- *Corresponding Author:
- V.H. Sato
Department Pharmacology, Faculty of Pharmacy, Mahidol University, Bangkok, 10400, Thailand
E-mail: vilasinee.sat@mahidol.ac.th
| Date of Submission | 01 June 2016 |
| Date of Revision | 17 July 2016 |
| Date of Acceptance | 01 August 2016 |
| Indian J Pharm Sci 2016;78(4):547-552 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows other the remix, tweak, and build up to the non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Antihyperuricemic effect Aquilaria crassna leaf extract has been commercially advertised to markedly reduce uric acid level after drinking with hot water in Thailand, whereas its experimental evidence has not been published yet. The present study was therefore conducted to investigate pharmacological activities of the water extract of A. crassna leaves, focusing on its inhibitory effect on xanthine oxidase activity in vitro and antihyperuricemic effect in plasma uric acid level in oxonate-induced hyperuricemic mice. Moreover, the residual xanthine oxidase inhibitory effect in the liver of mice treated with the extract was also determined. We found that A. crassna inhibited xanthine oxidase activity in with IC50 of 1.35±0.03 mg/ml. Lineweaver- Burk analysis showed that the inhibition of xanthine oxidase was non-competitive with Ki of 1.72 mg/ml. Oral administration of 3000 mg/kg of A. crassna extract significantly prevented the increase of uric acid level induced by potassium oxonate in mice, however, the lower doses (500 and 1000 mg/kg) showed no effect. Livers of mice treated with the extract at the doses of 1000 and 3000 mg/kg significantly decreased the production of uric acid through inhibition on xanthine oxidase activity approximately 47.2% and 63.6%, as compared with untreated hyperuricemic mice (P<0.01). However, the concentrations of A. crassna of which exhibit antihyperuricemic effect observed in the study were much higher than that of recommend dosage written in the commercial, therefore, we concluded that A. crassna leaves extract could not inhibit xanthine oxidase activity and the effect on urate excretion in the kidney by long term administration the extract is further needed.
Keywords
Aquilaria crassna, xanthine oxidase, oxonate-induced hyperuricemia, uric acid
Recently, Aquilaria crassna Pierre ex Lecomte leaves have been commercially advertised that the leaf extract markedly reduces plasma uric acid level after drinking with hot water. However, direct experimental evidence for the extract to demonstrate the hypouricemic effect has not been published yet. A. crassna is one of the plant species which belong to Thymelaeaceae family, largely distributed many parts of Thailand. A. crassna extract is known as one of the compositions in Ya-hom, a traditional herbal remedy in Thailand for treatment of symptoms of fainting, as a mild cardiac stimulant, and as a remedy for stomach discomfort. It is listed in Herbal Medicine Products, National List of Essential Medicines, Ministry of Public health, Thailand [1]. Previous phytochemical studies have identified several bioactive compounds in A. crassna leaves [2,3]. Among the bioactive compounds present in A. crassna, mangiferin is the major constituent of the leaves which expresses an antioxidant effect [4], hypouricemic effect [5], and inhibitory effect on renal urate reabsorption [6]. Therefore, the present study was designed to examine whether the aqueous extract of A. crassna leaves possess antihyperuricemia effect or not. The effect on plasma uric acid level in hyperuricemic mice and intrinsic activity of the extract to inhibit xanthine oxidase (XOD) activities in vitro were also carried out
A. crassna young leaves (first five youngest leaves) were collected from Raifawaree farm at Lop Buri Province, Thailand. The authentic samples were identified by the Office of the Forest Herbarium, Ministry of Natural Resources and Environment, Bangkok, Thailand with the voucher number BKF NO.191083 and the reference specimen was deposited at their herbarium. Plant materials were cleaned, cut, and dried at temperature of 50° for 8-12 h under air-drying condition (Model UF110, Memmert, Schwabach, Germany). Then, dried powdered leaves (300 g) were extracted in boiling water (10 l) for 15 min. The pooled extracts were filtered through a cotton cloth and then concentrated at 50° using a rotary evaporator under low pressure (rotary evaporator with cooling system, Model Rotavapor R-124, Büchi, Switzerland). The residue was freeze- dried in a lyophilizer and the extracts were stored at 4° until used. Yield of the final extract was found to be 29.5% w/w.
