- *Corresponding Author:
- Limei Deng
Department of Cardiology, The Second Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang 150001,China
E-mail: zyk506846456@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “91-100” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the research effects of compound Danshen dripping pills on the changes of electrocardiogram and hemorheology in the treatment of angina pectoris in coronary heart disease is the objective of the study. In this study we have collected papers from PubMed, Web of Science, Cochrane library, Google Scholar, China National Knowledge Infrastructure, Wanfang, Embase and virus protein domain database and the retrieval time limit is from the establishment of the database to May 2023. In this study we used ReviewManager 5.4 for meta-analysis. 9 studies were included here. In terms of various efficacy indicators, 8 studies clearly indicated that the clinical efficacy of the treatment group was better than that of the control group (relative risk=1.18, 95 % confidence interval: 1.10-1.26). Two of the studies measured the efficacy of electrocardiogram and the results showed that the treatment group had better electrocardiogram efficacy after compound Danshen dripping pills treatment (relative risk=1.39, 95 % confidence interval: 1.20-1.62). Three studies mentioned the frequency and duration of angina pectoris, all of which were significantly improved after compound Danshen dripping pills treatment (standardized mean difference=-1.35, 95 % confidence interval: -1.62 to -1.08, p<0.01) and (standardized mean difference=-2.15, 95 % confidence interval: -2.54 to -1.77, p<0.01); both high sensitivity c-reactive protein and endothelin were significantly decreased in the treatment group (standardized mean difference=-1.79, 95 % confidence interval: -2.18 to -1.40, p<0.01) and (standardized mean difference=-3.24, 95 % confidence interval -3.75 to -2.73, p<0.01). Two studies showed changes in hemorheology and the index of hemorheology in the treatment group was lower than that in the control group. The 9 included studies showed that compound Danshen dripping pills are safe and effective in treating patients with coronary heart disease and angina pectoris. Compared with conventional treatment alone, the clinical efficacy can be further improved using this.
Keywords
Coronary heart disease, angina pectoris, compound Danshen dripping pills, electrocardiogram, hemorheology
Coronary Heart Disease (CHD) has always been one of the public health problems of global concern. Since the 21st century, the morbidity and mortality of CHD have been at a relatively high level. Along with the trend of the world’s aging development, cardiovascular and cerebrovascular diseases have become the main factors that endanger human health, and CHD and angina pectoris have become common diseases of middle-aged and elderly people. The progress of society, the enrichment of material life and the change of diet structure make the total blood cholesterol or low-density lipoprotein-cholesterol increase, leading to an increase in the incidence and mortality of CHD. According to statistics from the Ministry of Health, in the past 16 y, the mortality rate of CHD in my country has increased by 2.5 times; (China Cardiovascular Disease Report 2008-2009) data show that in 2007, the mortality rate of CHD among urban residents in my country was 64.67/100 000, CHD has become a major disease threatening human health.
At present, the drugs for the treatment of CHD are mainly Western medicines such as nitrates, statins and anti-platelet preparations. Traditional Chinese medicine has been used to prevent and treat diseases for thousands of years. Compared with synthetic chemical drugs, traditional Chinese medicine is a natural medicine and these natural medicines have been more and more widely used due to their low toxicity, small side effects and high safety. Among them, Compound Danshen Dripping Pills (CDDP) is a new type of compound medicine based on compound Danshen tablets, which is produced with international leading technology. It is mainly refined from traditional Chinese medicine Danshen, sanqi and borneol. Dripping pills are solid dispersion preparations formed by melt-dispersing, dripping and condensing the drug and matrix. The hydrophilic matrix of CDDP is conducive to the rapid dissolution, release and rapid onset of action of the drug. After the drug of CDDP was melted and dispersed with the polyethylene glycol matrix, the drug was highly uniformly dispersed in the matrix and could be completely dissolved in 5-7 min, which was far better than the control limit requirement of <60 min in the Chinese Pharmacopoeia[1]. CDDPs are characterized by high drug loading, small dose and hydrophilic preparation, which is also very suitable for sublingual administration. Through the sublingual administration, the active ingredients are directly absorbed into the blood through the mucosa, while avoiding the first-pass effect of the liver and stomach. The degradation of intestinal digestive juice improves the utilization rate of drugs, which is beneficial to the rapid relief of patients with acute diseases. Clinical data and animal experiments have confirmed that CDDP have the effects of dilating coronary arteries, reducing myocardial oxygen consumption, improving myocardial ischemia, and anti-platelet surface activity and aggregation[2-4]. As a typical traditional Chinese medicine, CDDP has achieved remarkable results in the treatment of cardiovascular diseases such as CHD and angina pectoris. Among them, the adverse reactions of CDDP are only occasional gastrointestinal discomfort, head swelling, flushing, etc., and most of them can be relieved by themselves. CHD is mainly a series of diseases caused by Atherosclerosis (AS) caused by blockage or compression of coronary arteries, and Acute Coronary Syndrome (ACS) is a serious type of CHD. According to traditional Chinese medicine, ACS is part of the categories of “chest pain and heart pain”, and its pathogenesis belongs to “yang micro-yin string”. Studies have shown that chronic inflammation is closely related to AS[5]. CDDP can reduce serum Interleukin-6 (IL-6) novel in elderly patients with CHD and has a significant anti-inflammatory effect, thereby exerting a preventive effect on AS[6]. Another pathological feature of ACS is unstable coronary atherosclerotic plaques with platelet aggregation. CDDP can reduce the level of C-Reactive Protein (CRP) in patients with CHD complicated with carotid atherosclerotic plaque and protect endothelial cells by inhibiting Endothelin-1 (ET-1)[7]. Dripping pills can significantly reduce blood viscosity and improve hemodynamics[8]. The levels of inflammatory factors and the degree of vascular endothelial injury are different in different syndromes of CHD, among which the blood stasis type is von Willebrand factor, P-selectin, fibrinogen and thrombus.
