- *Corresponding Author:
- Vladimir Biocanin
Faculty of Stomatology in Pancevo, Zarka Zrenjanina, Pancevo 26000, Serbia
E-mail: vladimirbiocanin@gmail.com
| Date of Received | 05 May 2025 |
| Date of Revision | 09 May 2025 |
| Date of Acceptance | 16 June 2025 |
| Indian J Pharm Sci 2025;87(3):102-107 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was to compare the effects of infiltration anaesthesia and intraseptal anaesthesia on the pulpal blood flow and gingival blood flow in healthy human subjects. Articaine (4 %) with adrenaline (1:100 000) was administered to 10 participants (20-25 y old) using the intraseptal anaesthesia technique (0.6 ml) in the region of upper central incisor. 2 w later, 1.8 ml of the same anaesthetic was administered using the infiltration anaesthesia technique. Laser Doppler flowmeter was used to measure pulpal blood flow and gingival blood flow. The infiltration anaesthesia resulted in a significant reduction of pulpal blood flow compared to baseline, in contrast to intraseptal anaesthesia. There was no significant difference in pulpal blood flow between intraseptal anaesthesia and infiltration anaesthesia. Laser Doppler flowmeter detected statistically significant reduction in gingival blood flow both for intraseptal anaesthesia and infiltration anaesthesia compared to basal values. The gingival blood flow levels between intraseptal anaesthesia and infiltration anaesthesia were not significantly different. High levels of individual variability between participants were detected for both pulpal blood flow and gingival blood flow. No statistically significant differences were observed in the pulpal or gingival blood flow, induced by intraseptal and infiltration anaesthesia. There was a tendency of more reduced gingival blood flow after intraseptal anaesthesia. Lower pulpal blood flow reduction caused by intraseptal anaesthesia could be important in cases of compromised pulps with already reduced blood flow. On the other hand, slightly higher gingival blood flow reduction caused by intraseptal anaesthesia may be favourable for better visualization of the operating field during surgical procedures.
Keywords
Pulpal blood flow, gingival blood flow, intraseptal anaesthesia, infiltration anaesthesia, Laser Doppler Flowmetry
Effective anaesthesia of the dental pulp, periodontal ligament and surrounding bony structures is essential for painless dental procedures. Infiltration Anaesthesia (IA) is a conventional method used to anaesthetise maxillary teeth with a high rate of success[1,2]. However, it may produce unpleasant numbness of the lip and cheek and long duration of soft tissue anaesthesia[2]. As an alternative anaesthetic technique Intraseptal Anaesthesia (ISA) can be used. In addition, in the case of failure of standard intraoral local anaesthetic techniques, it has been shown that the application of ISA, can achieve clinically desirable results[3-5].
ISA involves injecting the anaesthetic solution subperiosteally, and directly into the bone of the interdental septa. The target area is located 2-3 mm apical to the tip of the interdental papilla, centred between two adjacent teeth[3]. In a review of the injection technique by Woodmansey, the author also suggests delivering the intraseptal injection at mesial and distal aspects of the tooth to gain pulpal anaesthesia[6]. In the bonny area at the base of the interdental septum, there are numerous perforations of the nutrient canals. These perforations provide pathways where the anaesthetic solution caninterdental septa. The target area is located 2-3 mm apical to the tip of the interdental papilla, centred between two adjacent teeth[3]. In a review of the injection technique by Woodmansey, the author also suggests delivering the intraseptal injection at mesial and distal aspects of the tooth to gain pulpal anaesthesia[6]. In the bonny area at the base of the interdental septum, there are numerous perforations of the nutrient canals. These perforations provide pathways where the anaesthetic solution can penetrate and diffuse through the cancellous part of the bone, reaching the apical foramen of the tooth. ISA has some advantages over IA, such as shorter latency period, shorter duration, absence of unpleasant anaesthesia of the lips and cheeks and smaller amount of local anaesthetic needed, as well as the vasoconstrictor[3,5].
