- *Corresponding Author:
- Lihua Guo
Traditional Chinese Medicine Hospital of Yunnan Province, Kunming, Yunnan 650118, People’s Republic of China
E-mail: ynszyyguolihua@163.com
| Date of Received | 03 February 2023 |
| Date of Revision | 05 September 2023 |
| Date of Acceptance | 24 January 2024 |
| Indian J Pharm Sci 2024;86(1):187-192 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To examine the impact of p21-activated kinase 1 on the growth, migration, apoptosis and invasion of hepatocellular carcinoma cells and its related mechanism. Hep38 was selected and divided into blank group, transfection control group and transfection group. The blank group was not given any treatment, the transfection control group was transfected with universal nonsense sequence, and the transfection group was given p21-activated kinases 1 sequence. The proliferation ability of cell counting kit-8 cells was compared by cell proliferation assay at different points in time after transfection. The cell cycle and apoptosis rate were detected by flow cytometry, the ability of cell migration was detected by scratch test, the p21-activated kinases 1/extracellular signal-regulated kinase pathway- related protein expression was found using the Western blot method, and the invasiveness of the cells in each group was assessed using the Transwell invasion test. The ability of cell proliferation in the transfection group was reduced than the blank group and the transfection control group at each time point. The proportion of cells in G1 phase in transfection group was reduced than blank group and transfection control group, while the proportion of G2/M phase in transfection group was higher than blank group and transfection control group. The apoptosis rate in the transfection group was higher than the blank group and the transfection control group. After 72 h of transfection, the number of invasive cells in the transfection group was reduced than the blank group and the transfection control group. After 24 h and 48 h of transfection, the cell migration ability of the transfection group was reduced than that blank group and the transfection control group. The expression of phosphorylated-p21-activated kinases 1 and phosphorylated-extracellular signal-regulated kinase 1/2 protein in the transfection group was reduced than the blank group and the transfection control group. Silencing the expression of the p21-activated kinases 1 gene can hinder hepatoma cell motility and invasion, decrease hepatoma cell proliferation, and cause apoptosis by causing cell arrest in the G2 shock M phase. Its suppression of the expression of proteins connected to the p21-activated kinases 1/extracellular signal-regulated kinase pathway could be the mechanism.
Keywords
p21-activated kinases 1, extracellular signal-regulated kinase pathway, hepatoma cells, metastasis, mortality, apoptosis, carcinoma
The 4th most common cause of cancer-related mortality worldwide and the 5th biggest solid tumor is Hepatocellular Carcinoma (HCC). The occurrence of HCC is closely related to hepatitis B, hepatitis C virus infection, liver cirrhosis and Aflatoxin B1 (AFB1) uptake, but the pathogenesis of HCC is not completely clear[1]. HCC has the characteristics of high morbidity, high degree of malignancy and high mortality. At present, the clinical treatment of HCC mainly includes surgical resection, local ablation and intervention, radiotherapy and chemotherapy and other comprehensive treatment, but the recurrence rate of traditional surgery is high, and the effect of radiotherapy and chemotherapy is not satisfactory, so it is crucial to investigate the pathophysiology of HCC and identify novel, targeted treatment targets[2,3]. p21 is highly expressed in human melanoma, breast cancer, ovarian cancer, gastrointestinal cancer and other tumor cells, it has a tight connection to the formation and occurrence of malignancies[4]. The overexpression of p21-Activated Kinases 1 (PAK1) protein may have a direct correlation to the malignant biological phenotype of colorectal cancer and may facilitate the onset and progression of the disease[5]. Ma et al.[6] studies and Liu et al.[7] found that PAK1 was highly expressed in HCC, but the related mechanism was not clear. The purpose of this work was to exam the mechanism behind the impact of PAK1 on the metastasis, apoptosis, and proliferation of the human HCC line Hep38. The overexpression of PAK1 protein may have a significant influence on the onset and progression of ovarian epithelial tumors, and may also participate during the invasion and spread of the tumor. PAK1 was highly expressed in HCC cells, but the related mechanism was not clear. The purpose of those work were to investigate the mechanism behind the impacts of PAK1 on the invasion, migration, apoptosis, and proliferation of the human HCC line Hep38.
