- *Corresponding Author:
- Min Feng
Department of Radiology, First Affiliated Hospital of Huzhou University, Huzhou, Zhejiang 313000, China
E-mail: fm_hzyy@163.com
| This article was originally published in a special issue, “Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(2) Spl Issue “11-16” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of regional arterial infusion chemotherapy combined with laparoscopic surgery on the prognosis of patients with colorectal cancer and the serum interleukin-8, insulin-like growth factor binding protein-3, blood vascular endothelial growth factor and E-cadherin. From January 2019 to January 2020, 122 cases of colorectal cancer patients who planned to undergo laparoscopic surgery in our hospital were selected as the research objects and the research objects were divided into observation group and control group according to the random principle, with 61 cases in each group and control group. The patients in the group received laparoscopic surgery and the patients in the observation group received regional arterial infusion chemotherapy before operation. The total incidence of complications after operation was compared between the two groups and the serum tumor marker levels before and after operation was compared between the two groups, including interleukin-8, E-cadherin, insulin-like growth factor binding protein-3 and vascular endothelial growth factor; 3 y after operation, the comparison survival rates of patients in the two groups. The total incidence of postoperative complications in the observation group was 8.20 %, the interleukin-8 level was 92.16±11.1 pg/ml, the positive rate of E-cadherin was 37.70 % and the insulin-like growth factor binding protein-3 level was 4.08±0.72 ng/ml. The overall incidence of complications in control group was 14.75 %, the interleukin-8 level was 93.08±11.54 ng/ml, the positive rate of E-cadherin was 34.43 % and the insulin-like growth factor binding protein-3 level was 4.11±0.74 ng/ml. There was no statistically significant difference between the two groups (p>0.05). The vascular endothelial growth factor level in the observation group was 129.54±16.98 pg/ml, which was significantly lower than that in the control group (192.72±17.25 pg/ml) and the difference was statistically significant (p<0.05). During the 3 y follow-up, the difference in survival rate between the two groups was statistically significant (p>0.05). Regional arterial infusion chemotherapy combined with laparoscopic resections is safe and feasible because it can reduce the vascular endothelial growth factor level in colorectal cancer patients and improve the 3 y survival rate of patients without increasing the incidence of surgical complications and inflammation.
Keywords
Regional arterial infusion chemotherapy, laparoscopic surgery, colorectal cancer, adenoma
Colorectal Cancer (CRC) is the third most common cancer worldwide. Because CRC develops from normal mucosa to adenoma, it can develop into invasive adenocarcinoma as the disease progresses. According to research reports, the estimated development time can be 5 y-10 y[1]. If the diagnosis is in the early stage of CRC, the 5 y survival rate can reach 90 %; if the diagnosis is in the advanced stage of CRC, the 5 y survival rate can drop to 14 %. Therefore, early diagnosis and effective treatment is the key to reducing mortality[2].
Laparoscopic resection is a common treatment for CRC. A number of large randomized controlled trials have proved that this method has many advantages compared with open surgery, such as less intraoperative blood loss, faster recovery of intestinal function and shorter hospital stay[3,4]. Regional Arterial Infusion Chemotherapy (RAIC) is a neoadjuvant chemotherapy method. With the help of interventional positioning technology, chemotherapy drugs are directly infused from tumor blood supply arteries to tumor tissue to ensure the highest local concentration of chemotherapy drugs in the tumor, avoiding the combination of chemotherapy drugs with plasma proteins. The combination leads to a reduction in the effect, which helps to improve the surgical treatment effect. RAIC combined with laparoscopic resection is widely used in the treatment of CRC. This treatment scheme can give full play to its respective advantages, but there are few reports on the long-term prognosis of this scheme. The serum tumor marker Interleukin-8 (IL-8), Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3), Vascular Endothelial Growth Factor (VEGF) and E-cadherin (E-cad) are also not yet clear. Thus, we conduct our experiments.
Materials and Methods
Research subject:
From January 2019 to January 2020, 122 patients with CRC who planned to undergo laparoscopic surgery in our hospital were selected.
Inclusion criteria: Patients diagnosed with CRC by pathological examination of colonoscopy before operation; no contraindications for angiography, no contraindications for RAIC and laparoscopic treatment and no relevant anti-tumor treatment before admission.