Amide anaesthetics cause vasodilation, thus reducing the effectiveness of local anaesthesia. Therefore, vasoconstrictor is often administered along with local anaesthetics to prolong the duration of action by reducing blood flow to the application area[7,8]. Despite its positive effect on anaesthesia, the vasoconstriction has been considered to have adverse effects particularly if prolonged or if used for successive dental procedures, causing inflammatory changes in the surrounding tissues[9,10]. This is particularly underlined by the fact that the dental pulp is enclosed within a rigid structure of dentin, unable to expand or permeate other tissues[11].
Having in mind that preserved microcirculation is a prerequisite for tissue vitality, it is generally accepted that the Pulpal Blood Flow (PBF) may be the only available true indicator of the actual state of pulpal health[12-14]. Pulpal circulation can be assessed reliably and non-invasively using Laser Doppler Flowmetry (LDF) technique that is also utilized for monitoring the blood flow in other microvascular beds of the human body[15,16]. It has been shown that adrenaline in local anaesthetics reduce both PBF and Gingival Blood Flow (GBF), regardless of whether it is administered by IA or a nerve block[9]. In addition, due to its effect on the GBF, the best clinical outcomes of the minimally invasive non-surgical periodontal treatment is achieved only when local anaesthetics without vasoconstrictors are used[17]. However, it is not clear yet how different anaesthetic techniques, especially ISA with adrenaline, influence pulpal and gingival microcirculation while achieving an adequate anaesthetic effect at the same time.
To date, no study in humans has evaluated the influence of ISA administration on PBF and GBF using LDF monitoring. Therefore, the aim of this study was to assess and compare the effects of ISA and IA on PBF and GBF of the upper central incisor region in healthy human subjects.
Materials and Methods
Participants:
Participants were healthy adults (ASAI), of both gender (5 female, 5 male), between 20-25 y of age, with good oral hygiene. The general exclusion criteria, based on medical history, were: presence of any systemic disease, use of any medication, smoking, pregnancy or breastfeeding. The examined teeth were intact with positive electrical pulp testing response, healthy surrounding tissues and gingival sulcus depth less than 3 mm with no bleeding on probing. The presence of caries, restorations, tooth sensitivity, gingivitis, periodontitis, fixed orthodontic appliances, radiographic signs of pulp chamber calcification or resorption were local exclusion criteria.
Compliance with ethical standards: The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments and was approved by the Ethical Committee of the Faculty of Stomatology in Pancevo, University of Business Academy in Novi Sad, Serbia (No. 440/2-2024). Informed consent was obtained from all participants verbally.
Anaesthesia:
At the first appointment, ISA was administered for the upper left central incisor in all ten patients. Patients received 0.6ml of 4 % articaine with adrenaline (1:100 000). Anaesthetic solution was injected with Computer Controlled Local Anaesthetic Delivery System (CCLADS) (Anaeject®, Septodont, Sallanches, France) with constant pressure, speed, and dose, approximately 0.005 ml/s. Both mesial and distal sites of ISA injection per tooth were used (2-3 mm from the tip of the papilla) and the doses were divided into equal halves per site (0.3 ml). Total time of application for ISA was 2 min. After 2 w, IA was administered for the same tooth in all the patients around the apex of the root. Patients received 1.8 ml of 4 % articaine with adrenaline (1:100 000) with a conventional carpule syringe, for 30 s. A 30 g short needle (Septodont®, Dental Needle, Guyancourt, France), for both the ISA and IA, was used. Electric pulp tester (Vitality Scanner Model 2006®, Kerr, Sybron Endo, SAD) was used to check for the success of pulpal anaesthesia following ISA and IA.
Laser Doppler Flowmetry:
Before recordings started, participants were resting in a quiet room at a constant ambient temperature in a comfortable position in a dental chair for 10 min, after which all recordings were obtained by the same dentist for at least 3 min.