The high level of element B2 indicates that the stasis in "yinxuan" blocks the heart and reduces the antithrombotic ability, leading to the formation of AS[9]. The intervention of CDDP in patients with CHD and blood stasis syndrome can significantly reduce the messenger Ribonucleic Acid (mRNA) level of pro-apoptotic genes and inhibit myocardial ischemia and vascular endothelial immune response by regulating apoptosis[10].
CDDP have been in the market for many years and are widely used in China. Various application reports have also been found in many medical journals. However, due to the small number of samples, insufficient randomness and failure to exclude other complications in Randomized Controlled Trials (RCTs), there are no accurate and reliable conclusions about its efficacy, safety and strength of evidence-based medicine. Therefore, this study hopes to conduct a systematic review by searching the relevant literature in the treatment of CHD and angina pectoris with CDDP published in domestic professional journals in recent years through the method of meta-analysis to further understand the clinical efficacy and safety of CDDP in the treatment of angina pectoris in CHD.
Materials and Methods
Literature search strategy:
Specific and systematic searches were carried out on the webpage, databases like PubMed, Embase, Web of Science, Google Scholar, China National Knowledge Infrastructure (CNKI), Wanfang and Virus Protein (VIP) domain databases; the search terms were Adriamycin (ADM), Doxorubicin (DOX), ADM, DOX, cardiotoxicity, traditional Chinese medicine, cardiac adverse reactions, clinical research and meta-analysis; the search time limit is from the establishment of the database to May 2023, the search results are limited to clinical research and are not restricted by language or race, and manual searches are performed by reading relevant works and summarizing references. Search strategies need to be adjusted for complying with the relevant regulations in every database.
Inclusion criteria:
RCTs, no matter whether it is single-blind, double-blind or non-blind; the trial includes a parallel control group, receiving other drugs (including Western medicine and traditional Chinese medicine), placebo or blank control; intervention measures which used CDDP to treat CHD angina pectoris and treatment time is not <14 d.
Exclusion criteria:
Non-randomized trials; duplicate publications or data duplication; studies without a control group; animal experiments; research methods, results and conclusions that cannot be explained or do not correspond to each other; statistical methods and data analysis that have obvious errors; literature with imperfect experimental design; literature for which data could not be extracted or data was incomplete; review, animal experiments, special adverse reaction reports and pharmacology, pharmacokinetics and other non-clinical research; patients with severe hepatic and renal insufficiency etc.; the test results and conclusions are obviously inconsistent with the reality; the number of dropped cases reaches >20 %, including death, loss of follow-up, automatic stoppage of the test and failure to meet the requirements which participated in the research etc.
Literature screening and data extraction:
Literature screening: Two researchers on the basis of the inclusion and exclusion criteria independently screened the literature, targeting titles and abstracts, including primary screening, secondary screening and cross-checking to determine possible relevant studies. Firstly, conduct a preliminary screening that includes reading and analyzing the titles and the abstracts of the articles, and eliminating the literature that apparently does not include in the inclusion criteria or duplicating studies. 2nd, re-screening, which involved to read the full text of the papers obtained from the primary screening and then further screen the literature according to the inclusion criteria. Finally, to check the papers includes cross-checking of the obtained literature. For documents with incomplete or questionable information, it is necessary to contact the corresponding authors for detailed information. Finally, it was judged whether the literature was included in the study. If two researchers have different opinions on some articles, they will discuss together until a consensus is reached; if no consensus can be reached, a third researcher will participate in the judgment. Finally, the selected documents are included in the table for extraction and summary.
Data extraction: The content of data extraction includes title, first author, year of publication, research type and observation indicators.
Intervention:
The intervention in the treatment group was CDDP and the control group was treated with Xiaoxintong (using only chemical drugs or chemical drugs+placebo to treat CHD) which was also the conventional treatment.
The clinical curative effect was calculated according to the efficacy index; clinical efficacy; Electrocardiogram (ECG) efficacy; frequency of angina attacks; duration of angina attacks; platelet aggregation rate; hypersensitivity CRP (hs-CRP); ET; platelet Granule Membrane Protein (GMP-140) and fibrinogen level. Among them the clinical curative effect was observed. The clinical efficacy refers to the comprehensive evaluation including the improvement of the pain degree of angina pectoris and the reduction of the number of attacks. The judgment of clinical curative effect refers to "judgment criteria for curative effect of CHD, angina pectoris and ECG"; "clinical disease diagnosis based on curing and improvement criteria" and "clinical guidelines for new drugs of traditional Chinese medicine". Whole blood viscosity, platelet aggregation rate, erythrocyte aggregation index and erythrocyte deformation index are determined by hemorheology. The improvement or judgment standard of ECG is markedly effective.