Exclusion criteria: Patients were informed and agreed to participate; abnormal liver and kidney function, combined with severe anemia or coagulation disorders and other serious diseases; stop treatment midway or transfer to another hospital and combined with other malignant tumors. According to the treatment methods, 122 patients with CRC were divided into groups. Patients who received RAIC combined with laparoscopic resection were selected into the observation group (61 cases) and patients who received laparoscopic resection were selected into the control group (61 cases). There was no significant difference in general information such as gender, age, tumor location, diameter and Tumor, Nodes and Metastases (TNM) stage between the two groups (p>0.05) as shown in Table 1.
| Baseline data | Observation group (n=61) | Control group (n=61) |
|---|---|---|
| Average age (years) | 50.78±5.84 | 49.25±6.18 |
| Gender (male/female) | 35/26 | 33/28 |
| Tumor location (colon/rectum) | 32/29 | 35/26 |
| TNM stage (II/III) | 38/23 | 40/21 |
| Tumor size (cm) | 3.52±0.53 | 3.38±0.56 |
Note: *p<0.05, compared with the control group
Table 1: Baseline Data (n (%), x̄±s).
Treatment methods:
The control group was treated with laparoscopic surgery. All patients underwent general anesthesia with endotracheal intubation. The operation was performed according to the standard guidelines for laparoscopic surgery and the principle of tumor-free operation. The tumor and infiltrated tissues were completely removed to ensure that the intestinal tract at both ends of the tumor was resected at an appropriate length. The resected tumor was ligated with a plastic bag after removal, the lymph nodes were fully dissected, digestive tract reconstruction was performed for patients with colon cancer, and bowel anastomosis was performed for patients with rectal cancer. Antibiotics, nutritional support and other symptomatic treatment, and close care were given postoperatively.
The observation group was treated with RAIC combined with laparoscopic surgery. Preoperatively, the Seldinger technique was used to intubate the right femoral artery of the patient and the intubation method was selected according to the tumor site; sigmoid colon cancer through the inferior mesenteric artery to the sigmoid colon, right colon cancer through the superior mesenteric artery to the right colic artery and rectal cancer through the inferior mesenteric artery to the superior rectal artery. After intubation, digital subtraction angiography was used to insert a micro catheter into the tumor blood supply artery. The chemotherapy method was 1000 mg 5-fluorouracil+10 mg mitomycin+50 mg cisplatin. For patients with rectal cancer, 40 % lipiodol is injected into local blood vessels for embolization. After infusion chemotherapy, the tube is extubated and disinfected. Laparoscopic surgery can be performed after 1 w and the method is the same as that of the control group.
Observation indicators:
Adverse reactions: The possible complications of the two groups were compared after operation, including incision infection, urinary system infection, urinary retention and intestinal obstruction.
Serum tumor markers: Before and after operation, the changes of IL-8, IGFBP-3, VEGF levels and the positive rate of E-cad in two groups of patients.
Postoperative follow-up: 3 y postoperative follow-up to compare the survival rates of the two groups.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 software was used for statistical analysis of all data. Measurement data were expressed as mean±standard deviation (x̄±s) and t-test was used for comparison between groups; enumeration data were expressed as case number and rate (%) and comparison between groups, the Chi-square (χ²) test was used; p<0.05 was considered statistically significant.
Results and Discussion
The total incidence of complications in the observation group was 8.20 % and that in the control group was 14.75 %, with no significant difference between the two groups (χ²=1.291, p=0.2559) as shown in Table 2.
| Complications | Observation group (n=61) | Control group (n=61) |
|---|---|---|
| Incision infection | 3 (4.92) | 4 (6.56) |
| Urinary tract infection | 1 (1.64) | 2 (3.28) |
| Urinary retention | 1 (1.64) | 1 (1.64) |
| Intestinal obstruction | 1 (1.64) | 2 (3.28) |
| Total prevalence complications | 5 (8.20) | 9 (14.75) |
Table 2: Complications of patients in the two groups (n (%)).