PBF were measured on maxillary central incisors using a laser Doppler flowmeter PeriFlux PF 5001 (Perimed, Jarfalla, Sweden) and software Perisoft (Version 2.50; Perimed, Stockholm, Sweden). Before blood flow measurements, the flowmeter was calibrated using Perimed Motility Standard (Jarfalla, Sweden), a latex particles colloidal suspension. The probe 407-2 (Perimed) emitting a red laser light (623.8 nm) and the probe holder ph 07-6 (Perimed, Jarfalla, Sweden) were stabilized by a custom-made clear plastic stent (Bioplast; Schen-Dental, Iserlohn, Germany) with appropriately placed holes that matched the probe holder. These individual stents covered the examined teeth enabling repeatable and firm position of the probe on the labial cervical third of the crown, perpendicular to the enamel, over the long axis of the tooth. Stents also covered labial gingiva with incorporated probe holder in order to place the probe perpendicular to the basis of the interdental papilla. For PBF measurements, rubber dam was applied under the stent, in order to isolate the pulpal signal from contribution of blood flow through surrounding tissues. Blood flow levels were displayed in PU and recordings that contained the artefacts caused by participants movements were excluded. The PBF and GBF were measured before and 5 min after the ISA and IA.
Statistical analysis:
The average values of PBF and GBF levels were obtained in basal conditions, after ISA and after IA and expressed as mean±standard deviation. Kolmogorov-Smirnov test was used to check for normality distribution. Differences between the groups were analysed with one-way analysis of variance, and pair-wise comparisons were made with the Tukey post hoc test. We used (Statistical Package for the Social Sciences (SPSS) software (Version 16.0; SPSS Inc, Chicago, IL) for the data analysis and a p value <0.05 was considered statistically significant. Individual differences between participants were described too.
Results and Discussion
All participants had successful anaesthesia, confirmed by electric pulp testing. At 4 min after the injection, none of the participants showed response to maximum 80 of the electric pulp tester.
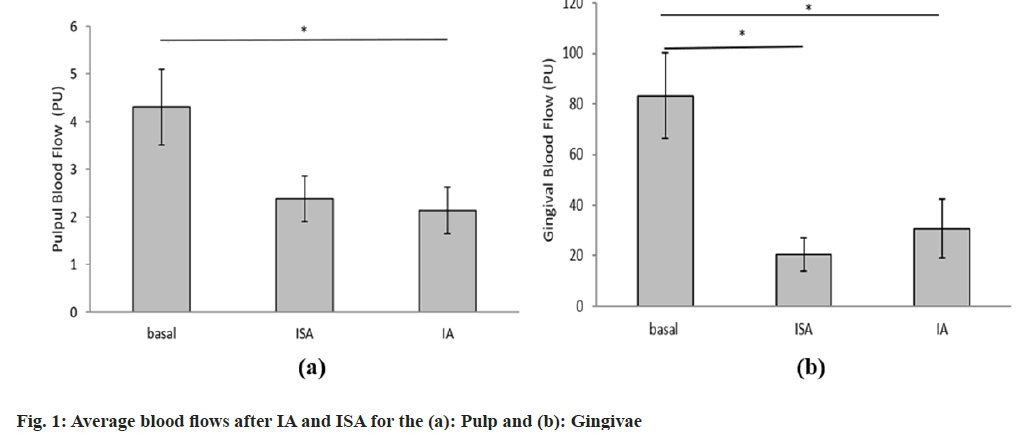
The average basal PBF for all participants was 4.3 PU and it significantly decreased to 2.1 PU after the infiltration anaesthetic technique (Table 1). Similarly, after the ISA, the PBF decreased to 2.4 PU which was not statistically significant from the basal rate. The average detected gingival basal flow rate was 83.2 PU and it significantly decreased to 30.8 PU after infiltration and even more after the intraseptal injections to 20.5 PU. There were no statistically significant differences between post-IA and post-ISA on the pulpal or GBF. The graphical representation of the results with statistical differences is depicted in fig. 1.
