of the TG. Meanwhile, the proportion of positive cells in the MGTs of the two groups, which was the PR, was calculated and analyzed, and the results were shown in fig. 3. The PR of FasL PE was 20.25 % in the CG and 89.65 % in the TG, there was a statistically obvious difference (p<0.01). The expression rate of Bcl-2 protein in the MGTs of the TG was greatly higher than that of the CG and the PR of FasL PE in the MGTs of the TG and CG were 87.96 % and 31.48 % respectively, and there was a highly obvious difference (p<0.01).
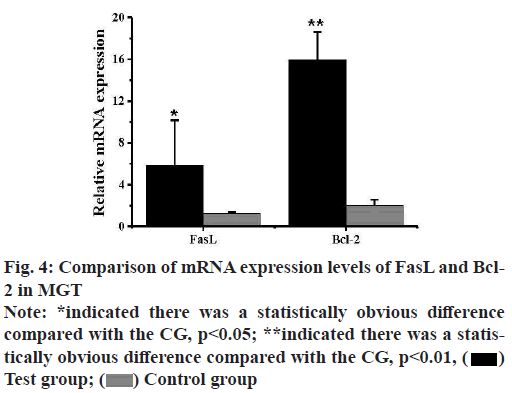
The expression levels of mRNA of FasL and Bcl-2 in the MGTs of mice in the TG and CG were analyzed by qPCR and the results were shown in fig. 4. The expression level of FasL mRNA in the MGTs of mice in the TG was 5.82±4.37, while that of mice in the CG was 1.27±0.12 and there was a statistically obvious difference (p<0.05). The expression level of Bcl-2 mRNA in the MGTs of mice in the TG was 18.97±2.65, while that in the MGTs of mice in the CG was 2.02±0.54 and there was an extremely obvious difference (p<0.01).
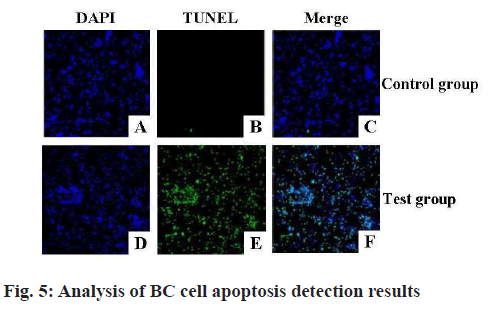
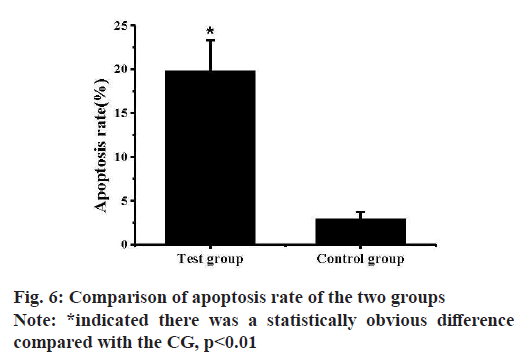
Breast tissue sections of the two groups of mice were examined by TUNEL method to detect the apoptosis of mice breast cells and the results were shown in fig. 5. Almost none of the mammary gland cells of the CG mice were stained by TUNEL, indicating that there was no apoptosis in the CG cells. Almost all the mammary gland cells of the TG mice were stained by TUNEL, indicating that the mammary gland cells in the TG entered apoptosis state. And the apoptosis rates of cells in the MGTs of the two groups of mice were calculated and analyzed, the results were shown in fig. 6. The apoptosis rate of mice mammary gland cells in the TG was (19.79±3.53) % and that of the CG was (2.93±0.28) % and there was an extremely obvious difference (p<0.01).
Apoptosis and proliferation of tumor cells play an important role in the carcinogenesis and development of tumor. Studies showed that Fas and ligand FasL were important regulating factors inducing apoptosis and maintaining the body’s balance[22,23]. The effect of Dimethyl acetylenedicarboxylate (DMAD) on FasL and Bcl-2 expression level and breast cell apoptosis were studied by inducing BC in mice of the TG through DMBA[24]. The results showed that the expression level of FasL protein in the MGTs of the CG was greatly lower than that of the TG and the PR of FasL expression detected by the immunohistochemical method was 20.25 %, which was statistically significant compared with the PR of 89.65 % in the TG (p<0.01). The relative expression of FasL was further analyzed by qPCR and it was found that the mRNA expression of FasL in the MGTs of mice in the TG was 5.82±4.37, which was greatly different from that in the CG 1.27±0.12 (p<0.05). At present, a large number of studies showed that[25,26] the PR of FasL in BC tissues was greatly higher than that in normal MGT. And studies have found that FasL expression level was greatly different in different MGTs, with the highest expression level in BC tissues, followed by breast tumors, both of which were greatly higher than that in normal MGTs[27]. Fan et al.[28] also found that in the immunohistochemistry results, the expression rate of FasL positive cells increased in normal cells, mammary gland hyperplasia and mammary gland. The results of this study also showed that FasL expression in BC tissues was higher than that in normal MGT, which was consistent with the current research results. This indicated that the expression of FasL had a certain correlation with the tumor progression of BC[29]. TUNEL method was used to detect the apoptosis of mice breast cells, it was found that almost none of the cells in the CG was stained by TUNEL, while almost all the cells in the TG were stained by TUNEL. Cubero et al.[30] found that in the immunohistochemical detection of MGT, a large number of cells in FasL positive MGT showed apoptosis, indicating that FasL expression was high and a large number of cells in MGT showed apoptosis compared with the normal CG. The results of this study were consistent with those of Cubero et al.