After treatment, the level of IL-8 in the observation group was 92.16±11.1 pg/ml, the positive rate of E-cad was 37.70 %, the level of IGFBP-3 was 4.08±0.72 ng/ml and the level of IL-8 in the control group was 93.08±11.54 ng/ml, E-cad positive rate was 34.43 %, IGFBP-3 level was 4.11±0.74 ng/ml, the difference was not statistically significant (p>0.05); after treatment, VEGF in the observation group was 129.54±16.98 pg/ml, VEGF in the control group was 192.72±17.25 pg/ml and the difference between the two groups was statistically significant (p<0.05) as shown in Table 3.
| Serum tumor markers | Observation group | Control group | ||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| IL-8 (pg/ml) | 45.13±11.05 | 92.16±11.17 | 46.22±10.98 | 93.08±11.54 |
| E-cad (+) | 22 (36.07) | 23 (37.70) | 23 (37.70) | 21 (34.43) |
| IGFBP-3 (ng/ml) | 3.97±0.78 | 4.08±0.72 | 4.20±0.75 | 4.11±0.74 |
| VEGF (pg/ml) | 195.15±16.97 | 129.54±16.98* | 201.07±17.11 | 192.72±17.25 |
Note: *p<0.05, compared with the control group
Table 3: Comparison of serum tumor marker levels between the two groups before and after treatment (n (%), x̄±s).
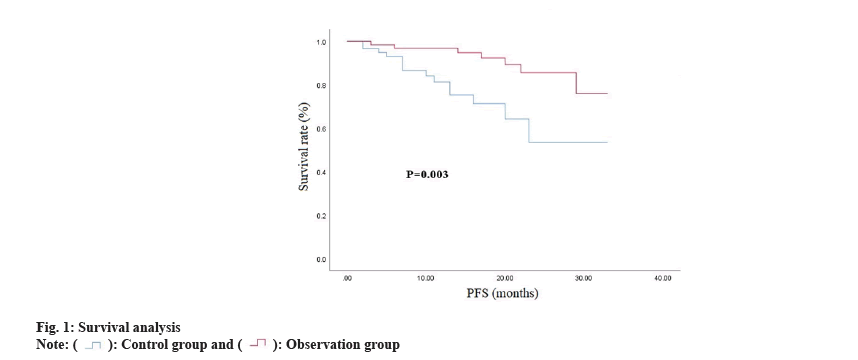
All patients were followed up until 36 mo after treatment. During the follow-up, a total of 11 (18.03 %) patients in the observation group relapsed and 15 (24.59 %) patients in the control group relapsed, with no significant difference between the groups (p>0.05). Kaplan-Meier analysis showed that the 3 y survival rate of the observation group was 88.52 % and the average Progression-Free Survival (PFS) was (30.11±1.007) mo; the 3 y survival rate of the control group was 77.05 % and the average PFS was (24.18±1.951) mo. Significantly (p<0.05) as shown in Table 4 and fig. 1.
| Group | Average PFS | 95 % CI | p |
|---|---|---|---|
| Observation group | 30.11±1.007 | 28.14~32.09 | 0.0031 |
| Control group | 24.18±1.951 | 20.35~28.00 |
Table 4: Survival Analysis of observation group and control group after 3 Y follow-up.
CRC is the 5th most common cause of death in China and a major public health problem. The traditional treatment method is to use open surgery to remove the cancerous tissue of the patient. With the advancement of minimally invasive surgery techniques and equipment, laparoscopic resection has gradually become an alternative to open surgery. Some studies[5-7] have also confirmed that compared with traditional surgical methods, the recovery period after laparoscopic surgery is faster, reflected in less postoperative pain and blood loss, shorter hospital stay and faster return to normal activities. However, a large number of clinical data in recent years have found that for some patients, the effect of single surgical treatment and postoperative chemotherapy is limited, especially for patients with middle and advanced CRC, the treatment effect is not satisfactory. In order to improve the treatment effect, some of the study proposes to combine multiple neoadjuvant chemotherapy methods for comprehensive treatment of patients. In addition, for inoperable patients, neoadjuvant chemotherapy improves the respectability. RAIC refers to injecting chemotherapeutic drugs directly into the tumor tissue through the tumor blood supply artery with the assistance of interventional positioning technology. This method increases the concentration of chemotherapeutic drugs around the tumor, effectively reduces the binding rate of drugs and plasma proteins and thus improves the therapeutic effect. Reaction, minimize the adverse reaction of the drug. The study of Peng et al.[8] showed that for CRC patients, preoperative interventional arterial infusion chemotherapy is safe and feasible, and it is more effective than conventional preoperative systemic chemotherapy in improving quality of life, improving clinical control, and reducing adverse reactions and complications. The study of Meng et al.[9] also showed that the addition of regional intra-arterial chemotherapy to the standard treatment can transform the tumor into a resectable tumor, which can effectively create good surgical conditions for patients with locally advanced rectal cancer. Although the efficacy of preoperative RAIC combined with laparoscopic resection in the treatment of CRC has been confirmed by many studies, there are still few research reports on its long-term prognosis.