| Tooth (pulp) | Gingiva | |||||
|---|---|---|---|---|---|---|
| Basal | ISA | IA | Basal | ISA | IA | |
| Mean±SD (PU) | 4.3±2.51 | 2.4±1.5 | 2.1±1.5* | 83.2±53.4 | 20.5±20.1* | 30.8±37.4* |
Table 1: Comparison of Pulpal and GBF after ISA and IA
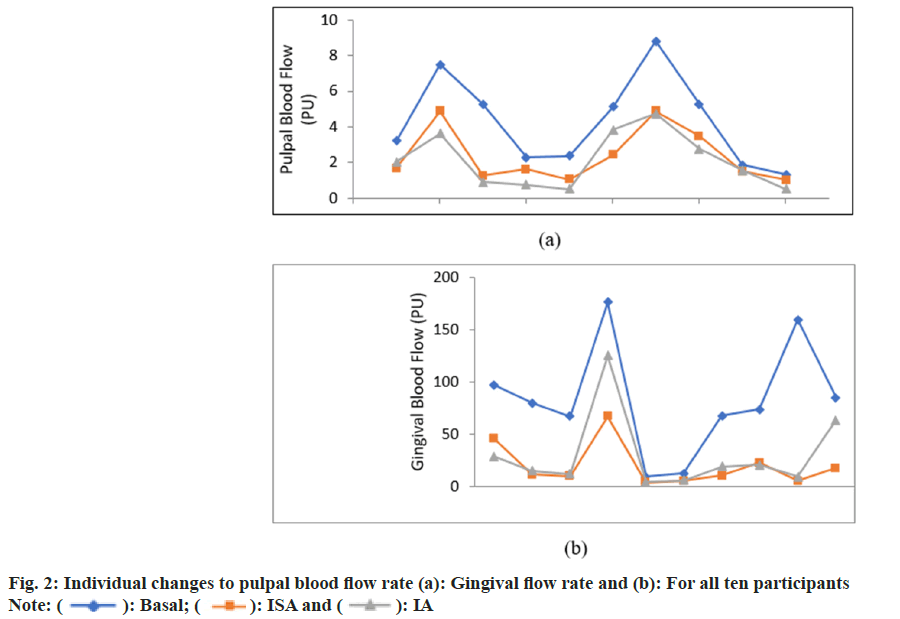
Individual differences to basal and post-anaesthesia blood flows were observed among the participants for both PBF and GBF. While the decrease in the PBF s was proportionate to the basal levels for all participants, GBF changes were less influenced by the basal gingival flow rate (fig. 2).
High perfusion rate of pulpal circulation, in physiological conditions, is required for the elimination of various irritants from the pulp chamber and root canals, as well as for the homeostasis of periodontal tissues[9]. Prolonged reduction in PBF and GBF, caused by IA with a vasoconstrictor, may impair toxin clearance resulting in excessive accumulation of inflammatory mediators and possible corresponding tissue damage[9]. To the best of our knowledge, there is no study that evaluated PBF and GBF after ISA using LDF monitoring. So far, only one study evaluated blood flow reduction after ISA in dogs, but using radioisotope-labelled microsphere injection method[18]. In the mentioned study, Kim et al.[18] used 2 % lidocaine with adrenaline 1:100 000, and compared PBF reduction after Mandibular Block Anaesthesia (MBA), IA and ISA. They found that the most drastic reduction of PBF was obtained after administration of ISA (90.4 % in mandibular molars and 69.8 % in mandibular canines). Our study showed that the ISA-caused PBF reduction (44.65 %) was not statistically significant from the basal levels, as opposed to the PBF reduction caused by the IA (51.47 %). This lower reduction of PBF after ISA may be explained by slower injection of local anaesthetic with the CCLADS and the smaller amount of anaesthetic used. This lower reduction of PBF with ISA compared to IA imply good safety profile of the ISA technique.
Previous studies, using different anaesthetic techniques, showed differential PBF reduction in maxillary teeth[19][20][21][22]. Kim et al.[23] showed that the intra-periodontal injection using 2 % lidocaine with 1:100 000 adrenaline caused a complete cessation of PBF in the rat incisor teeth and dog’s teeth for more than 20 min. The study of Vongsavan et al.[24] in humans, demonstrated that intraosseous anaesthesia with 4 % articaine with adrenaline 1:100 000 in mandibular first molars with normal pulp caused 60 % reduction of PBF.