Materials and Methods
Experimental materials:
Cell line: Human hepatoma cell line Hep38 was selected and provided by the cell bank of Shanghai Academy of life sciences.
Reagent: Small interference Ribonuclic Acid (RNA) (including identical sequence and siPAK1 sequence), provided by Shanghai Jikai Genome Medical Technology Co., Ltd. Bicinchoninic Acid (BCA) protein quantitative kit, provided by Beijing Yita Biotechnology Co., Ltd. Transwell Chamber, provided by Shanghai Yanhui Biotechnology Co., Ltd. Matrigel glue room.
Instrument: Enzyme labeling instrument, provided by Beijing Ammeg Trading Co., Ltd. Inverted microscope, provided by Shanghai Fuze Trading Co., Ltd. Ultra-low temperature refrigerator, provided by Shanghai Zuoming Machinery and Equipment Trading Co., Ltd. Ultraviolet spectrophotometer. Small desktop centrifuge, provided by Beijing Boyuhang instrument Co., Ltd. Super clean worktable, provided by Beijing Aerospace Keen Security Lab Equipment Engineering Technology Co., Ltd.
Experimental methods:
Cell culture: Human hepatoma cell line Hep38 was grown in 10 % Fetal Bovine Serum (FBS)- containing Dulbecco’s Modified Eagle Medium (DMEM) high glucose medium and cultured in incubator at 37° and 5 % Carbon dioxide (CO2).
Cell transfection: 1 500 000 cells per well of a 12-well plate were used for the inoculation of logarithmic growth phase cells. After adhering to the wall, the cells were separated into Blank Group (BLG), Transfection Control Group (TCG) and Transfection Group (TRG). The BLG was not given any treatment, the TCG was transfected with universal nonsense sequence, and the TRG was given siPAK1 sequence.
Cell Counting Kit 8 (CCK8): Human hepatoma cell line Hep38 was selected and laid in a 96-well plate with 1×105/well, with 5 multiple holes in each group. 10 μl of CCK8 reagent was added 24 h, 48 h and 72 h after transfection. 2 h later, the absorbance value of 450 nm wavelength was detected by enzyme labeling instrument. Taking the 24 h absorbance of blank control group as internal reference, the relative percentage of cell activity of each group was calculated.
Detection of cell cycle by flow cytometry: Human hepatoma cell line Hep38 was selected and laid in 96-well plate with 1×105/well, with 5 multiple holes in each group. The cells were digested into Eppendorf (EP) tube 24 h after transfection, and the operation was detected by flow cytometry. Finally, flow cytometry was used to identify the cell cycle, and the cell cycle percentage of each group was calculated.
Apoptosis rate detection using flow cytometry: Human hepatoma cell line Hep38 was selected and laid in a 96-well plate with 1×105/well, with 5 multiple holes in each group. After transfection and culture for 72 h, 0.5 μg Annexin V and 0.5 μ Lysinuric Protein Intolerance (LPI) were added and incubated and away from light for 10 min, respectively, and 500 μl of Phosphate Buffer Solution (PBS) was added to detect cell apoptosis. Repeat measurement 3 times to take the mean value.
Apoptosis rate=(Number of early apoptotic cells+late apoptotic cells)/total fine number×100 %
Scratch test: Marker pen was used to mark the back of 6 cm cell petri dish, grouping and transfection. 24 h later, the sterilized 20 μl gun head was used to draw lines vertically in a 6-hole plate. The cells drifted by scratches were washed with PBS, added with high sugar medium containing DMEM, photographed under microscope, cultured at 37° and 5 % CO2 incubator, marked on the 0th d, washed with PBS every 24 h, replaced with fresh medium containing DMEM high sugar, and photographed.
Transwell: The cells were broken down 12 h after transfection. The lower compartment received 700 μl of DMEM high glucose growth media with 30 % FBS, whereas the top chamber received 250 μl of cell suspension without FBS. After 24 h, fixing and staining, and the cells in the upper ventricle were removed.