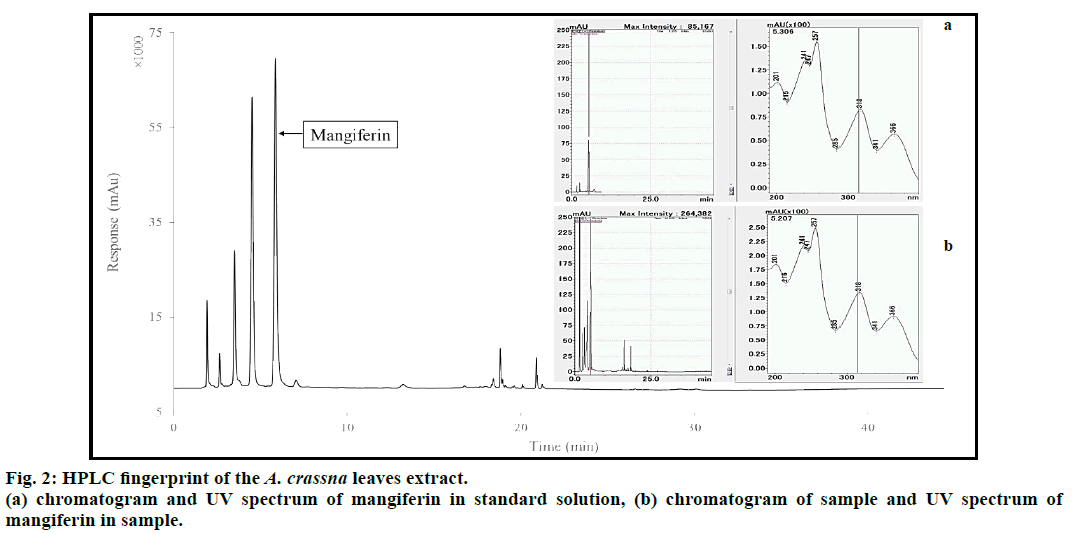
Mangiferin was used as a standardized marker compound for the quality control of the extract. A quantitative analysis of mangiferin contained in the water extract of A. crassna leaves was performed on the Prominence liquid chromatographic system (Shimadzu Corp., Kyoto, Japan) consisting of a quaternary pump liquid chromatographic (LC-20AT) with degasser (DGU-20A3), photodiode array detector (SPD- M30A), column oven (CTO-20A), and communication bus module (CBM-20A). A TSK-gel ODS-100Z column (150×4.6 mm, 5 μm; Tosoh Bioscience, Tokyo, Japan) was used as an analytical column. The analytical method was modified from Yu et al. [7]. The elution was performed with a constant flow rate of 1.0 ml/min at 25° using the mobile phase consisting of 0.05% v/v TFA (solvent A) and acetonitrile (solvent B) with the gradient program as follows: 0-10 min, 15% solvent B; 10-18 min, 15-45% solvent B; 18-25 min, 45-80% solvent B; 25-27 min, 80% solvent B; 27-35 min, 80-45% solvent B; 35-40 min, 45-15% solvent B; 40-45 min, 15% solvent B. The samples (20 μl) were introduced into the HPLC system and the elution was recorded at 315 nm. A peak purity determination and UV-spectrum (200-400 nm) of mangiferin in the samples were characterized using Shimadzu Lab Solution software by which a corresponding peak of mangiferin in the standard solution was consistently compared. Moreover, amount of mangiferin in the extract was calculated from the standard calibration line.