Our study also demonstrated that both ISA and IA caused significant reduction of GBF. However, ISA produced more pronounced reduction of GBF compared to IA. This finding may have a positive effect, leading to less bleeding and better visualization of the operating field during surgery. The reduction of GBF with ISA (75.35 %) in our study indicated that ISA could take priority over IA (63.02 % reduction) in periodontal surgery[25].
Limitations of our study are the small number of participants, and because of that, this study may be considered preliminary. Likewise, further information is needed concerning the possible damaging effects of double or triple doses of ISA and IA with adrenaline on dental pulp and gingiva.
In conclusion, we found that ISA and IA produced PBF reduction of 44.65 % and 51.47 % respectively, and were effective in achieving 100 % success rate of anaesthesia. Lower PBF reduction caused by ISA in comparison to IA, could be clinically important in cases of compromised pulps with already reduced blood flow. On the other hand, higher GBF reduction caused by ISA (75.35 %) in comparison to IA (63.02 %) may be favourable during surgical procedures, but possibly without negative effects as gingivae have an extensive collateral circulation.
Ethical approval:
Faculty of Stomatology in Pancevo, University of Business Academy in Novi Sad, Serbia (No. 440/2- 2024). Informed consent was obtained from all participants verbally.
Funding:
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Woo A, Nusstein J, Drum M, Fowler S, Reader A, Ni A. Success of pulpal anesthesia following buccal infiltration of the maxillary first molar with 1.8 ml and 3.6 ml of 4 % articaine with 1: 10 00 00 epinephrine: A prospective, randomized crossover study. Anesthesia Prog 2023;70(3):110.
[Crossref] [Google Scholar] [PubMed]
- Malamed SF. Handbook of Local Anaesthesia, 7th edition: South Asia Edition-E-Book. Elsevier India; 2019.
- Djoric J, Djinic Krasavcevic A, Barac M, Kuzmanovic Pficer J, Brkovic B, Nikolic-Jakoba N. Efficacy of intraseptal anesthesia obtained by computer-controlled articaine with epinephrine delivery in scaling and root planing. Clin Oral Invest 2023;27(6):2913-22.
[Crossref] [Google Scholar] [PubMed]
- Pan J, Wang Y, Qian Y, Zou J, Zhang Q. Comparison of dental anesthetic efficacy between the periodontal intraligamentary anesthesia and other infiltration anesthesia: A systematic review and meta-analysis. PeerJ 2023;11:e15734.
[Crossref] [Google Scholar] [PubMed]
- Biocanin V, Brkovic B, Milicic B, Stojic D. Efficacy and safety of intraseptal and periodontal ligament anesthesia achieved by computer-controlled articaine+epinephrine delivery: A dose-finding study. Clin Oral Invest 2013;17(2):525-33.
[Crossref] [Google Scholar] [PubMed]
- Woodmansey K. Intraseptal anesthesia: A review of a relevant injection technique. Gen Dent 2005;53(6):418-20.
[Google Scholar] [PubMed]
- Malamed SF, GAGNON S, Leblanc D. Efficacy of articaine: A new amide local anesthetic. J Am Dental Assoc 2000;131(5):635-42.
[Crossref] [Google Scholar] [PubMed]
- Thayer S, Townsend JA, Peters M, Yu Q, Odom M, Sabey KA. Kovanaze intranasal spray vs traditional injected anesthetics: A study of pulpal blood flow utilizing laser Doppler Flowmetry. Anesth Prog 2022;69(1):31.
[Crossref] [Google Scholar] [PubMed]
- Ahn J, Pogrel MA. The effects of 2 % lidocaine with 1:100,000 epinephrine on pulpal and gingival blood flow. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85(2):197-202.
[Crossref] [Google Scholar] [PubMed]
- Zheng QH, Hong QC, Zhang L, Ye L, Huang DM. A clinical study on the effect of injection sites on efficacy of anesthesia and pulpal blood flow in carious teeth. Operative Dentistry 2018;43(1):22-30.
[Crossref] [Google Scholar] [PubMed]
- Kijsamanmith K, Sriworapongpun C, Pawasut N, Huayhongthong N, Sakulyuenyong T, Krongyoungyuen P, et al. The effect of single buccal infiltration anesthesia of 4 % articaine with either 1: 100 000 or 1: 200 000 epinephrine on pulpal blood flow and anesthesia of maxillary first molars and second premolars in humans. Clin Oral Invest 2022;26(1):343-51.