Western blot: Collect cells and the 30 min was lysed on ice by adding the cell lysate and transferred to the EP tube. The protein concentration in the supernatant was determined after centrifugation. Take the same amount of protein, after electrophoresis in 10 % Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel, transfer the membrane to Polyvinylidene Difluoride (PVDF) membrane, seal for 1 h, incubate the first antibody, and spend the night at 4°. Tris-Buffered Saline with Tween® 20 detergent (TBST) washed the film for 3 times, incubated the second antibody, washed the film 1 h later, and exposed.
Statistical method:
The study’s data were analyzed using the Statistical Package for the Social Sciences (SPSS) 20.0 software program; all measurement data that fit a normal distribution were compared using (x±s); and pairwise comparisons were made using the Student–Newman–Keuls (SNK)-q test. Compared with the blank group, ap<0.05 and compared with the TCG, bp<0.05.
Results and Discussion
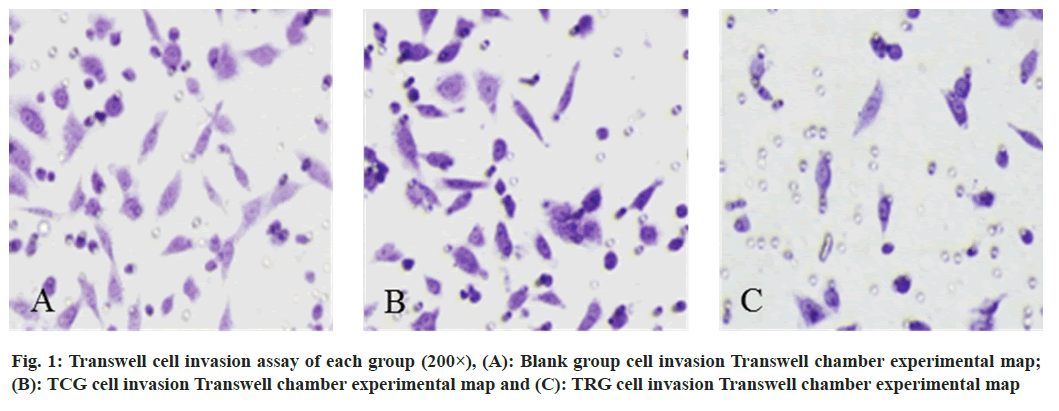
The cell proliferation ability of the TRG was reduced than the BLG and the TCG at each time point (Table 1). In the TRG, the ratio of G2/M phase was much greater than in the BLG and TCG, but the proportion of cells in the G1 phase was reduced than in both groups (Table 2). The apoptosis rate in the TRG was higher than the BLG and the TCG (Table 3). 72 h after transfection, compared to the BLG and the TCG, the TRG’s number of invasive cells was noticeably lower (fig. 1).
| Group | 24 h | 48 h | 72 h |
|---|---|---|---|
| BLG | 100.00±10.26 | 97.64±12.64 | 97.52±10.64 |
| TCG | 99.21±9.46 | 97.12±8.57 | 96.54±14.25 |
| TRG | 42.61±7.64ab | 40.18±6.85ab | 38.64±8.74ab |
