- *Corresponding Author:
- Yingjue Wang
Departments of Traditional Chinese Medicine, Putuo District people’s Hospital of Shanghai City, Shanghai, P.R. China
E-mail: binniao686318@163.com
| This article was originally published in a special issue, “XXXXXX” |
| Indian J Pharm Sci 2020:82(1)spl issue1;1-7 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was to investigate the role of the extracts of Cyathula officinalis Kuan on blood pressure in spontaneously hypertensive rats and the possible mechanisms involved. Blood pressure was recorded, renal vascular remodeling was visualized through hematoxylin and eosin staining and the expression of aldosterone, renin and angiotensin II in serum and kidney of spontaneously hypertensive rats was measured by real-time polymer chain reaction and enzyme-linked immuno-sorbent assay, respectively. The extracts of Cyathula officinalis Kuan significantly decreased the high blood pressure and reduced renal artery narrowing in spontaneously hypertensive rats. Moreover, Cyathula officinalis Kuan extracts also decreased the expression of aldosterone, renin and angiotensin II in both the serum and the kidney and activated ERK1/2 and p38 signaling pathways in kidney of spontaneously hypertensive rats. However, the toxicity to rat liver and kidney did not differ significantly between the extracts of Cyathula officinalis Kuan and enalapril, a well-known prodrug providing antihypertensive actions. These results demonstrated that the extracts from Cyathula officinalis Kuan can ameliorate hypertension-induced renal vascular remodeling in a rat models through inhibiting the expression of aldosterone, renin and angiotensin II and activating ERK1/2 and p38 signaling pathways.
Keywords
Cyathula officinalis Kuan, spontaneously hypertensive rats, renal vascular remodelling, reninangiotensin- aldosterone system
Clinical hypertension is classified as essential hypertension and secondary hypertension. Essential hypertension is an independent disease that defined high blood pressure as the main clinical manifestation due to nonspecific lifestyle and genetic factors[1,2], accounting for more than 90% of all hypertensive patients, and its pathogenesis remains unknown. Hypertension is one of the most common cardiovascular disease, characterized by high incidence, high mortality and high disability, and the incidence of the disease in adults was 18.8 % according to the 2002 epidemiology research of China[3]. High blood pressure can affect the structure and function of important organs such as heart, brain and kidney. Various complications caused by hypertension seriously threaten the life and quality of life of patients[4,5]. Prevention and treatment of hypertension is thus not only a problem of lowering blood pressure, but also of slowing or preventing damage to target organs. It is a hot issue in the world to find a new target to protect the target organ and to prevent the complications of hypertension[6]. Currently, it is generally believed that vascular remodelling is a structural and functional change of blood vessels in the process of growth, development, aging and diseases[7].
In the context of hypertension, vascular remodelling allows arteries to withstand the increased pressure load, but as a result, the vessels typically become more rigid than in their native state, and the reduced compliance decreases their ability to dampen the cyclical changes in blood pressure[8]. Vascular remodelling is not only an important pathophysiological change and the basis of pathogenesis of some cardiovascular diseases such as hypertension and diabetes, but also one of the important pathogenic mechanisms that cause damage to target organs[9,10]. Changes in blood pressure, which can lead to changes in wall thickness, are the first factors considered to be associated with vascular diameter and function[11]. Hypertension can cause vascular remodelling, and vascular remodelling can also cause increased vascular resistance. The vicious cycle of the two can eventually cause damage to target organs and functional insufficiency[12].