Bcl-2 gene also known as apoptosis suppressor gene, is currently recognized as a tumor cell apoptosis suppressor[31]. Bcl-2 can induce apoptosis through a variety of pathways and mechanisms and is involved in the carcinogenesis and development of tumors[32,33]. In this study, the expression rate of Bcl-2 protein in the MGTs of the two groups of mice was analyzed and it was found that the positive expression rate of Bcl- 2 protein in the TG was 87.96 %, which was greatly higher than that in the CG (31.48 %) and there was an extremely obvious difference between the two groups (p<0.01). The expression level of Bcl-2 mRNA was analyzed by qPCR and it was found that the expression level of Bcl-2 mRNA in the MGTs of mice in the TG was 18.97±2.65, while that in the MGTs of mice in the CG was 2.02±0.54 and there was an obvious significant difference (p<0.01). The results of Zhou et al.[34] showed that the PR of Bcl-2 in MGT was 28.42 % and there was no statistically obvious difference from the control normal MGT, which was contrary to the results of this study. The possible reason was that Bcl-2 had both anti-proliferation and anti-apoptosis effects in cells and was expressed in normal MGT, which could regulate the normal tissue to survive rather than apoptosis[35]. Studies showed that with the cell proliferation, the anti-proliferation effect of Bcl-2 gradually weakened, showing the anti-apoptotic activity, so the expression level of Bcl-2 in the early stage of the tumor was not greatly changed. It was also possible that the expression of Bcl-2 was related to the degree of tumor cell proliferation and the size of tumor cells[36], resulting in different research results. The results of this study showed that the apoptosis rate of mammary gland cells in the TG was (19.79±3.53) %, which was greatly higher than that in the CG (2.93±0.28) % and there was an extremely obvious difference (p<0.01). The study results of Senturk et al.[37] also showed that the apoptosis rate in BC tissues was greatly higher than that in normal tissues, which was consistent with the results of this study. The apoptosis rate was increased and the apoptosis was active. Meanwhile, the proliferation rate was accelerated and the DNA replication error rate was increased due to the rapid proliferation, which further increased the probability of normal cells turning into tumor cells. After the carcinogenesis of precancerous lesions, the apoptosis mechanism was inhibited, but the cells still proliferated at a fast speed, promoting the formation of tumors.
Based on the research results, DMBA upregulated FasL and Bcl-2 expression levels in tissues and cells, inhibited apoptosis of BC cells, resulting in BC. There were still some deficiencies in this study. The changes of DMBA on FasL and Bcl-2 expression were analyzed, yet the effects of different dose of DMBA on FasL and Bcl-2 expression were not studied. In the following work, the relationship between DMBA dose and FasL and Bcl-2 expression and apoptosis of BC cells will be studied. In conclusion, DMBA inhibited the apoptosis of BC cells by regulating the up-regulation of FasL and Bcl-2 expression.
Conflict of interests:
The authors declared no conflicts of interest.
References
- McKinney SM, Sieniek M, Godbole V, Godwin J, Antropova N, Ashrafian H, et al. International evaluation of an AI system for breast cancer screening. Nature 2020;577(7788):89-94.
[Crossref] [Google Scholar] [PubMed]
- Plichta JK, Ren Y, Thomas SM, Greenup RA, Fayanju OM, Rosenberger LH, et al. Implications for breast cancer restaging based on the 8th edition AJCC staging manual. Ann Surg 2020;271(1):169-76.
[Crossref] [Google Scholar] [PubMed]
- Mariotto A, Jayasekerea J, Petkov V, Schechter CB, Enewold L, Helzlsouer KJ, et al. Expected monetary impact of oncotype DX score-concordant systemic breast cancer therapy based on the TAILORx trial. J Natl Cancer Inst 2020;112(2):154-60.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Wu L, Shu XO, Cai Q, Shu X, Li B, et al. Genetically predicted levels of DNA methylation biomarkers and breast cancer risk: Data from 228 951 women of European descent. J Natl Cancer Inst 2020;112(3):295-304.