Quality evaluation:
Eligible literature was assessed for methodological quality using the Jadad scoring scale, scores on a scale of 1 to 7, assessing random sequence generation, blinding, allocation concealment and patient withdrawal or withdrawal. A Jadad score of 4-7 was considered high-quality literature and 1-3 was considered as low-quality literature[11].
Statistical analysis:
All analyzes were pooled using RevMan 5.4 statistical software with Weighted Mean Differences (WMD) and 95 % Confidence Interval (CI) for continuous data and Relative Risk (RR) and 95 % CI for dichotomous data. The heterogeneity Index (I2) is used to evaluate the heterogeneity of the treatment effect. When there is no significant heterogeneity among the studies (I2<50 %), the fixed effect model is used; when there is significant heterogeneity among the studies (I2≥50 %), use a random effects model. Egger’s test was used to assess the potential risk of publication bias, with a test level of p=0.05. Sensitivity analysis was performed on factors that may cause heterogeneity and literature with high sensitivity was excluded. A descriptive analysis was performed for those who could not perform a meta-analysis[12].
Results and Discussion
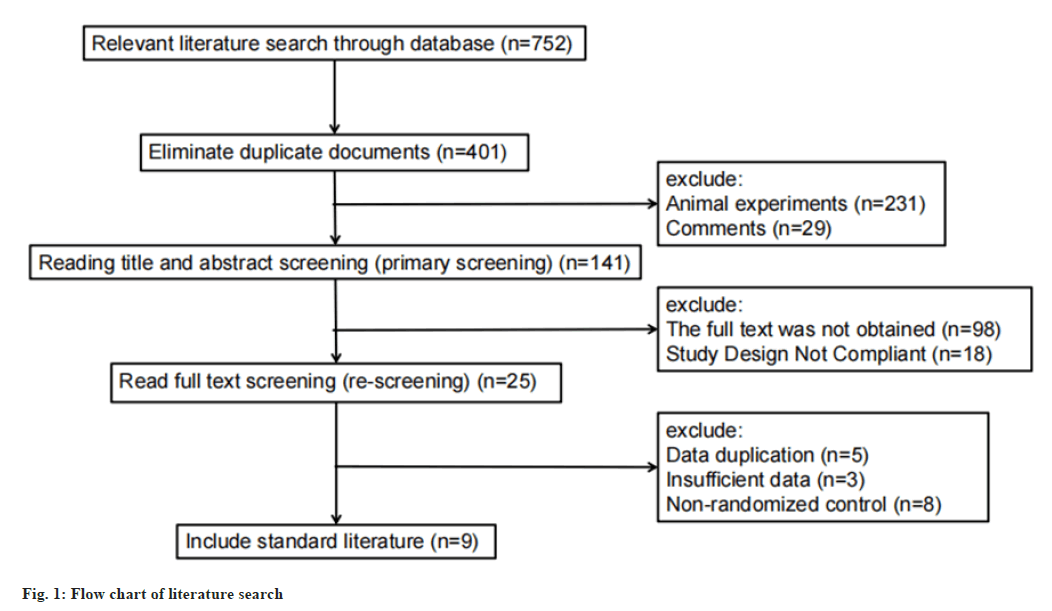
We systematically retrieved the original literature on CHD, angina pectoris and CDDP, published in databases such as CNKI, Wanfang, VIP, Embase, Web of Science, and PubMed, using subject headings combined with free words for systematic retrieval and manually retrieved 752 literature; 29±231 articles that were repeatedly published or animal experiments were obtained, and 124 articles were obtained; after reading the full text, 98±18 literatures that could not obtain the full text and incomplete experimental design were eliminated and finally 9 literatures were obtained. The literature screening process is shown in fig. 1.
Basic characteristics and quality evaluation of included literature were as follows. The demographic characteristics and baseline characteristics of the patients are shown in Table 1[13-21]. In the included literature, the control group used conventional chemical medicine treatment, while the treatment group used CDDP on the basis of the control group. Compared with the control group, the Jadad score of the included literature was 4 to 5, which was high-quality literature and none of the 8 included studies had withdrawal.