Extensive systemic artery involvement is the direct cause of pathological changes in target organs in hypertension. Small artery lesions are the most important pathological changes in hypertension. The small artery lesions in the tissues and organs can promote the maintenance and development of hypertension, and eventually lead to the ischemic injury of tissues and organs[13], among which renal arterial lesions are the most obvious injuries[14], and these verity of lesions is closely related to the blood pressure and the duration of the disease[15]. Persistent hypertension results in the vascular wall thickening, lumen stenosis, glomerular fibrosis, atrophy and renal arteriosclerosis, which ultimately lead to the renal failure[16]. The root of Cyathula officinalis Kuan is a widely used medicinal herb in China with a wide range of pharmacological activities[17]. The main components of C. officinalis extracts are alkaloids, including cyasterone, inokosterone and ecdysterone. The spontaneously hypertensive rat (SHR) is a well-characterized model representing primary hypertension of humans, which has obvious structural and functional changes in the early stage of hypertension. In larger renal cortical artery of 4-w old SHR, the area of the vessel middle wall and ratio of wall to cavity were increased and obviously with age accompanied by the damage and dysfunction of target organ[18,19]. C. officinalis extracts could decrease blood pressure and influence myocardial cell diameter in SHR, and the mechanism of antihypertensive effect might be associated with the inhibition of angiotensin converting enzyme and Ang II production, which leads to vasodilation and a reduction of blood pressure[9]. However, it is still unknown how C. officinalis extracts inhibit high blood pressure via its influences on vascular remodelling in the kidneys of SHR. To elucidate the effects of C. officinalis extracts on blood pressure and renal vascular remodelling in SHR, the expression of aldosterone (ALD), renin and angiotensin II (Ang II) and the activation of ERK1/2 and p38 signaling pathways were measured. In this investigation, C. officinalis extracts significantly ameliorated hypertension-induced renal vascular remodeling through inhibiting the expression of ALD, renin and Ang II and the activation of ERK1/2 and p38 signaling pathways.
Materials and Methods
Experimental animals:
Forty four male SHR rats (12 w, 256±20 g) were purchased from the Shanghai Institutes for Biological Sciences (Shanghai, China), and housed in polypropylene cages with sawdust bedding in hygienically controlled environment (23-25°) with a 12-h light/dark cycle throughout the study period. All animal care and procedures were in strict accordance with the China Laboratory Animal Use Regulations and were approved by the Institutional Animal Care and Use Committee at Putuo District people’s Hospital of Shanghai City (Shanghai, China).
Extraction procedures:
The dried C. officinalis plant material (1.6 kg) was powdered and refluxed with 85 % ethanol for 10 times (3 times×1.5 h). The extracts were concentrated under vacuum and dried, and the so obtained C. officinalis extract yield was 79.2 g. C. officinalis extract (47 g) was resuspended in distilled water and submitted to sequential extraction with petroleum ether, dichloromethane, and n-butanol (5 times), to obtain the n-butanol fraction, which was concentrated under vacuum and dried, obtaining the ACO (33.5 g). The samples were stored at -4° until used.
Animal treatments:
Animals were randomly divided into 5 groups, SHR control group (n=8); 2.5 mg/kg enalapril-treated SHR group (Shanghai Shyndec Pharmaceutical Co., Ltd, Shanghai, China; n=8); 3 g/kg C. officinalis extracts (Shaanxi Jiahe Phytochem Co., Ltd, Xian, China; n=9); 6 g/kg C. officinalis extracts (n=9); 12 g/kg C. officinalis extracts (n=10). Animals in the treatment group were administered intragastrically with 2.5 mg/kg enalapril, 3, 6 or 12 g/kg C. officinalis extracts once a day for 8 w. Blood pressure was measured once a week in the conscious state using a tail BP Series Automatic noninvasive blood pressure measuring system (BP-300A; Chengdu Techman Software Co., Ltd, Chengdu, China). Prior to the measurement of blood pressure, rats were trained to get accustomed to the equipment. Animals were anesthetized using intraperitoneal injection of 30 mg/kg pelltobarbitalum natricum (pentobarbital sodium) after 8 w treatment. The kidney was harvested and placed in 4 % paraformaldehyde for haematoxylin and eosin staining.