Effect of the aqueous extract of A. crassna leaves on XOD was assayed in vitro according to the method of Kong et al.[8] with some modifications. A. crassna extract was initially dissolved in DMSO (final concentration 0.2% in the reaction mixture) and diluted with distilled water to obtain the desired concentration. A reaction mixture containing 2.98 ml of 80 mM pyrophosphate buffer (pH 7.4) and 20 μl of freshly prepared XOD enzyme (0.08 U) dissolved in 0.1 M pyrophosphate (pH 7.4) was prepared, and mixed with 1 ml of various concentrations (0.25-4.5 mg/ml) of the A. crassna extract or buffer (as a negative control). Allopurinol (1- 10 μM) was added into the reaction tubes instead of the extract as a positive control. The reaction was initiated by the addition of 2 ml of xanthine (120 μM) dissolved in pyrophosphate buffer (pH 7.4), and incubated at 25° for 10 min. The reaction was terminated by adding 1 ml of 1 N HCl, and concentrations of uric acid were determined in triplicate by HPLC, as described later.
Percent inhibition of the XOD-mediated uric acid formation, by A. crassna extract or allopurinol was calculated by using the following equation: Percentage inhibitory activity=(1– UAsample/UAcontrol)×100. Where, UAcontrol is the concentration of uric acid in the negative control and UAsample is the concentration of uric acid in the sample.
IC50 of the herb extract, defined as the herb concentration required to inhibit the XOD activity by 50% was calculated by a linear regression analysis of the dose- response curve plotting between the percentage of inhibitory activity and log-concentration.
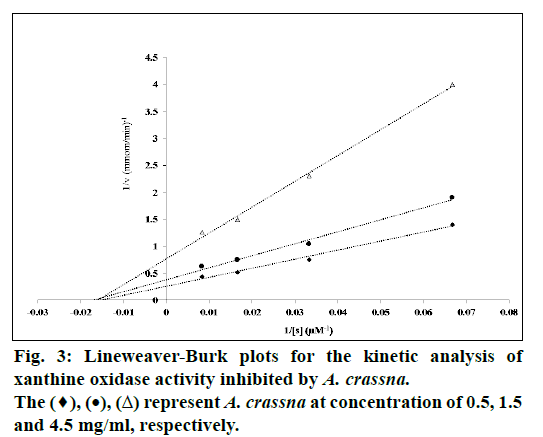
Lineweaver-Burk plot analysis was performed to determine the type of XOD inhibition by A. crassna. The assay was carried out in the absence or presence of 3 concentrations of A. crassna extract (0.5, 1.5, and 4.5 mg/ml) in different concentrations of a substrate xanthine (15, 30, 60, and 120 μM). The enzyme reaction was performed as described above. The inhibitory constant (Ki) for the XOD inhibition by A. crassna extract was determined by a non-least squares regression of the observed data to the following equation, using Solver Add-in equipped with Microsoft Excel 2010:
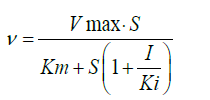
Where, v and vmax represent the initial and maximum velocities of the uric acid formation, respectively in mmol/min, Km represents Michaelis constant, and S and I represent the substrate (μM) and inhibitor concentrations (μg/ml), respectively. For the nonlinear optimization, the generalized reduced gradient (GRG) algorithm was employed.