IL-8, E-cad, IGFBP-3 and VEGF are common tumor markers, which are abnormally expressed in malignant tumor tissues such as CRC and gastric cancer and their expression levels are closely related to the occurrence and progression of the disease. IL-8, also known as neutrophil chemokine, is produced by immune cells and tumor cells. It is a member of the neutrophil-specific CXC chemokine family involved in the initiation and expansion of inflammatory processes, inducing chemotaxis of different granulocytes, especially neutrophils, but also in stimulating phagocytosis. Currently, IL-8 has been revealed in the proliferation, invasion and metastasis of CRC cells. In addition, some studies have described the correlation between its overexpression and poor prognosis of CRC patients[10]. E-cad, a member of the calcium-dependent cell adhesion proteins, is a key component of cell-to-cell adhesions (adherens junctions), which assemble between adjacent cells in epithelial tissues and have been previously shown. The loss of E-cad will lead to the loss of adhesion between CRC cells, thereby promoting tumor cell invasion and migration[11]. IGFBP-3 can affect a variety of molecular mechanisms or signaling pathways that determine cell death or survival, especially in the context of cancer. GFBP-3 is a major secreted protein whose primary role is to bind and sequester circulating like Insulin Growth Factor (IGF), thereby extending its half-life while controlling its bioavailability. In CRC, previous reports have shown that the IGF system affects CRC cell proliferation and low expression of IGFBP-3 is one of the risk factors for lymph node metastasis in CRC[12]. The VEGF family affects the process of tumor angiogenesis, thereby promoting tumor growth and metastasis formation. An increase in its expression correlates with the progression of neoplastic disease. Many studies on tumors of various types and locations have shown that the high expression of VEGF and its receptors has an impact on disease development. Some researchers have shown that higher preoperative VEGF levels are thought to be associated with recurrence of CRC after treatment and that higher expression of VEGF is a marker of poor prognosis and poor survival in CRC. In addition, relevant data have shown that VEGF levels are associated with tumor stage and disease progression[13].
The results of this study showed that the total incidence of postoperative complications in the observation group was 8.20 % and that in the control group was 14.75 %, with no significant difference between the two groups (p>0.05). IL-8 level was 92.16±11.1 pg/ml, E-cad positive rate was 37.70 %, IGFBP-3 level was 4.08±0.72 ng/ml, compared with control group (IL-8 level was 93.08±11.54 ng/ml, E-cad positive rate was 34.43 %, and the IGFBP-3 level was 4.11±0.74 ng/ml) and the difference was not statistically significant (p>0.05). The above results show that performing RAIC before laparoscopic resection does not increase the risk of complications and inflammation, nor does it reduce the expression rates of E-cad and IGFBP-3, which is safe and feasible. The view of the feasibility and effectiveness of RAIC has also been proved by foreign scholar Huang[14].
The results of this study also showed that after treatment, VEGF in the observation group was 129.54±16.98 pg/ml, which was significantly lower than that in the control group (192.72±17.25 pg/ml) and the difference between the two groups was statistically significant. Analysis of the reasons may be tumor tissue; the main source of VEGF, chemotherapy drugs cause tumor cell apoptosis and VEGF levels decrease; anti-tumor drugs have anti-angiogenic effects, which reduce VEGF levels; chemotherapy drugs can inhibit endothelial cell proliferation and block endothelial cell growth factor-mediated angiogenesis. This suggests that RAIC may have anti-tumor effects by inhibiting tumor angiogenesis. The research results of Raphael et al.[15] and Buisman et al.[16] are consistent with ours. On the other hand, this study found that the combination of RAIC and laparoscopic resection in the treatment of CRC patients can improve their survival rate through the survival analysis of the two groups of subjects.