RNA extraction and real-time reverse transcription polymerase chain reaction:
Total RNA from the kidney was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) and 2 μg of RNA was reverse transcribed with PrimeScript RT reagents Kit according to the manufacturer’s instructions. Real-time PCR was carried out using SYBR Green (Takara, Otsu, Shiga, Japan) and performed using the GeneAmp PCR Systems 2700 (Applied Biosystems). The primer sequences for polymerase chain reaction (PCR) were as follows; ALD forward, 5’-CTCAGCACCAAAGCACAAATC-3’; ALD reverse, 5’-AGTAGGCACAACCCAGTAATC-3’; renin forward, 5’-CGGCATAACAATCGCATC-3’; renin reverse, 5’-AAGGGACAAGCACTCATC-3’; GAPDH forward, 5’-GTCGGTGTGAACGGATTTG-3’; GAPDH reverse, 5’-TCCCATTCTCAGCCTTGAC-3’. Expression values were calculated using the comparative Ct method and the GAPDH gene was used as endogenous control.
Western blot:
The whole cell extracts from the kidney were prepared using RIPA buffer (Beyotime, Shanghai, China). After electrophoresis, proteins were electroeluted at 120 volts onto a polyvinylidene difluoride membrane. The membrane was incubated with primary antibodies against antiALD (Life Span BioSciences, Inc, LS-C27137), antirenin (Abcam, ab176127), antiphospho-ERK1/2 (CST,#9101), antiERK1/2 (Abcam, ab17942), antiphospho-p38 (CST,#9211), antip38 (CST,#9212), and antiGAPDH (CST, #5174) overnight at 4° and then incubated with a secondary antibody. GAPDH was used as a control for total protein input.
Enzyme-linked immunosorbent assay (ELISA):
Secretions of ALD, renin and Ang II were determined by ELISA. Briefly, plasma collected from SHR with different treatments were mixed with 15 μl 10 % EDTA and 20 μl 200 IU aprotinin and then centrifuged at 400×g at 4° for 10 min. The serum was harvested and stored at -20°. The relative content of ALD, renin and Ang II in the serum of SHR was measured using an ELISA kit according to the manufacturer’s protocol.
Measurement of the toxicity of C. officinalis extracts:
The content of plasma alanine aminotransferase (ALT) and creatinine (CRE) was measured by Beckman Coulter Chemistry analyser AU5800 Series (Beckman Coulter Commercial Enterprise (China) Co. Ltd). The content of plasma C-reactive protein (CRP) and brain natriuretic peptide (BNP) was measured using ELISA as previously described.
Statistical analysis:
Results were shown as mean±SD. All data were analyzed by SPSS 18.0 software (SPSS, Inc., Chicago, USA). Comparison was done with one-way ANOVA followed by post hoc test. P value of less than 0.05 was considered statistically significant.
Ethical consideration:
All in vivo experiments were performed in accordance with the regulations for the administration of experimental animals in our university. The animal protocols were approved by the Animal Care and Use Committee (IACUC) of our University under reference No.7643/MMU/CH.
Results and Discussion
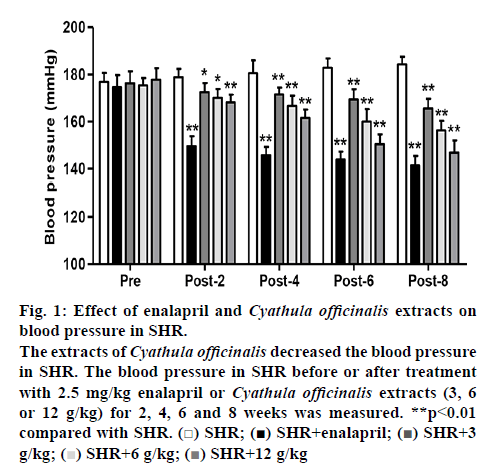
In the SHR with 2.5 mg/kg enalapril treatment for 2, 4, 6 and 8 w, the blood pressure was measured and found to be significantly decreased by 16.4, 19.2, 21.3 and 23.3 % compared to the SHR before enalapril treatment. SHR treated with different doses of C. officinalis extracts (3, 6 and 12 g/kg) treatment for 2, 4, 6 and 8 w, exhibited a significant fall in the blood pressure compared to SHR before enalapril treatment, with the most reduction in blood pressure was detected in the 12 g/kg C. officinalis extracts-treated group (figure 1). In the SHR treated with 12 g/kg C. officinalis extracts for 2, 4, 6 and 8 weeks, the blood pressure was significantly decreased by 6.1, 10.5, 17.8 and 23.3 % compared to that of the SHR before treatment, respectively (figure 1).