| Researcher | Number of patients (observation group/control group) | Age (years) Control group/treatment group | Control group drug | Treatment group drugs | Efficacy index | Course of treatment/day | Jadad score |
|---|---|---|---|---|---|---|---|
| Chen et al.[13] | 40/40 | 64.7±10.2/66.8±11.6 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1, 5 | 180 | 4 |
| Feng[14] | 32/32 | 31-78/35-70 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1 | 30 | 4 |
| Gu[15] | 30/30 | -/- | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1, 3, 4 | 28 | 4 |
| Liu[17] | 32/32 | 47-76/45-77 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1, 6, 8 | 60 | 4 |
| Ke[16] | 62/66 | 63±8/63±10 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1, 3, 4, 9 | 28 | 5 |
| Xu[18] | 74/83 | 61-94/61-94 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1, 2, 7, 9 | 30 | 5 |
| Xu[19] | 30/30 | 57-79/55-78 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1, 2 | 28 | 5 |
| Xu[21] | 40/40 | 51-78/48-79 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 3, 8 | 60 | 4 |
| Zhu et al.[20] | 31/31 | 46-82/44-83 | Conventional chemotherapy | Conventional chemotherapy+CDDP | 1, 5 | 56 | 4 |
Note: (-): Not available
Table 1: Basic Characteristics and Jadad Score of Included Studies
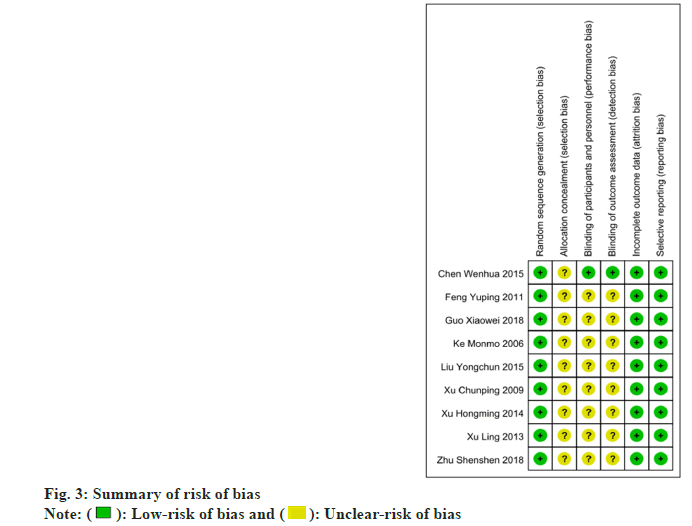
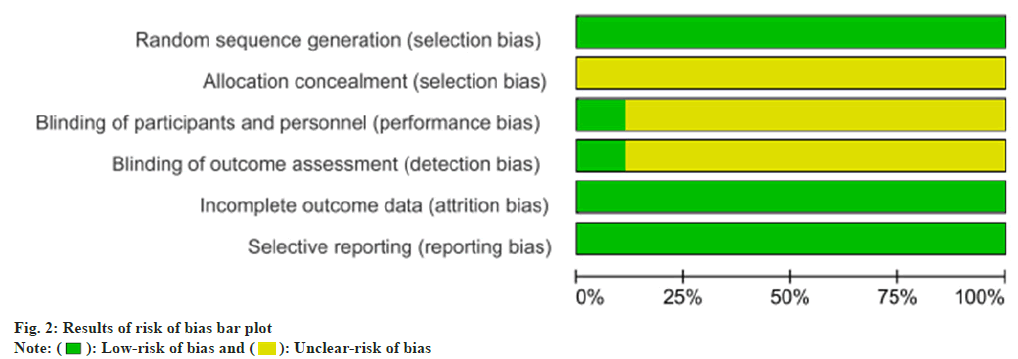
Risk of bias results was as follows. In order to assess the risk of bias, we used the Cochrane risk assessment tool to conduct an item-by-item evaluation of each included study through the following 6 evaluation criteria. They include random sequence generation; allocation concealment; blinding of participants and personnel’s; blinding of outcome assessment; incomplete outcome data and selective reporting.
The analysis results of the risk of bias (fig. 2 and fig. 3) showed that each study included in the study properly described the generation of the random sequence and had relatively comprehensive outcome data. As for allocation concealment, implementer-participant double-blinding is not described very comprehensively in most studies.
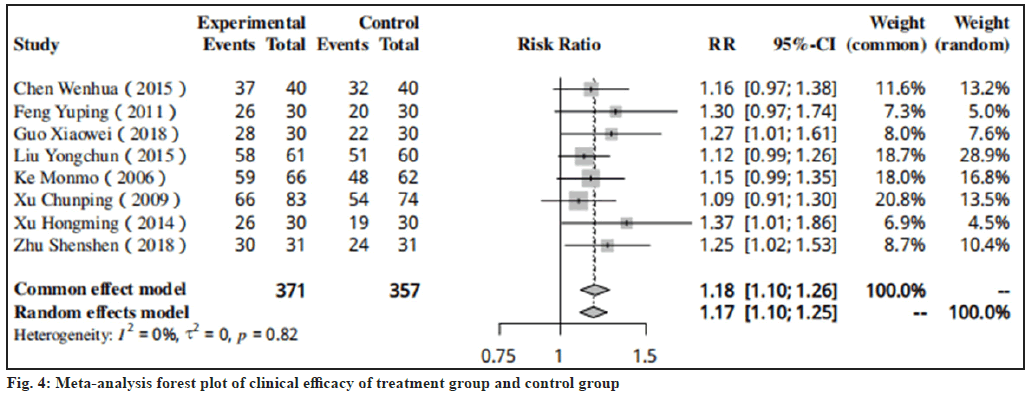
Efficacy index results were as follows. Results of clinical efficacy were shown in fig. 4 which was mentioned in the 8 studies[13-20]. After the treatment with CDDP, the clinical efficacy of the treatment group was higher than that of the control group in all included studies (RR=1.18, 95 % CI: 1.10-1.26).