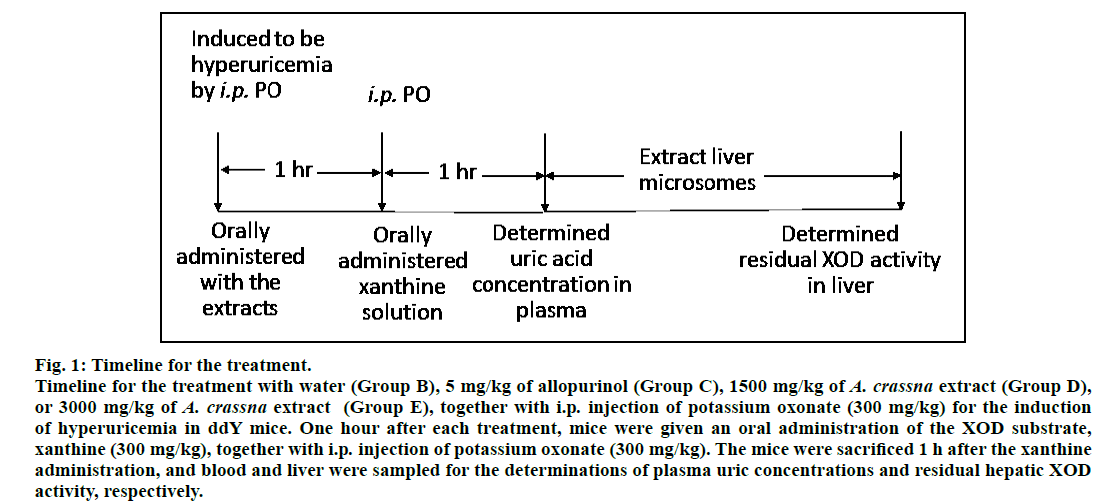
Male ddY mice (30-40 g, 6 week-old) were housed at the animal center of Showa University, Tokyo, Japan, at a constant temperature (25±1°) with a 12 h lightdark cycle, and had free access to standard diet and water ad libitum. Mice were acclimatized for 7 days prior to start the experiment. The animal care and experimental protocol were approved by the Animal Ethics Committee of Showa University. The animals were deprived of food at least 3 h before the experiment, and the mice were randomly divided into six groups (6 mice per group), nominated as groups A, B, C, D, E and F. Group A corresponds to the mice pretreated orally with water and no further treatment, designated as normal control mice. Hyperuricemic mice model was developed according to the procedure of Smith et al.[9] with slight modifications. Groups B, C, D, E and F of mice were given two intraperitoneal (i.p.) injections of 300 mg/kg of potassium oxonate (as an uricase inhibitor) dissolved in normal saline for the induction of hyperuricemia, and also given an oral dose of 300 mg/kg xanthine (as XOD substrate) dispersed in 1% CMC-Na. Group B was pretreated orally with water as placebo, designated as untreated hyperuricemic mice. Group C corresponds to the hyperuricemic mice pretreated orally with 5 mg/kg of allopurinol, named as positive control mice. Groups D, E and F are the hyperuricemic mice pretreated orally with 500, 1000 and 3000 mg/kg of the aqueous extracts of A. crassna leaves, respectively. Two hour after the pretreatment, the mice were sacrificed with anesthesia using diethyl ether, and whole blood was collected from superior vena cava into a tube containing heparin. The blood samples were centrifuged at 3000 g for 5 min, and obtained plasma samples were stored at -4° before analysis. Consecutively, mice livers were rapidly excised, washed in cold normal saline, and stored at -4° until use. Fig. 1 illustrates the timeline for each animal experiment except for the normal control mice.
Figure 1: Timeline for the treatment.
Timeline for the treatment with water (Group B), 5 mg/kg of allopurinol (Group C), 1500 mg/kg of A. crassna extract (Group D), or 3000 mg/kg of A. crassna extract (Group E), together with i.p. injection of potassium oxonate (300 mg/kg) for the induction of hyperuricemia in ddY mice. One hour after each treatment, mice were given an oral administration of the XOD substrate, xanthine (300 mg/kg), together with i.p. injection of potassium oxonate (300 mg/kg). The mice were sacrificed 1 h after the xanthine administration, and blood and liver were sampled for the determinations of plasma uric concentrations and residual hepatic XOD activity, respectively.