Figure 1: Effect of enalapril and Cyathula officinalis extracts on blood pressure in SHR.
The extracts of Cyathula officinalis decreased the blood pressure in SHR. The blood pressure in SHR before or after treatment with 2.5 mg/kg enalapril or Cyathula officinalis extracts (3, 6 or 12 g/kg) for 2, 4, 6 and 8 weeks was measured. **p<0.01 compared with SHR.  SHR;
SHR;  SHR+enalapril;
SHR+enalapril;  SHR+3 g/kg;
SHR+3 g/kg;  SHR+6 g/kg;
SHR+6 g/kg;  SHR+12 g/kg
SHR+12 g/kg
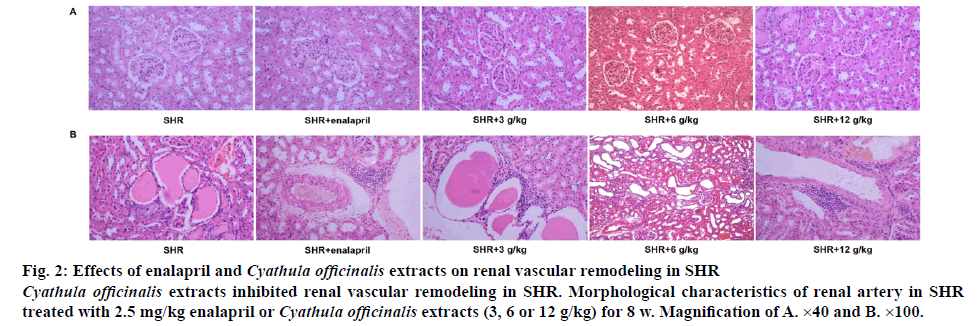
The structure of kidney and renal arteries was examined on histological sections. As shown in figure 2A, SHR with different doses of C. officinalis extracts significantly prevented the glomerular fibrosis and sclerosis as well as renal atherosclerosis compared with the SHR without treatment, with the most effective prevention detected in 6 g/kg C. officinalis extracts-treated group, which had a similar effect as 2.5 mg/kg enalapril in SHR. Furthermore, SHR with different doses of C. officinalis extracts also significantly reduced the thickness of the vascular wall and inhibited the decreased retinal arterial diameter compared with the SHR without treatment, with the most effective prevention detected in 6 g/kg C. officinalis extracts group, which had a similar effect as 2.5 mg/kg enalapril in SHR (figure 2B).
Figure 2: Effects of enalapril and Cyathula officinalis extracts on renal vascular remodeling in SHR
Cyathula officinalis extracts inhibited renal vascular remodeling in SHR. Morphological characteristics of renal artery in SHR treated with 2.5 mg/kg enalapril or Cyathula officinalis extracts (3, 6 or 12 g/kg) for 8 w. Magnification of A. ×40 and B. ×100.