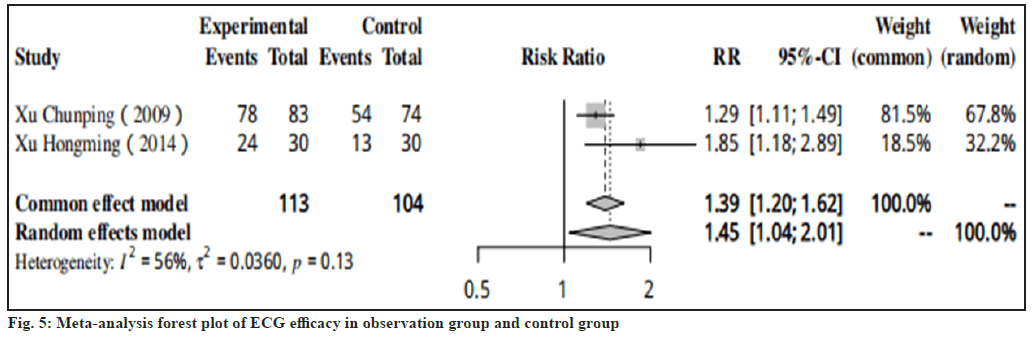
Results of ECG efficacy were shown in fig. 5. In two studies, it was specifically described that the ECG efficacy of the control group after treatment with CDDP was much worse than that of the treatment group (RR=1.39, 95 % CI: 1.20-1.62).
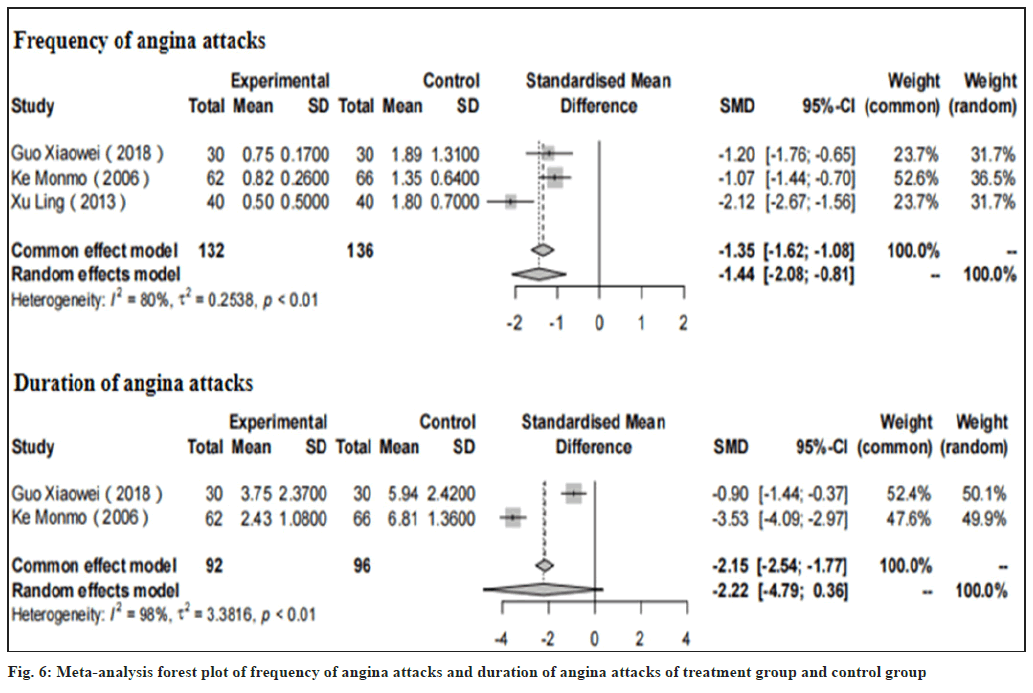
Frequency of angina attacks and duration of angina attacks were shown in fig. 6. In 3 studies[15,16,21], it has been fully demonstrated that after the treatment with CDDP, the frequency of angina pectoris in the treatment group was lower than that in the control group Standardized Mean Difference (SMD) of -1.35, 95 % CI: -1.62 to -1.08, with p<0.01) and the duration of angina attack was also significantly reduced after the comparison between the 2 groups having SMD of -2.15, 95 % CI: -2.54 to -1.77, with p<0.01 (fig. 6).
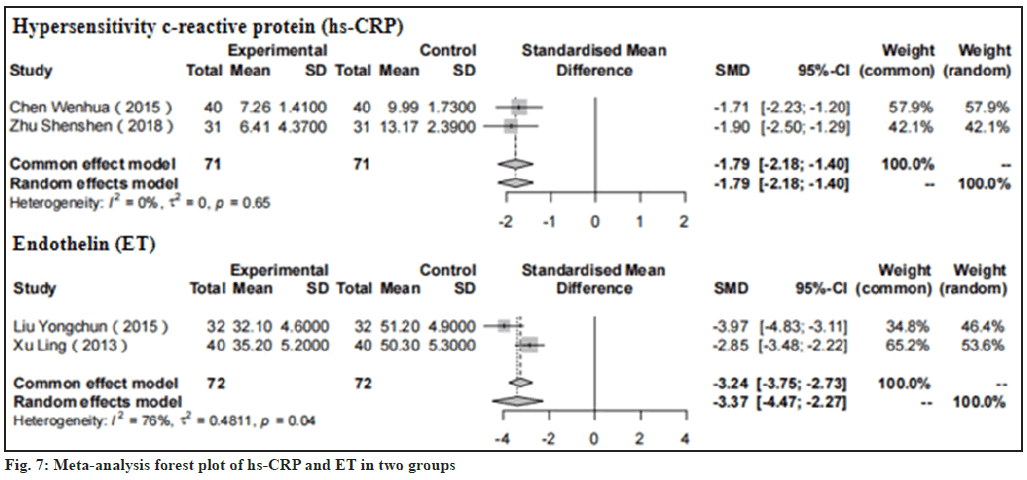
Results of hs-CRP and ET were shown in fig. 7. In two studies explained by Chen et al.[13] and Zhu et al.[13,20], we could observe that the hs-CRP in the treatment group was significantly less than that in the control group having SMD of -1.79, 95 % CI: -2.18 to -1.40, p<0.01). And we can also conclude that the ET value of the treatment group after treatment with CDDP in the two studies (Liu[17] and Xu[21]) is also lower than that of the control group having SMD of -3.24, 95 % CI: -3.75 to -2.73, p<0.01).