| F | 257.16 | 233.605 | 173.694 |
| p | <0.001 | <0.001 | <0.001 |
Note: abp<0.05, compared with the TCG
Table 1: Comparison of Cell Proliferation among Different Groups (x͞ ±s)
| Group | G1 | S | G2/M |
|---|---|---|---|
| BLG | 52.89±1.52 | 12.56±1.02 | 34.55±1.85 |
| TCG | 52.16±1.48 | 12.64±1.84 | 35.20±2.46 |
| TRG | 36.52±2.15ab | 13.32±2.85 | 50.16±3.74ab |
| F | 562.433 | 0.832 | 199.431 |
| p | <0.001 | 0.439 | <0.001 |
Note: abp<0.05, compared with the TCG
Table 2: Comparison of Cell Cycle Ratio (x͞ ±s)
| Group | Apoptosis rate |
|---|---|
| BLG | 5.41±1.16 |
| TCG | 5.94±0.85 |
| TRG | 11.23±0.76ab |
| F | 234.863 |
| p | <0.001 |
Note: abp<0.05, compared with the TCG
Table 3: Comparison of Cell Proliferation among Different Groups (x͞ ±s)
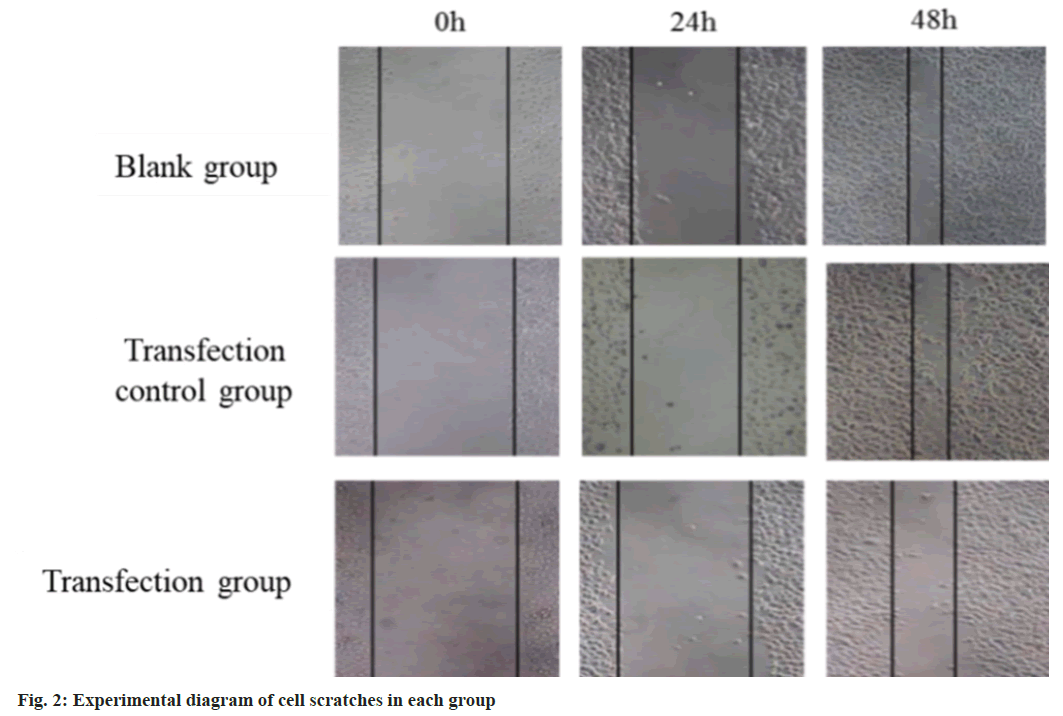
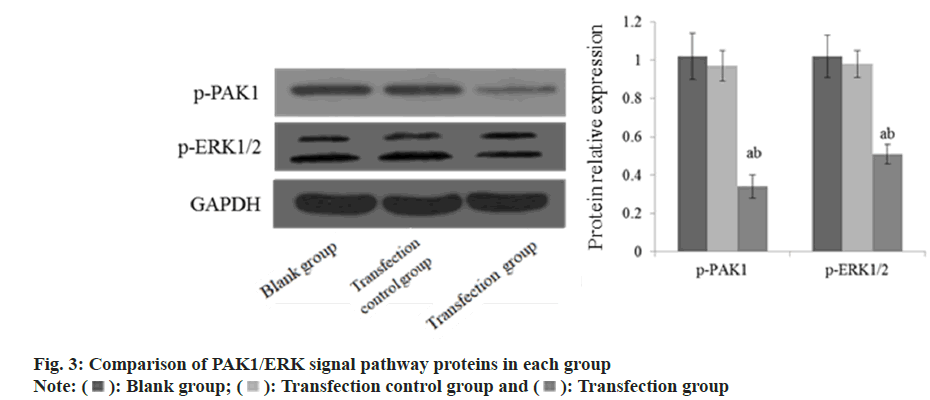
24 h and 48 h after transfection, the cell migration ability of the TRG was reduced than the BLG and the TCG (fig. 2). The p-PAK1 and p-EPK1/2 protein in the TRG was lower than that in the BLG and the TCG (fig. 3).
A malignant tumor with a high incidence rate in China is called HCC. Surgical resection and liver transplantation are the only cure for early HCC. However, because the early symptoms of HCC are relatively secret, the patients are found to be in the middle and late stage, and the surgical effect is poor, so they often use comprehensive treatment based on chemotherapy[8]. However, HCC patients are prone to multi-drug resistance to chemotherapeutic drugs, and the chemotherapeutic effect is poor, so the search for new HCC molecular targeted therapeutic drugs has been the attention of both domestic and international medical researchers.