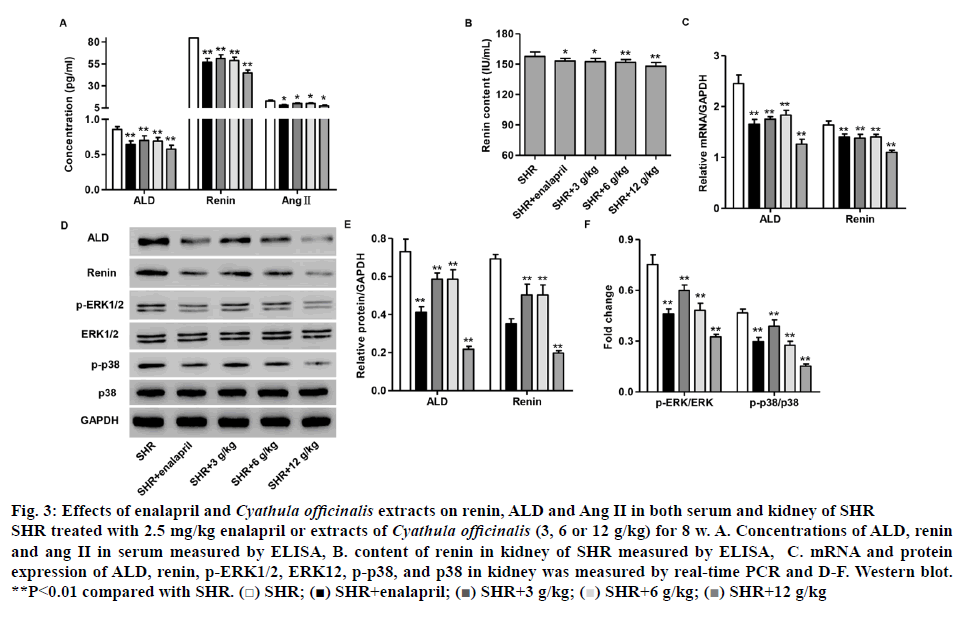
Abnormal activity of the renin-angiotensin-aldosterone system (RAAS) leads to the development of hypertension, atherosclerosis, myocardial infarction, stroke, congestive heart failure and renal disease[20,21]. Therefore, the expression of ALD, renin and Ang II in serum and kidney of SHR with different treatment was measured by real-time PCR and ELISA, respectively. The concentration of serum ALD, renin and Ang II of SHR with 2.5 mg/kg enalapril or different doses of C. officinalis extracts (3, 6 and 12 g/kg) treatment was significantly decreased compared to that in SHR without treatment (figure 3A). As shown in (figure 3B), the content of renal renin in 2.5 mg/kg enalapril or different doses of C. officinalis extracts (3, 6 and 12 g/kg) group showed significant decrease compared with that in SHR without treatment. The mRNA and protein expression of ALD and renin in kidney of SHR with 2.5 mg/kg enalapril or different doses of C. officinalis extracts (3, 6 and 12 g/kg) treatment was significantly decreased compared with that in SHR without treatment (figures 3C-E).
Figure 3: Effects of enalapril and Cyathula officinalis extracts on renin, ALD and Ang II in both serum and kidney of SHR
SHR treated with 2.5 mg/kg enalapril or extracts of Cyathula officinalis (3, 6 or 12 g/kg) for 8 w. A. Concentrations of ALD, renin and ang II in serum measured by ELISA, B. content of renin in kidney of SHR measured by ELISA, C. mRNA and protein expression of ALD, renin, p-ERK1/2, ERK12, p-p38, and p38 in kidney was measured by real-time PCR and D-F. Western blot. **P<0.01 compared with SHR.  SHR;
SHR;  SHR+enalapril;
SHR+enalapril;  SHR+3 g/kg;
SHR+3 g/kg;  SHR+6 g/kg;
SHR+6 g/kg;  SHR+12 g/kg
SHR+12 g/kg
Given the role that ERK1/2/p38 pathways as downstream effectors of RAAS mediated vascular biology and physiology, particularly, vascular remodeling[21], the expression of ERK1/2 as well as p38 and their phosphorylation levels was also examined by western blotting. As shown in (figures 3D and F), the expression of p-ERK1/2and p-p38 in kidney of SHR with 2.5 mg/kg enalapril or different doses of C. officinalis extracts (3, 6 and 12 g/kg) treatment significantly decreased compared to that in SHR without treatment. However, the expression of ERK1/2 and p38 was not changed after treatment. These results suggested that C. officinalis extracts inhibited the activation of ERK1/2 and p38 signaling in SHR.