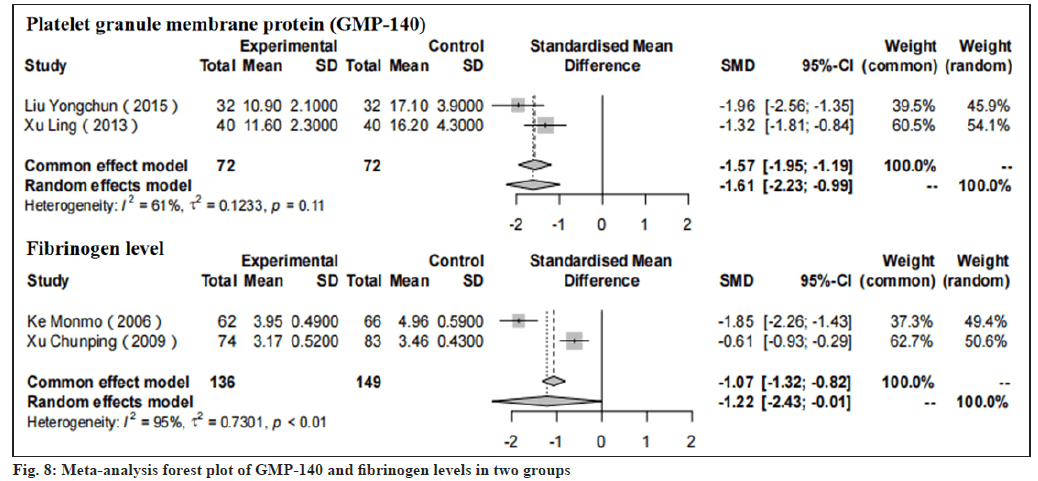
Results of GMP-140 and fibrinogen levels were shown in fig. 8. In two studies by Liu[17] and Xu[21], we could observe that GMP-140 in the treatment group was significantly less than the control whose SMD was -1.57, 95 % CI: -1.95 to -1.19, p<0.01). Moreover, in the two studies explained by Ke[16] and Xu[18], the fibrinogen level of the treatment group after treatment with CDDP was also significantly lower than that of the control group with SMD of -1.07, 95 % CI: -1.32 to -0.82, p<0.01).
Platelet aggregation rate and the changes in hemorheology were explained here. Among the 9 literatures, 2 studies (Ke[16] and Xu[18]) described very specifically the changes in hemorheology after the patients received the treatment of CDDP. Changes in hemorheology include blood viscosity, red blood cell accumulation, plasma viscosity, platelet aggregation rate and so on. In the study of Xu[18], after various treatments, the whole blood viscosity/mPa.s of control group decreased by 0.82±0.27, while the treatment group decreased by 1.50±0.41, the plasma viscosity/mPa.s of control group decreased by 0.38±0.21, while the treatment group decreased by 0.69±0.32, the hematocrit (×10-2/l) of control group decreased by 1.51±0.84, while the treatment group decreased by 3.03±1.97, the platelet aggregation rate (×10-2/l) of control group decreased by 7.82±1.79, while the treatment group decreased by 11.56±2.52. Through the comparison of these data, we can clearly see that the effect of CDDP treatment is significantly better than that of conventional chemotherapy. And in the study of Ke[16], the result obtained showed that these values have decreased to a certain extent after CDDP treatment. Therefore, we can say with certainty that hemorheology has also changed significantly after receiving CDDP treatment.
CDDP is a new type of commonly used clinical traditional Chinese medicine successfully developed by applying the theory of traditional Chinese medicine to modern medical technology. Its components have the effects of expanding coronary arteries, increasing blood flow, anti-hypoxia, enhancing contractility, anticoagulation and lowering lipids[22] and also has liver protection, anti-tumor, anti-inflammatory, antibacterial and improving blood circulatory system, etc. It has significant effects in the treatment of cardiovascular diseases, liver cirrhosis, ulcer diseases, tumors and neonatal hypoxic encephalopathy[23].
In this study, the 9 included clinical studies were analyzed through meta-analysis. The results showed that on the basis of routine Western medicine treatment, the addition of CDDPs had a positive effect on the clinical curative effect, electrocardiographic curative effect, duration of angina pectoris attack and angina pectoris attack frequency. The platelet aggregation rate and hs-CRP, ET, GMP-140, and fibrin levels were improved in the treatment, and the platelet aggregation rate, ET and GMP-140 levels were significantly reduced in the control group.