The residual activity of XOD in the excised liver was determined in vitro by measuring uric acid concentrations generated from xanthine, using the method of Huang et al. [10] Briefly, the livers were homogenized in 5 volumes of cold 80 mM sodium pyrophosphate buffer (pH 7.4), and centrifuged at 3000 G for 10 min at 4°. After the lipid layer was removed, the remaining part was further centrifuged at 10 000 G for 60 min at 4°, and the obtained supernatants were used for measuring the residual XOD activity and protein concentrations. The reaction tube containing 100 μl of liver homogenate and 800 μl of 80 mM sodium pyrophosphate buffer (pH 7.4) was incubated at 25° for 10 min. The mixtures were then added with 500 μl of 120 μm xanthine solution, mixed and incubated for 0 and 30 min, respectively. The reaction was terminated after 0 and 30 min by adding 100 μl of 1 N HCl. Thereafter, the samples were centrifuged at 3000 G for 10 min and uric acid concentrations were measured by HPLC. Total protein concentration in liver was determined spectrophotometrically, according to the method of Bradford. The residual XOD activity was expressed as nanomoles of uric acid formed per minute per milligram protein.
Uric acid concentrations in vitro and plasma were analyzed by a chromatographic method reported previously by Carro et al. [11]. Briefly, 100 μl of the plasma sample was thoroughly mixed with 100 μl acetonitrile, centrifuged at 3000 G for 5 min at 4°, and the resultant supernatant was filtered through 0.45 μm PTFE syringe filter (RephiLe Bioscience, China). Twenty microliter of the filtrate was injected in triplicate into the system and eluted with the mobile phase containing acetonitrile and 1 mm sodium octane sulfonic acid in 7 mm K2HPO4, pH 3.0 (2.5:97.5) at a flow rate of 1.0 ml/min. The absorbance was monitored at 292 nm. Uric acid found in samples was calculated with respect to calibration curve.
The results were expressed as the mean±standard error of the mean (SEM). Data were analyzed by one-way analysis of variance (ANOVA) followed by post-hoc analysis for comparing the difference of each group using student’s t test using JMP Pro (version 12, SAS Institute Japan Inc., Tokyo, Japan). Differences in the mean values were considered to be statistically significant at P < 0.05.
The corresponding peak of mangiferin in the aqueous extract of A. crassna leaves was identified and quantified based on the retention time of mangiferin and UV spectrum in standard solution, as shown in fig. 2. The calibration line of standard mangiferin solutions (6.2=31.0 μg/ml) was expressed as the equation, y=46003x+40324, with a good correlation (r2=0.9998). The precision of each concentration was lower than 0.99 percentage relative standard deviation (n=3). The amount of mangiferin in the extract was determined to be 1.44±0.01% w/w.
A. crassna extract inhibited the XOD activity in vitro in a concentration-dependent manner, and the IC50 was determined to be 1.35±0.03 mg/ml, while that of the positive control, allopurinol, was 3.78±0.08 μg/ ml. Lineweaver-Burk analysis of the enzyme kinetics data showed that the inhibition of XOD activity by A. crassna extract was in a non-competitive fashion (fig.3). The Ki and Km value were determined to be 1.72 and 0.087 mg/ml, respectively.
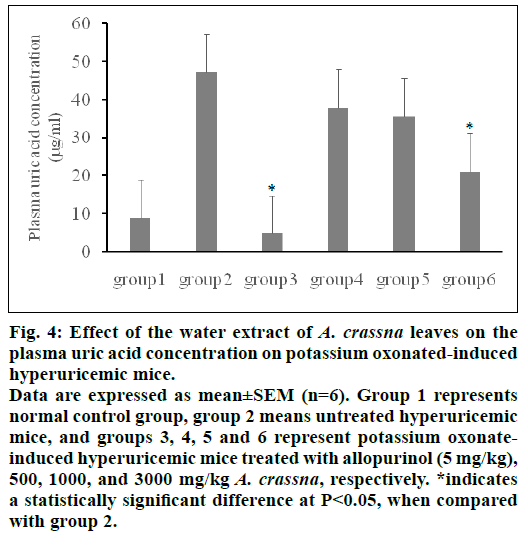
Fig. 4 summarized the in vivo actions of A. crassna extract on plasma uric acid concentration. Plasma uric acid level in the control hyperuricemic group (no treatment) was elevated markedly after treatment with potassium oxonate by approximately 5 times as compared with normal control group (47.2±15.7 vs. 8.8±3.1 μg/ml, P< 0.01), indicating that hyperuricemic mice were establised. Pretreatment with allopurinol significantly reduced plasma uric acid concentration by 90% comparing with the hyperuricemic control group (P< 0.01). Pretreatment with 500 and 1000 mg/kg of A. crassna decreased plasma uric acid concentration but the plasma uric acid concentration was not significantly different compared with the hyperuricemic control group. Plasma uric acid concentration was reduced 55% when concentration of A. crassna was increased to 3000 mg/kg, as compared with hyperuricemic control group (P< 0.05). However, significant differences were not observed between each dose of treatment groups.