To investigate the toxicity of C. officinalis extracts on liver and kidney function, the content of ALT, CRE, CRP and BNP was measured in plasma of SHR. As shown in (Table 1), enalapril or different doses of C. officinalis extracts significantly increased the content of CRE, decreased the content of CRP and BNP and had no effect on ALT content in SHR compared to the SHR without treatment. However, there were no significant differences on the content of ALT, CRE, CRP and BNP between enalapril and different doses of C. officinalis extracts.
Table 1: Effect of Cyathula officinalisKuan extracts on the Content of ALT, CRE, CRP and BNP
| Group | ALT (IU/ml) | Cre (mM) | CRP (ng/ml) | BNP (ng/ml) |
|---|---|---|---|---|
| SHR | 30.76±6.87 | 38.60±3.14 | 74.06±3.49 | 53.46±1.85 |
| SHR+12 g/kg | 28.56±6.17 | 48.56±4.75** | 51.23±3.96** | 41.53±4.20** |
| SHR+6 g/kg | 26.68±5.50 | 47.98±3.09** | 52.25±4.93** | 40.92±0.91** |
| SHR+3 g/kg | 26.91±6.46 | 48.40±3.51** | 51.07±2.41** | 42.63±3.58** |
| SHR+enalapril | 28.52±7.93 | 48.01±2.76** | 49.17±3.79** | 40.17±1.46** |
SHR- spontaneously hypertensive rat, ALT- alanine aminotransferase, CRE- creatinine, CRP- plasma C-reactive protein and BNP- brain natriuretic peptide. **P<0.01 compared with SHR
To the best of our knowledge, the present study is the first to determine the effects of C. officinalis extracts on renal vascular changes in hypertensive animals. The main results and findings of this work are as follows, firstly, C. officinalis extracts decrease blood pressure in SHR; secondly, these extracts prevented the glomerular fibrosis and sclerosis as well as renal atherosclerosis in SHR; thirdly, these extracts reduced the thickness of the vascular wall and inhibited the decreased retinal arterial diameter in SHR; fourthly, and most importantly, C. officinalis extracts inhibited the expression of ALD, renin and Ang II in both serum and kidney of SHR.
Hypertension is associated with decreased renal function and renal failure. The kidney is an important organ to regulate the balance of water and electrolyte and has a variety of endocrine functions. It is not only an important organ of blood pressure regulation, but also one of the main target organs of hypertension[22,23]. It is well known that resistance vessels become thicker or encroach into the lumen in kidneys[24], which occurs mainly in the preglomerular vessels at the prehypertensive or early stage of hypertension and that promotes hypertension by increasing renal vascular resistance and reducing both glomerular filtration and sodium excretion in SHR[25]. Hypertension increases peripheral vascular resistance by vascular remodelling, including vascular smooth muscle cell proliferation, hypertrophy, vascular compliance and narrow vessel lumen. In previous studies, it was also reported that vascular remodelling ultimately leads to target organ damage, such as myocardial hypertrophy, myocardial fibrosis and glomerular hyalinization in SHR[26]. Recent study have reported that C. officinalis extracts significantly reduced the expression of renal TGF-1 in SHR through inhibiting renal fibrosis, renin release and Ang II production, thus leading to vasodilation and a decrease in blood pressure[27]. In the present study, the protective effect of C. officinalis extracts against retinal vascular remodeling was confirmed, indicating the potential protection against end-organ damage induced by hypertension, such as renal damage.
The RAAS, one of the most important endocrine systems, is responsible for the regulation of blood pressure, fluid volume, sodium and potassium balance in cardiovascular, renal and adrenal glands[20]. It plays an important role in the occurrence and development of hypertension, leads to the development of cardiovascular and renal diseases and contributes to the drug resistance[21]. In the present study, C. officinalis extracts were found to significantly decrease the expression of ALD, renin and Ang II in both serum and kidney of SHR. The primary function of renin is to eventually cause an increase in blood pressure, leading to restoration of perfusion pressure in the kidneys. Renin inhibition is indeed associated with lowering of Ang II levels and blood pressure reduction[28]. Ang II, the most active substance of RAAS, has a strong role in vascular remodeling involving vasoconstriction and promotion of development of atherosclerosis, endothelial dysfunction, hypertension and related diseases such as metabolic syndrome, through a variety of inflammation and coagulation mechanism[29,30]. Another effector of the RAAS, ALD, plays a central role in the regulation of plasma Na+, extracellular K+ and arterial blood pressure[31].