Gu et al.[24] research found that CDDP has the pharmacological effect of blocking calcium channels. CDDP expands coronary arteries, increases coronary blood flow, reduces vascular resistance and promotes collateral circulation without increasing myocardial oxygen consumption thereby alleviating myocardial ischemia. Zhuge et al.[25] found that in a study of 93 patients of ACS, CDDP can inhibit inflammation, stabilize atherosclerotic plaque and reduce the occurrence of ACS. Chen et al.[26] found that CDDP has a positive regulatory effect on vascular endothelial function in elderly patients with imbalance.
CDDP have pharmacological effects such as inhibiting platelet aggregation, inhibiting adhesion factors, promoting fibrinolysis and anticoagulation, thereby inhibiting thrombus formation and exerting an effect on hemorheology. Feng et al.[27] found that CDDP had significant inhibitory effects on rat platelet aggregation induced by Adenosine Diphosphate (ADP), thrombin and collagen in the study of the effect of CDDP on platelet aggregation in rats and showed dose-dependent relationship. Wei[28] administered CDDP (10 capsules each time, 3 times a day, for 2 mo) to 81 patients with definite diagnosis of CHD. Platelet aggregation and other drugs that improve blood rheology (such as aspirin, etc.) are used. After treatment, hemorheological indicators such as whole blood viscosity, plasma viscosity and fibrinogen were significantly improved (p<0.01). Rong et al.[29] treated 75 elderly patients with CHD by taking CDDP and isosorbide dinitrate tablets orally for 4 w, the blood viscosity, plasma viscosity, hematocrit, fiber proteinogen was significantly lower than that in the isosorbide dinitrate tablet group (p<0.05). Wang et al.[30] conducted a group study on 40 patients with unstable angina pectoris and found that the addition of CDDP on the basis of conventional treatment can reduce the level of plasma platelet GMP-140 in patients with unstable angina pectoris. The content of plasminogen activator was decreased and the activity of tissue Plasminogen Activator (t-PA) was significantly enhanced, and its effect was significantly better than the control.
CDDP is the first drug in China to pass the clinical trial of the United States (U.S.) Food and Drug Administration (FDA) and also the first Chinese patent medicine in the world to pass the review for the treatment of cardiovascular diseases[31]. It has obvious advantages in the treatment, prevention and first aid of cardiovascular diseases. At present, CDDP has been widely used in the field of CHD as the basic drug for clinical treatment of angina pectoris. At the same time, in recent years, researchers have also expanded the scope of application of CDDP, applied to a variety of disease areas[32] and achieved exciting success.
However, the specific safety and effectiveness of CDDP need to be further verified by clinical trials with larger sample size, more rigorous design and more detailed records of adverse reactions.
Most of the studies on CDDP are based on empirical medication and there is no objective and fair systematic evaluation system for its curative effect; coupled with the lack of existing evidence, more high-quality, large-sample, multi-center clinical studies are needed to verify. We need to use the methods and concepts of evidence-based medicine to conduct meta-analysis, explore its laws and provide more reliable and authentic evidence for further clinical promotion and application.
The incidence of CHD is increasing year by year and the mortality rate and disability rate are extremely high, which seriously threatens human health. The prevention and treatment of CHD with traditional Chinese medicine has shown its own unique advantages. We should further develop the advantages of traditional Chinese medicine to benefit more patients with CHD.
Author’s contributions:
Yukai Zhao, Xuedong Hao, Yuan Gao studied about conceptualization, methodology, software; Yumo Zhao, Dongmei Wan were involved in data curation, writing-original draft preparation; Qian Xu helped in visualization, investigation; Wenji Zhai was involved in supervision; Yukai Zhao studied about software and validation, and Yukai Zhao and Jing Gao were involved in writing-reviewing and editing.
Conflict of interests:
The authors declared no conflict of interest.
References
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press; 2015. p. 1219-20.
- Hong X, Mi SQ, Wang NS. Study on the pharmacokinetics of Danshensu in compound Danshen dropping pills. New Chinese Medicines and Clinical Pharmacology. 20th anniversary celebration conference and world federation of Chinese medicine pharmacology professional committee academic annual meeting; 2011. p. 113-6.
- Sun SB, Li MM, Zhang LH, Yang A, Fan HY. New clinical application of DSP. Chin Herb Med 2001;9:3-4.
- Ge YX, Duan YY, Ruan LT, Cao TS, Lu FQ, Zhou XY. Protective effect of compound Danshen dropping pills on vascular endothelial function. 2008;241:124-6.
- Crea F, Liuzzo G. Anti-inflammatory treatment of acute coronary syndromes: The need for precision medicine. Eur Heart J 2016;37(30):2414-6.
[Crossref] [Google Scholar] [PubMed]
- Li GZ, Wang JJ. Application value of DSP in treating CHD and improving serum inflammatory factor levels. Chin J Gerontol 2017;37(11):2663-4.