PAK1 is a serine/threonine kinase that participates in the dynamics of cytoskeleton. Regulation of apoptosis, cell proliferation and other cellular functions[9]. In breast cancer lesions, PAK1 is abundantly expressed, and its level is correlated with alterations in the progesterone and estrogen receptors[10]. By controlling the expression of the Bcl-2 protein, PAK1 can control the apoptosis of human melanoma cells[11]. Pyo et al.[12] suggested that PAK1 can promote the proliferation of colorectal cancer by forming kinase cascade reaction. These results imply that PAK1 has a role in the emergence and progression of a number of malignant cancers. Najahi et al.[13] found that PAK1 was highly expressed in the serum of patients with HCC, and its alterations were directly correlated with the patient’s clinical status and length of survival. In this experiment, the ability of cell proliferation in the TRG was reduced than the BLG and the TCG. The proportion of cells in G2/M phase in the TRG was higher than the BLG and the TCG. It is suggested that the inhibition of PAK1 expression can inhibit the proliferation of HCC by inducing the arrest of HCC cells in G2/M phase. In this experiment, flow cytometry showed that the apoptosis rate in the TRG was higher than the BLG and the TCG. It is suggested that silencing PAK1 can induce apoptosis in HCC cells, which is comparable to the outcomes of Bondar et al.[14].
Invasion and metastasis are common biological characteristics of cancer cells, which is a complex and multi-step cascade process, which directly affects the prognosis of patients[15]. HCC cells are loosely arranged and easy to fall off, and they can be metastasized to a distance or directly with the blood, lymph and bile duct system, etc. One crucial stage in the spread of cancer cells is their metastasis, which is of great significance to endothelial cell angiogenesis and tumor growth and metastasis[16]. In this experiment, 72 h after transfection, it was discovered that the TRG’s number of invasive cells was much fewer than that of the TCG and the BLG. Further Transwell invasion assay revealed that the cell migration ability of TRG was reduced than BLG and TCG at 24 h and 48 h after transfection. It is recommended that silencing the PAK1 can reduce the metastasis ability of HCC cells, thus inhibit the metastasis of cancer cells.
Extracellular Signal-Regulated Kinase (ERK) molecule and its signal pathway are the basic signal pathways necessary for cell growth and survival. Recent studies have shown that PAK1 is located in the upstream of ERK signal pathway and can encourage the growth and metastasis of tumor cells by controlling ERP signal pathway[17]. Inoue et al.[18] found that high expression of PAK1 can affect the biological activity of breast cancer cells through PAK1/ERK signal pathway. In this experiment, the p-PAK1 and p-EPK1/2 protein in TRG was reduced than that TCG and BLG. It is suggested that silencing PAK1 can inhibit the growth and metastasis of HCC cells and induce apoptosis, which may be connected to the inhibition of proteins related to PAK1/ERK pathway.
To sum up, silencing the PAK1 gene can reduce the proliferation ability of HCC cells, reduced the metastasis ability of HCC cells, and induce apoptosis by inducing cell arrest in G2/M phase, and its mechanism might have something to do with suppressing the expression of proteins involved in the PAK1/ERK pathway.
Funding:
This work was supported by the Science Research Fund Project of Yunnan Provincial Health Department Establishment of Research Institutes (No. 2016NS099) and the Science Research Fund Project of Yunnan Provincial Department of Education (No. 2018JS225).
Author’s contributions:
Ruilian Zhao and Ying Gao have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl J Med 2018;379(1):54-63.
[Crossref] [Google Scholar] [PubMed]
- Bosetti C, Bianchi C, Negri E, Colombo M, La Vecchia C. Estimates of the incidence and prevalence of hepatocellular carcinoma in Italy in 2002 and projections for the years 2007 and 2012. Tumor J 2009;95(1):23-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Qian S, Yang G, Zhu L, Zhou B, Wang J, et al. microRNA-199 suppresses cell proliferation, migration and invasion by downregulating RGS17 in hepatocellular carcinoma. Gene 2018;659:22-8.