ERK1/2 and p38 MAPK signalling pathways play important roles in cell proliferation and extracellular matrix deposition during hypertensive cardiovascular remodeling[21,32]. In the present study, C. officinalis extracts significantly inhibited the activation of ERK1/2 and p38 signalling pathways. ALD mediates vascular biology and physiology, particularly, vascular remodelling, fibrosis and vascular tone by regulating ERK1/2 and p38 signalling pathways[21]. p38 MAPK inhibition improves vascular remodeling and vascular function in Ang II-induced hypertension[33]. Therefore, it can be postulated that C. officinalis extracts might inhibit hypertension-induced renal vascular remodeling in rat models through inhibiting the expression of ALD, renin and Ang II and downstream effectors ERK1/2 and p38 signaling pathways.
In order to further evaluate the potential of this drug in clinic, the toxicity of C. officinalis extracts on human liver and kidney was studied by measuring the plasma level of ALT, CRE, CRP and BNP in SHR. The results clearly showed that the C. officinalis extracts significantly decreased ALT, CRP and BNP plasma level and increased CRE content in plasma in SHR. However, compared to enalapril, a well-known antihypertensive, C. officinalis extracts did not affect the plasma level of these indexes, suggesting little toxicity of C. officinalis extracts to human liver and kidney.
In conclusion, this work demonstrated for the first time that C. officinalis extracts ameliorated hypertensioninduced renal vascular remodelling in the SHR model through inhibiting the expression of ALD, renin and Ang II and activation of ERK1/2 and p38 signalling pathways. It suggested that C. officinalis extracts have potential beneficial effects on the renal vascular remodelling induced by hypertension.
Conflict of interest
No conflict of interest between any of the authors.
Acknowledgements
This work was funded by the Research Project of Shanghai Municipal Health and Family Planning Commission (20144Y0134), Key projects in Yangpu District (YP16ZC04) and Construction Project of Key Clinical Specialty of Traditional Chinese Medicine in Putuo District (PTZYLCZDZK-2017006).
References
- Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 2015;386:801-12.
- Onal EM, Sag AA, Sal O, Yerlikaya A, Afsar B, Kanbay M. Erythropoietin mediates brain-vascular-kidney crosstalk and may be a treatment target for pulmonary and resistant essential hypertension. Clin Exp Hypertens 2017;39:197-209.
- Wu Y, Huxley R, Li L, Anna V, Xie G, Yao C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from the China National Nutrition and Health Survey 2012. Circulation 2018;118:2679-86.
- Muxfeldt ES, de Souza F, Margallo VS, Salles GF. Cardiovascular and renal complications in patients with resistant hypertension. Curr Hypertens Rep 2014;16:471.
- Tientcheu D, Ayers C, Das SR, McGuire DK, de Lemos JA, Khera A, et al. Target Organ Complications and Cardiovascular Events Associated With Masked Hypertension and White-Coat Hypertension: Analysis From the Dallas Heart Study. J Am Coll Cardiol 2015;66:2159-69.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens 2017;25:1751-62.
- Pullamsetti SS and Savai R. Macrophage Regulation during Vascular Remodeling: Implications for Pulmonary Hypertension Therapy. Am J Respir Cell Mol Biol 2017;56:556-8.
- Lemarie CA, Tharaux PL and Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol 2013;48:433-9.
- Kontaraki JE, Marketou ME, Zacharis EA, Parthenakis FI, Vardas PE. MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: potential markers of target-organ damage. J Am Soc 2014;8:368-75.