- Gao B, Song XY, Guo H, Bi Y, Zheng JC, Li L. Effect of compound Danshen dripping pills on the C-reactive protein and vascular endothelial function of patients with coronary heart disease and carotid atherosclerotic plaque. Prog Mod Biomed 2016;(15):2910-3.
- Yao YL, Yang B, Kang H, Zhang YM, He ZY. Effect of compound Danshen dripping pills on hemorheology in acute coronary syndrome patients before and after percutaneous coronary intervention. Chin J Mult Organ Dis Elderly 2015;14(9):678-82.
- Xu ZB, Wang WD, Zhang LF, Li J, Wang Y, Xi XX, et al. Clinical research on expression of vWF, Ps, hs-CRP, FIB, TXB2, 6-keto-PGF1α of differential TCM syndrome of coronary heart disease. Chin J Basic Med Tradit Chin Med 2015;(1):66-8.
- Yang G, Jiang Y, Li J, Xing YW, Li XQ. Effect of compound Danshen dropping pills in interfering apoptosis-related genes of coronary heart disease with blood stasis syndrome. Chin J Exp Tradit Med Formul 2016;22(20):153-7.
- Mohsina S, Gurushankari B, Niranjan R, Sureshkumar S, Sreenath GS, Kate V. Assessment of the quality of randomized controlled trials in surgery using Jadad score: Where do we stand? J Postgrad Med 2022;68(4):207-12.
[Crossref] [Google Scholar] [PubMed]
- Marees AT, de Kluiver H, Stringer S, Vorspan F, Curis E, Marie‐Claire C, et al. A tutorial on conducting genome‐wide association studies: Quality control and statistical analysis. Int J Methods Psychiatr Res 2018;27(2):e1608.
[Crossref] [Google Scholar] [PubMed]
- Chen WH, Rao LL, Yin ZE. The efficacy of compound Danshen dropping pills in the treatment of unstable angina and its effect on serum hs-CRP. Mod J Integr Tradit Chin West Med 2015;24(16):1773-4.
- Feng YP. Clinical observation on 32 cases of blood stasis type CHD angina pectoris treated by DSP. Chin J Basic Med Tradit Chin Med 2011;4:436-8.
- Guo XW. To explore the clinical effect of DSP in the treatment of angina pectoris of CHD. Digest World Latest Med Inf 2018;54:24-8.
- Ke MM. Clinical observation on 66 cases of unstable angina pectoris treated by DSP. J Youjiang Med Univ Natl 2006;23:42-9.
- Liu YC. The effect of DSP on unstable angina pectoris and its plasma. Guide Chin Med 2015;11:210-11.
- Xu CP. Curative effect observation on 83 cases of unstable angina pectoris treated by DSP. J Fujian Univ Tradit Chin Med 2019;11:79-83.
- Xu HM. Clinical treatment research of 60 patients with CHD and angina pectoris. Chin Foreign Med Res 2014;9:29-32.
- Zhu SS, Li L. Curative effect of DSP on unstable angina pectoris and its effect on serum hs-CRP. Med Health Technol 2018;15:220-23.
- Xu L. The effect of compound Danshen dropping pill in treatment of unstable angina pectoris and level change of plasma ET and GMP-140. Chin J Mod Drug Appl 2013;7(19):7-9.
- Chen XQ, Jin YY, Tang G. New pharmacology. 17th ed. Beijing: People’s Medical Publishing House; 2011.
- Zhou Y. Observation on pharmacological action and clinical application effect of Salvia miltiorrhiza. Tradit Chin Med J 2015;23:321-24.
- Gu H, Gao Y, Hou Z, Schoepf UJ, Snyder AN, Duguay TM, et al. Prognostic value of coronary atherosclerosis progression evaluated by coronary CT angiography in patients with stable angina. Eur Radiol 2018;28:1066-76.
[Crossref] [Google Scholar] [PubMed]
- Zhuge LM, Wu Q, Lou ZJ. Effect of Fufang Danshen diwan on C-reactive protein and endothelium-dependent vasodilatation in patient with acute coronary syndrome. J Zhejiang Coll Tradit Chin Med 2005;29(4):13-5.
- Chen HQ, Xiong XQ, Duan CZ, Han ZJ, Zhang XK, Zhao FR. Intervention of DSP on vascular endothelial function in CHD and its mechanism. J Sun Yat-Sen Univ Med Sci 2009;30(2):221-3.
- Feng J, Wang SC. Effect of Fufang Danshen Diwan to platelet aggregation function. Chin J MisDiagn 2006;6(12):2261-3.
- Wei J. Effects of DSP on hemorheology in patients with CHD. J Chin Microcirc 2019;5(2):146-7.
- Rong JH, Gu J, Qian XF. Clinical observation of DSP on elderly patients with CHD symptoms and hemorheological effects. J Nantong Med Univ 2004;24(4):448.
- Wang SL, Wang LX, Sun YH. Effect of compound Danshen dropping pills on platelet activation and fibrinolytic activity in patients with unstable angina pectoris. Chin J Cardiol 2003;8(5):354-6.
- Tang RL. General situation of clinical application of DSP. Eval Anal Drug Use Hosp China 2007;(5):399-400.
- Han GL. New progress in clinical application of DSP. Tianjin Pharm 2006;18(4):68-70.
 Unclear-risk of bias
Unclear-risk of bias