[Crossref] [Google Scholar] [PubMed]
- Livneh E, Ari AB, Zurgil U. Cell death and senescence: Role of protein kinase C in promoting senescence. Cancer Res 2018;78(13):464.
- Chishima F, Hayakawa S, Yamamoto T, Sugitani M, Karasaki-Suzuki M, Sugita K, et al. Expression of inducible microsomal prostaglandin E synthase in local lesions of endometriosis patients. Am J Reproductive Immunol 2007;57(3):218-26.
- Ma Y, Liang S, Zhang Y, Yang D, Wang R. Development of anti-fungal pesticides from protein kinase inhibitor-based anticancer agents. Eur J Med Chem 2018;148:349-58.
[Crossref] [Google Scholar] [PubMed]
- Liu YY, Tanikawa C, Ueda K, Matsuda K. INKA2, a novel p53 target that interacts with the serine/threonine kinase PAK4. Int J Oncol 2019;54(6):1907-20.
[Crossref] [Google Scholar] [PubMed]
- Muaddi H, Al-Adra DP, Beecroft R, Ghanekar A, Moulton CA, Doyle A, et al. Liver transplantation is equally effective as a salvage therapy for patients with hepatocellular carcinoma recurrence following radiofrequency ablation or liver resection with curative intent. Ann Surg Oncol 2018;25:991-9.
[Crossref] [Google Scholar] [PubMed]
- Yao X, Ei-Samahy MA, Fan L, Zheng L, Jin Y, Zhang G, et al. In vitro influence of selenium on the proliferation of and steroidogenesis in goat luteinized granulosa cells. Theriogenology 2018;114:70-80.
[Crossref] [Google Scholar] [PubMed]
- Lee HW, Rhee DK, Kim BO, Pyo S. Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways. Biomed Pharmacother 2018;102:670-80.
[Crossref] [Google Scholar] [PubMed]
- Kumar R, Li DQ. PAKs in human cancer progression: From inception to cancer therapeutic to future oncobiology. Adv Cancer Res 2016;130:137-209.
[Crossref] [Google Scholar] [PubMed]
- Pyo JS, Min KW, Oh IH, Lim DH, Son BK. Clinicopathological significance and the associated signaling pathway of p21-activated kinase 1 (PAK1) in colorectal cancer. Pathol Res Pract 2023;251:154820.
[Crossref] [Google Scholar] [PubMed]
- Najahi-Missaoui W, Quach ND, Jenkins A, Dabke I, Somanath PR, Cummings BS. Effect of P21-activated kinase 1 (PAK-1) inhibition on cancer cell growth, migration, and invasion. Pharmacol Res Perspect 2019;7(5):e00518.
[Crossref] [Google Scholar] [PubMed]
- Bondar VV, Adamski CJ, Onur TS, Tan Q, Wang L, Diaz-Garcia J, et al. PAK1 regulates ATXN1 levels providing an opportunity to modify its toxicity in spinocerebellar ataxia type 1. Hum Mol Genet 2018;27(16):2863-73.
[Crossref] [Google Scholar] [PubMed]
- Zhang YU, Wang X, Wang Z, Tang HU, Fan H, Guo Q. miR-182 promotes cell growth and invasion by targeting forkhead box F2 transcription factor in colorectal cancer. Oncol Rep 2015;33(5):2592-8.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022;7(1):3.
[Crossref] [Google Scholar] [PubMed]
- Zhang F, Lu YX, Chen Q, Zou HM, Zhang JM, Hu YH, et al. Identification of NCK1 as a novel downstream effector of STAT3 in colorectal cancer metastasis and angiogenesis. Cell Signal 2017;36:67-78.
[Crossref] [Google Scholar] [PubMed]
- Inoue K, Patterson EK, Capretta A, Lawendy AR, Fraser DD, Cepinskas G. Carbon monoxide–releasing molecule-401 suppresses polymorphonuclear leukocyte migratory potential by modulating F-actin dynamics. Am J Pathol 2017;187(5):1121-33.
[Crossref] [Google Scholar] [PubMed]
 Transfection control group and
Transfection control group and  Transfection group
Transfection group