- Jumar A, Ott C, Kistner I, Friedrich S, Michelson G, Harazny JM, et al. Early Signs of End-Organ Damage in Retinal Arterioles in Patients with Type 2 Diabetes Compared to Hypertensive Patients. Microcirculation 2016;23:447-55.
- Simon G. Pathogenesis of structural vascular changes in hypertension. J Hypertens 2014;22:3-10.
- Skov K and Mulvany MJ. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand 2014;181:397-405.
- Garovic VD and Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation 2005;112:1362-74.
- Gauthier-Bastien A, Ung RV, Lariviere R, Mac-Way F, Lebel M, Agharazii M. Vascular remodeling and media calcification increases arterial stiffness in chronic kidney disease. Clin Exp Hypn 2014;36:173-80.
- Herman WW, Konzelman Jr JL, Prisant LM. New national guidelines on hypertension: a summary for dentistry. J Am Dent Assoc 2004;135:576-84.
- Redon J, Pascual JM. Development of microalbuminuria in essential hypertension. Curr Hypertens Rep 2016;8:171-7.
- Feng H, Du X, Tang J, Cao X, Han X, Chen Z, et al. Enhancement of the immune responses to foot-and-mouth disease vaccination in mice by oral administration of a novel polysaccharide from the roots of Radix Cyathulae officinalis Kuan (RC). Cell Immunol 2013;281:111-21.
- Li QP, Leng J, Peng T, Rao MR. Regression of vascular remodeling in renovascular hypertensive rats by tetrandrine and enalapril. Yao xue xue bao Acta Pharmacol Sin 2013;38:328-32.
- Feng H, Du X, Liu J, Han X, Cao X, Zeng X. Novel polysaccharide from Radix Cyathulae officinalis Kuan can improve immune response to ovalbumin in mice. Int J Biol Macromol 2014; 65:121-8.
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 2016;98:121-8.
- Pacurari M, Kafoury R, Tchounwou PB, Ndebele K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam 2014;2014.
- Rossignol P, Massy ZA, Azizi M, Bakris G, Ritz E, Covic A, et al. The double challenge of resistant hypertension and chronic kidney disease. Lancet 2015;386:1588-98.
- Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens 2014;28:74.
- Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol 2012;110:49-55.
- Kinuno H, Tomoda F, Koike T, Takata M, Inoue H. Effects of uninephrectomy on renal structural properties in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2015;32:173-8.
- Chen H, Yin J, Deng Y, Yang M, Xu L, Teng F, et al. The protective effects of ginsenoside Rg1 against hypertension target-organ damage in spontaneously hypertensive rats. BMC Complement Altern Med 2012;12:53.
- Han X, Shen S, Liu T, Du X, Cao X, Feng H, et al. Characterization and antioxidant activities of the polysaccharides from Radix Cyathulae officinalis Kuan. Int J Biol Macromol 2015;72:544-52.
- Gradman AH, Vivas Y. New drugs for hypertension: what do they offer. Curr Hypertens Rep 2014;8:425-32.
- Dendorfer A, Dominiak P, Schunkert H. ACE inhibitors and angiotensin II receptor antagonists. Handb Exp Pharmacol 2015;170:407-42.
- Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci 2017;112:375-84.
- Bussemaker E, Hillebrand U, Hausberg M, Pavenstadt H, Oberleithner H. Pathogenesis of hypertension: interactions among sodium, potassium, and aldosterone. Am J Kidney Dis 2014;55:1111-20.
- Jing L, Zhang J, Sun J, Guo F, An X, Yang K, et al. Inhibition of extracellular signal-regulated kinases ameliorates hypertension-induced renal vascular remodeling in rat models. Int J Mol Sci 2011;12:8333-46.
- Potthoff SA, Stamer S, Grave K, Königshausen E, Sivritas SH, Thieme M, et al. Chronic p38 mitogen-activated protein kinase inhibition improves vascular function and remodeling in angiotensin II-dependent hypertension. J Renin-Angio-Aldo S 2016;17:1470320316653284.