Figure 4: Effect of the water extract of A. crassna leaves on the plasma uric acid concentration on potassium oxonated-induced hyperuricemic mice.
Data are expressed as mean±SEM (n=6). Group 1 represents normal control group, group 2 means untreated hyperuricemic mice, and groups 3, 4, 5 and 6 represent potassium oxonateinduced hyperuricemic mice treated with allopurinol (5 mg/kg), 500, 1000, and 3000 mg/kg A. crassna, respectively. *indicates a statistically significant difference at P< 0.05, when compared with group 2.
The liver homogenate of mice treated with A. crassna extract at the dose of 1500 and 3000 mg/kg significantly inhibited the formation of uric acid by inhibiting XOD activity about 47.2% and 63.6%, as compared with the untreated hyperuricemic group (P< 0.05, respectively) as shown in Table 1. Allopurinol markedly inhibited XOD activity by about 81.3% (P< 0.05).
| Groups | XOD activity (nanomole/min/mg protein) |
XOD Inhibition (%) (compared with group 2) |
|---|---|---|
| 1 | 0.99±0.09 | - |
| 2 | 2.14±0.28 | - |
| 3 | 0.40±0.08*,# | 81.3 |
| 4 | 1.7±0.51 | 20.5 |
| 5 | 1.13±0.22* | 47.2 |
| 6 | 0.78±0.09** | 63.6 |
Table 1: Effect of the aqueous extract of A. Crassna leaves on residual activity of xod in liver
Several bioactive compounds found in the aqueous extract of A. crassna leaves have been identified such as fluorofenone 3-C-β glucose, epicatechin gallate, epigallocatechin gallate (EGCG), mangiferin [3]. Previous studies supported that mangifenin and EGCG expressed a hypouricemic effect. However, the XOD inhibitory effect of EGCG was quite low with inhibition less than 5% [12]. Mangiferin was detected in the highest level among compounds found in the extract (1.44%). A previous study found that mangiferin had a potent hypouricemic effect associated with inhibiting the activity of xanthine dehydrogenase and XOD [5,6]. Therefore, we employed mangiferin as the standardized marker of the extract and thought that it may contribute for the antihyperuricemic effect of the extract in the present study.
Inhibition of XOD is one of the drug targets for the treatment of hyperuricemia. In vitro study suggested that A. crassna extract inhibited the XOD activity in a concentration-dependent manner with IC50 more than 1 mg/ml, indication of very weak XOD inhibitory effect. Oral administration of 3000 mg/kg of A. crassna extract significantly prevented the increase in plasma uric acid level induced by potassium oxonate in mice and distributed into the liver, and could inhibit the XOD activity in the liver, however, there were no significant differences in plasma uric acid concentration after administration of each dose of A. crassna extract (500, 1000, 3000 mg/kg). Taken together, it can be concluded that effect of A. crassna on XOD inhibition was negligible.
From our knowledge, there are two mechanisms to reduce plasma uric acid concentration. First mechanism is to inhibit XOD enzyme and the latter is to inhibit the reabsorption of urate in renal i.e. renal mRNA and protein levels of urate transporter 1 (URAT1), glucose transporter 9 (GLUT9), organic anion transporter 1 (OAT1) and organic cation/carnitine transporters (OCT1/2, OCTN1/2). Administrations of A. crassna extract showed possibility to decrease plasma uric acid concentration, although inhibitory on XOD might not be involved. The inhibitory effect on renal urate reabsorption of mangiferin, a major bioactive compound found in A. crassna, has been reported [6].