[Crossref] [Google Scholar] [PubMed]
- Costa C, Wang Y, Ly A, Hosono Y, Murchie E, Walmsley CS, et al. PTEN loss mediates clinical cross-resistance to CDK4/6 and PI3Kα inhibitors in breast cancer. Cancer Discov 2020;10(1):72-85.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 2019;20(3):175-93.
[Crossref] [Google Scholar] [PubMed]
- Birkinshaw RW, Gong JN, Luo CS, Lio D, White CA, Anderson MA, et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat Commun 2019;10(1):1.
[Crossref] [Google Scholar] [PubMed]
- Timucin AC, Basaga H, Kutuk O. Selective targeting of antiapoptotic BCL‐2 proteins in cancer. Med Res Rev 2019;39(1):146-75.
[Crossref] [Google Scholar] [PubMed]
- Jimbo H, Nagai H, Fujiwara S, Shimoura N, Nishigori C. Fas‐FasL interaction in cytotoxic T cell‐mediated vitiligo: The role of lesional expression of tumor necrosis factor‐α and interferon‐γ in Fas‐mediated melanocyte apoptosis. Exp Dermatol 2020;29(1):61-70.
[Crossref] [Google Scholar] [PubMed]
- Aboismaiel MG, El-Mesery M, El-Karef A, El-Shishtawy MM. Hesperetin upregulates Fas/FasL expression and potentiates the antitumor effect of 5-fluorouracil in rat model of hepatocellular carcinoma. Egypt J Basic Appl Sci 2020;7(1):20-34.
- Ding J, Yin T, Yan N, Cheng Y, Yang J. FasL on decidual macrophages mediates trophoblast apoptosis: A potential cause of recurrent miscarriage. Int J Mol Med 2019;43(6):2376-86.
[Crossref] [Google Scholar] [PubMed]
- Jin M, Xiao Z, Zhang S, Men X, Li X, Zhang B, et al. Possible involvement of Fas/FasL-dependent apoptotic pathway in α-bisabolol induced cardiotoxicity in zebrafish embryos. Chemosphere 2019;219:557-66.
[Crossref] [Google Scholar] [PubMed]
- Shati AA, Dallak M. Acylated ghrelin protects the hearts of rats from doxorubicin-induced Fas/FasL apoptosis by stimulating SERCA2a mediated by activation of PKA and Akt. Cardiovasc Toxicol 2019;19(6):529-47.
[Crossref] [Google Scholar] [PubMed]
- Sheokand S, Navik U, Bansal AK. Nanocrystalline solid dispersions (NSD) of Hesperetin (HRN) for prevention of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in Sprague-Dawley (SD) rats. Eur J Pharm Sci 2019;128:240-9.
[Crossref] [Google Scholar] [PubMed]
- Zhu S, Wang X, Zheng Z, Zhao XE, Bai Y, Liu H. Synchronous measuring of triptolide changes in rat brain and blood and its application to a comparative pharmacokinetic study in normal and Alzheimer’s disease rats. J Pharm Biomed Anal 2020;185:113263.
- Ishikawa M, Okamoto C, Shinoda K, Komagata H, Iwamoto C, Ohuchida K, et al. Detection of pancreatic tumor cell nuclei via a hyperspectral analysis of pathological slides based on stain spectra. Biomed Opt Express 2019;10(9):4568-88.
- Bakre MM, Ramkumar C, Attuluri AK, Basavaraj C, Prakash C, Buturovic L, et al. Clinical validation of an immunohistochemistry‐based can assist‐breast test for distant recurrence prediction in hormone receptor‐positive breast cancer patients. Cancer Med 2019;8(4):1755-64.
[Crossref] [Google Scholar] [PubMed]
- Wing L, Ding G, Xue J, Zhang YW. PKN2 regulates the survival of PC12 cells by activating the AKT/mTOR pathway. Indian J Pharm Sci 2020:8-11.
- Papaiakovou M, Gasser RB, Littlewood DT. Quantitative PCR-based diagnosis of soil-transmitted helminth infections: Faecal or fickle? Trends Parasitol 2019;35(7):491-500.
[Crossref] [Google Scholar] [PubMed]
- Shahzad K, Ghosh S, Mathew A, Isermann B. Methods to detect endoplasmic reticulum stress and apoptosis in diabetic nephropathy. Methods Mol Biol 2020;2067(1):153-73.