In summary, RAIC combined with laparoscopic resection can reduce the tumor marker levels of VEGF and IGFBP-3 in CRC patients, thereby improving the therapeutic effect, improving prognosis, and improving the survival rate of patients without increasing the incidence of surgical complications and inflammation risk, with sufficient safety and feasibility.
Author’s contributions:
Haibin Huang and Yinyuan Zheng contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, et al. Colorectal cancer epidemiology: Recent trends and impact on outcomes. Curr Drug Targets 2021;22(9):998-1009.
[Crossref] [Google Scholar] [PubMed]
- Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe and northern America. Cancer Lett 2021;522:255-68.
[Crossref] [Google Scholar] [PubMed]
- Araujo RL, Figueiredo MN, Sanctis MA, Romagnolo LG, Linhares MM, Melani AG, et al. Decision making process in simultaneous laparoscopic resection of colorectal cancer and liver metastases. Review of literature. Acta Cir Bras 2020;35(3):e202000308.
[Crossref] [Google Scholar] [PubMed]
- Syn NL, Kabir T, Koh YX, Tan HL, Wang LZ, Chin BZ, et al. Survival advantage of laparoscopic vs. open resection for colorectal liver metastases: A meta-analysis of individual patient data from randomized trials and propensity-score matched studies. Ann Surg 2020;272(2):253-65.
[Crossref] [Google Scholar] [PubMed]
- Luo W, Wu M, Chen Y. Laparoscopic vs. open surgery for elderly patients with colorectal cancer: A systematic review and meta-analysis of matched studies. ANZ J Surg 2022;92(9):2003-17.
[Crossref] [Google Scholar] [PubMed]
- Martínez-Martínez AB, Arbonés-Mainar JM. Colorectal cancer: Immune response in laparoscopic vs. open colorectal surgery. Cir Cir 2022;90(3):295-302.
[Crossref] [Google Scholar] [PubMed]
- Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, et al. Recent advances in the treatment of colorectal cancer: A review. J Nippon Med School 2022;89(3):246-54.
[Crossref] [Google Scholar] [PubMed]
- Peng HS, Mbarak HS, Li YH, Ma C, Shang QL, Chen Z, et al. Neoadjuvant intra-arterial vs. intravenous chemotherapy in colorectal cancer. Medicine 2021;100(51):e28312.
[Crossref] [Google Scholar] [PubMed]
- Meng W, Gao Y, Xie J, Wu S, Jian B, Li Q, et al. Intra-arterial chemoembolization with chemotherapy for unresectable locally advanced rectal cancer: A case report and literature review. Ann Palliat Med 2021;10(8):9281-7.
[Crossref] [Google Scholar] [PubMed]
- Li J, Huang L, Zhao H, Yan Y, Lu J. The role of interleukins in colorectal cancer. Int J Biol Sci 2020;16(13):2323.
- Zhao Z, Lu L, Li W. TAGLN2 promotes the proliferation, invasion, migration and epithelial-mesenchymal transition of colorectal cancer cells by activating STAT3 signaling through ANXA2. Oncol Lett 2021;22(4):737.
[Crossref] [Google Scholar] [PubMed]
- Hou YL, Luo P, Ji GY, Chen H. Clinical significance of serum IGFBP-3 in colorectal cancer. J Clin Lab Anal 2019;33(6):e22912.
[Crossref] [Google Scholar] [PubMed]
- Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int J Mol Sci 2018;19(4):1232.
[Crossref] [Google Scholar] [PubMed]
- Huang W, Wu J, Liu G, Lv Y, Huang J, Li W. Chemoradiotherapy with concurrent regional arterial chemotherapy for locally bulky unresectable rectal cancer: A case series. Oncol Res Treat 2019;42(12):678-83.
[Crossref] [Google Scholar] [PubMed]
- Raphael MJ, Karanicolas PJ. Regional therapy for colorectal cancer liver metastases: Which modality and when? J Clin Oncol 2022;40(24):2806-17.
[Crossref] [Google Scholar] [PubMed]
- Buisman FE, Filipe WF, Galjart B, Grünhagen DJ, Homs MY, Moelker A, et al. Adjuvant intra-arterial chemotherapy for patients with resected colorectal liver metastases: A systematic review and meta-analysis. HPB 2022;24(3):299-308.
[Crossref] [Google Scholar] [PubMed]
 .
.