- Yang K, Guo F, Zhou Z, Hui Z, Wang Z, Wang J, et al. Laser doppler flowmetry to detect pulp vitality, clinical reference range and coincidence rate for pulpal blood flow in permanent maxillary incisors in Chinese children: A clinical study. BMC Oral Health 2023;23(1):283.
[Crossref] [Google Scholar] [PubMed]
- Baumgardner KR, Walton RE, Osborne JW, Born JL. Induced hypoxia in rat pulp and periapex demonstrated by 3H-misonidazole retention. J Dental Res 1996;75(10):1753-60.
[Crossref] [Google Scholar] [PubMed]
- Alghaithy RA, Qualtrough AJ. Pulp sensibility and vitality tests for diagnosing pulpal health in permanent teeth: A critical review. Int Endodontic J 2017;50(2):135-42.
[Crossref] [Google Scholar] [PubMed]
- Nemeth L, Birk L, Birk L, Cankar K. Laser-Doppler microvascular flow of dental pulp in relation to caries progression. Lasers Med Sci 2022;37(3):1549-57.
[Crossref] [Google Scholar] [PubMed]
- Antic S, Markovic-Vasiljkovic B, Dzeletovic B, Jelovac DB, Kuzmanovic-Pficer J. Assessment of radiotherapy effects on the blood flow in gingiva and dental pulp-a laser Doppler flowmetry study. J Appl Oral Sci 2022;30:e20220329.
[Crossref] [Google Scholar] [PubMed]
- Mehta J, Montevecchi M, Garcia‐Sanchez R, Onabolu O, Liñares A, Eriksson F, et al. Minimally invasive non‐surgical periodontal therapy of intrabony defects: A prospective multi‐centre cohort study. J Clin Periodontol 2024;51(7):905-14.
[Crossref] [Google Scholar] [PubMed]
- Kim S, Edwall L, Trowbridge H, Chien S. Effects of local anesthetics on pulpal blood flow in dogs. J Dental Res 1984;63(5):650-2.
[Crossref] [Google Scholar] [PubMed]
- Pitt Ford TR, Seare MA, McDonald F. Action of adrenaline on the effect of dental local anaesthetic solutions. Dental Traumatol 1993;9(1):31-5.
[Crossref] [Google Scholar] [PubMed]
- Premdas CE, Pitt Ford TR. Effect of palatal injections on pulpal blood flow in premolars. Dental Traumatol 1995;11(6):274-8.
[Crossref] [Google Scholar] [PubMed]
- Chng HS, Pitt Ford TR, McDonald F. Effects of prilocaine local anaesthetic solutions on pulpal blood flow in maxillary canines. Dental Traumatol 1996;12(2):89-95.
[Crossref] [Google Scholar] [PubMed]
- Odor TM, Pitt Ford TR, McDonald F. Adrenaline in local anaesthesia: The effect of concentration on dental pulpal circulation and anaesthesia. Dental Traumatol 1994;10(4):167-73.
[Crossref] [Google Scholar] [PubMed]
- Kim S. Ligamental injection: A physiological explantation of its efficacy. J Endod 1986;12(10):486-91.
[Crossref] [Google Scholar] [PubMed]
- Vongsavan K, Samdrup T, Kijsamanmith K, Rirattanapong P, Vongsavan N. The effect of intraosseous local anesthesia of 4 % articaine with 1: 100 000 epinephrine on pulpal blood flow and pulpal anesthesia of mandibular molars and canines. Clin Oral Invest 2019;23:673-80.
[Crossref] [Google Scholar] [PubMed]
- Dodson TB, Bays RA, Paul RE, Neuenschwander MC. The effect of local anesthesia with vasoconstrictor on gingival blood flow during Le Fort I osteotomy. J Oral Maxillofac Surg 1996;54(7):810-4.
[Crossref] [Google Scholar] [PubMed]
 Basal;
Basal;  ISA and
ISA and  IA
IA