[Crossref] [Google Scholar] [PubMed]
- Yun Z, Hong Z, Dengchuan Z, Afang Z, Jiabin L. Risk factor analysis of pan-drug resistant Acinetobacter baumannii-induced ventilator-associated pneumonia in ICU. Indian J Pharm Sci 2020;82(1):8-11.
- Yang L, Chang B, Guo Y, Wu X, Liu L. The role of oxidative stress-mediated apoptosis in the pathogenesis of uric acid nephropathy. Ren Fail 2019;41(1):616-22.
[Crossref] [Google Scholar] [PubMed]
- Skoumal M, Woodward KB, Zhao H, Wang F, Yolcu ES, Pearson RM, et al. Localized immune tolerance from FasL-functionalized PLG scaffolds. Biomaterials 2019;192(1):271-81.
[Crossref] [Google Scholar] [PubMed]
- Dang LW, Min Y. The efficacy and safety of rivaroxaban in preventing deep vein thrombosis after hip replacement. Indian J Pharm Sci 2020;82(3):11-6.
- Song B, Aoki S, Liu C, Ito K. A toll-like receptor 9 agonist sensitizes mice to mitochondrial dysfunction-induced hepatic apoptosis via the Fas/FasL pathway. Arch Toxicol 2019;93(6):1573-84.
[Crossref] [Google Scholar] [PubMed]
- Xiao B, Li X, Feng XY, Gong S, Li ZB, Zhang J, et al. Restraint stress of male mice induces apoptosis in spermatozoa and spermatogenic cells: Role of the FasL/Fas system. Biol Reprod 2019;101(1):235-47.
- Akhavan Sales Z, Tahoori MT, Sheikhha MH, Seifati SM, Bitaraf Sani M. Identification of a FAS/FASL haplotype associated with endometriosis in Iranian patients. Gynecol Endocrinol 2020;36(3):261-4.
[Crossref] [Google Scholar] [PubMed]
- Fan L, Zhang CJ, Zhu L, Chen J, Zhang Z, Liu P, et al. FasL-PDPK1 pathway promotes the cytotoxicity of CD8+ T cells during ischemic stroke. Transl Stroke Res 2020;11(4):747-61.
[Crossref] [Google Scholar] [PubMed]
- Yu Z, Sendong H. Comparative efficacy and safety of high-viscosity vs. low-viscosity bone cement in the treatment of osteoporotic vertebral fractures. Indian J Pharm Sci 2020;82(1):12-5.
- Cubero FJ, Woitok MM, Zoubek ME, de Bruin A, Hatting M, Trautwein C. Disruption of the FasL/Fas axis protects against inflammation-derived tumorigenesis in chronic liver disease. Cell Death Dis 2019;10(2):1-2.
- Sun Q, Wang Y, Desgrosellier JS. Combined Bcl-2/Src inhibition synergize to deplete stem-like breast cancer cells. Cancer Lett 2019;457:40-6.
[Crossref] [Google Scholar] [PubMed]
- Wu X, Zheng Y, Yang D, Chen T, Feng B, Weng J, et al. A strategy using mesoporous polymer nanospheres as nanocarriers of Bcl-2 siRNA towards breast cancer therapy. J Mater Chem B 2019;7(3):477-87.
- Wang H, Xu B, Shi J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene 2020;722:144076.
[Crossref] [Google Scholar] [PubMed]
- Zhou W, Fang H, Wu Q, Wang X, Liu R, Li F, et al. Ilamycin E, a natural product of marine actinomycete, inhibits triple-negative breast cancer partially through ER stress-CHOP-Bcl-2. Int J Biol Sci 2019;15(8):1723.
[Crossref] [Google Scholar] [PubMed]
- Cao RJ, Li K, Xing WY, Du S, Li Q, Zhu XJ, et al. Disabled‐1 is down‐regulated in clinical breast cancer and regulates cell apoptosis through NF‐κB/Bcl‐2/caspase‐9. J Cell Mol Med 2019;23(2):1622-7.
[Crossref] [Google Scholar] [PubMed]
- Gao X, Wang M, Zhang Y, Xu Z, Ding J, Tang J. MicroRNA-16 sensitizes drug-resistant breast cancer cells to Adriamycin by targeting Wip1 and Bcl-2. Oncol Lett 2019;18(3):2897-906.
[Crossref] [Google Scholar] [PubMed]
- Senturk M, Ercan F, Yalcin S. The secondary metabolites produced by Lactobacillus plantarum downregulate BCL-2 and BUFFY genes on breast cancer cell line and model organism Drosophila melanogaster: Molecular docking approach. Cancer Chemother Pharmacol 2020;85(1):33-45.
[Crossref] [Google Scholar] [PubMed]
 Control group
Control group