Acute toxicity test of A. crassna extract in mice showed no sign of toxicity or death at the doses up to 15000 mg/kg body weight [13]. In practice, the dose of A. crassna extract recommended commercially in 10 mg of tea with 500 ml of hot water [14] is much lower than the effective doses observed in the present study. We conclude from the results obtained from the present study that A. crassna leaf extract could not be used as alternative medicine for treating of acute hyperuricemia and the study of long term administration on urate reabsorption effect is needed.
Acknowledgements
We would like to deeply thank Prof. Gary H. Smith, Department of Pharmacy Practice and Science, University of Arizona, College of Pharmacy, Tucson, AZ for kindly reviewing our manuscript.
Financial support and sponsorship
This work was financial supported by School of Pharmacy, Showa University, Japan.
Conflicts of interest
There are no conflicts of interest.
References
- National list of Essential Medicines (List of Herbal Medicinal Products). Ministry of Public Health, Thailand; 2012.
- Tay PY, Tan CP, Abas F, Yim HS, Ho CW. Assessment of extraction parameters on antioxidant capacity, polyphenol content, epigallocatechingallate (EGCG), epicatechingallate (ECG) and iriflophenone 3-C-β-glucoside of agarwood (Aquilariacrassna) young leaves. Molecules 2014;19:12304-9.
- Ito T, Kakino M, Tazawa S, Watarai T, Oyama M, Maruyama H, et al. Quantification of polyphenols and pharmacological analysis of water and ethanol-based extracts of cultivated agarwood leaves.J NutrSciVitaminol (Tokyo) 2012;58:136-42.
- Prabhu S, Jain M, Sabitha KE, Devi CS. Role of mangiferin on biochemical alterations and antioxidant status in isoproterenol-induced myocardial infarction in rats. J Ethnopharmacol 2006;11:126-33.
- Niu Y, Lu W, Gao L, Lin H, Liu X, Li L. Reducing effect of mangiferin on serum uric acid levels in mice. Pharm Biol2012;50:1177-82.
- Yang H, Gao L, Niu Y, Zhou Y, Lin H, Jiang J, et al. Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biol Pharm Bull 2015;38:1591-8.
- Yu Q, Qi J, Yu HX, Chen LL, Kou JP, Liu SJ, et al. Quantitative and qualitative analysis of phenolic compounds in the leaves of Aquilariasinensisusing liquid chromatography-mass spectrometry. Phytochem Anal 2013;24:349-56.
- Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol 2000;73:199-207.
- Smith RD, Essenburg AD, Kaplan HR. The oxonate pretreated rat as a model for evaluating hyperuricemic effects of antihypertensive drugs. ClinExpHypertens1979;1:487-504.
- Huang CG, Shang YJ, Zhang J, Zhang JR, Li WJ, Jiao BH. Hypouricemic effects of phenylpropanoid glycosides acteoside of Scrophularianingpoensison serum uric acid levels in potassium oxonate-pretreated Mice. Am J ChinMed 2008;36:149-57.
- Carro MD, Falkenstein E, Radke WJ, Klandorf H. Effects of allopurinol on uric acid concentrations, xanthine oxidoreductase activity and antioxidative stress in broiler chickens. Comp BiochemPhysiol C Pharmacol2010;151:12-7.
- Kurisawa M, Chung JE, Uyama H, Kobayashi S. Oxidative coupling of epigallocatechingallate amplifies antioxidant activity and inhibits xanthine oxidase activity. ChemCommun (Camb)2004;3:294-5.
- Kamonwannasit S, Nantapong N, Kumkrai P, Luecha P, Kupittayanant S, Chudapongse N. Antibacterial activity of Aquilariacrassnaleaf extract against Staphylococcus epidermidisby disruption of cell wall. Ann ClinMicrobiolAntimicrob 2013;12:20.
- http://krisanatea.blogspot.in/2012/08/krisana-green-gold-tea_20.htm