- *Corresponding Author:
- Q. Y. Jiang
Department of Cardiology, Pudong Hospital Affiliated to Fudan University, Hongfeng, Shanghai 201206, China
E-mail: jiangqingy@126.com
| This article was originally published in a special issue, “Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “269-276” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Early studies suggested that right ventricular apical pacing could have non-beneficial effects on left ventricular function. While right ventricular non-apical pacing may have significantly beneficial effects and could be a good alternative to right ventricular apical pacing is unknown. A systematic review and meta-analysis were conducted to compare the mid and long-term effects of right ventricular non-apical pacing and right ventricular apical pacing on left ventricular function. We conducted randomized controlled trials and retrospective cohort studies on patients with pacemakers to compare right ventricular apical and right ventricular non-apical. We evaluated patient’s left ventricular ejection fraction, 6 min walk test, blood N-terminal pro-brain peptide, or brain natriuretic peptide. The results are expressed as the mean difference of the 95 % confidence interval. A total of 1181 references were included for evaluating the relevance. From this, 10 studies with 1246 patients have fulfilled the inclusion criteria (mean age ranged from 55 y to 85 y; male 62 %; 622 (50 %) right ventricular apical; 624 (50 %) right ventricular non-apical). There was no difference between the right ventricular apical and right ventricular non-apical groups in baseline left ventricular ejection fraction groups (mean difference=0.32, 95 % confidence interval (-0.70, 1.34), p=0.98, random effect model) and postoperative 6 min walking test (mean difference=5.14, 95 % confidence interval (-6.60, 16.89), p=0.51, I2=0 %). In terms of left ventricular ejection fraction index and N-terminal pro-brain natriuretic peptide or brain natriuretic peptides, the right ventricular non-apical group was better than the right ventricular apical group (mean difference=-0.74, 95 % confidence interval (-2.24, 0.77), p<0.0001, I2=56 %). Sensitivity analysis and funnel chart showed that our research is robust and has low publication bias. Our results showed that right ventricular non-apical pacing had significant beneficial effects on postoperative left ventricular ejection fraction than right ventricular apical pacing in patients with a pacemaker implanted for more than 1 y.
Keywords
Right ventricular non-apical, atrial fibrillation, right ventricular apical, pacemaker, heart failure
Since applying the transvenous pacing system in the clinic, it has been considered the most suitable pacing site due to the stability and reliability of Right Ventricular Apex (RVA) pacing[1,2]. However, many clinical trials show that right ventricular apical pacing is defective, which can increase the incidence of atrial fibrillation and heart failure. Therefore, ventricular pacing should be minimized in patients with normal atrioventricular function. In contrast, patients with atrioventricular block who need ventricular pacing need to choose other ideal pacing sites to replace them[3-5].
Right Ventricular Non-Apical (RVNA) (mainly including right ventricular outflow tract, high septum, and median septum) pacing has become a research hotspot because it is closer to physiological conduction[6-8]. With the development of cardiac pacing technology, the indications of pacemaker placement are also expanding, and the number of pacemakers is increasing. Therefore, it is essential to follow up with the patients regularly. We should know whether the performance and working condition of the pacemaker are average and know the heart’s function[9,10].
Studies have shown that the left ventricular systolic asynchrony caused by RVA pacing is significantly correlated with long-term follow-up mortality and hospitalization rate of heart failure. Myocardial relaxation depends on preload, afterload, and the consistency of myocardial contraction to peace[11-13]. RVA pacing can lead to the asynchrony of the systolic process, which further impairs the function of the ventricular diastole[14-16].
This systematic review study aims to evaluate the effects of RVNA pacing and RVA pacing on the medium and long term of left ventricular function.
Materials and Methods
Literature search strategy:
A comprehensive literature search was conducted on the abstracts and full-text articles published in the electronic databases MEDLINE, EMBASE, Scopus, Web of Science, and Cochrane Central Register of controlled trials (central) from June 2006 to March 2016. Right ventricular apical; left ventricular function; meta and pacing. These keywords were combined with the Boolean operators 'AND' or 'OR' to search literature.
A comprehensive search of the literature has been carried out, and there are no restrictions on the publication status. Two individual reviewers identified and reviewed full-text articles and abstracts deemed relevant by screening the list of titles. Disagreements between the two reviewers were resolved with consensus.
Study selection:
Randomized clinical trials were included if they were published in English. If studies provided relevant information on the effects of RVNA pacing and right ventricular apical pacing on left ventricular cardiac function (i.e., participants, interventions, comparisons, results, and study design), these studies were included in this systematic review and meta-analysis.
After the preliminary screening study, the possible relevant research texts were reviewed, and the included studies need to meet the following criteria.
Inclusion criteria: Patients with cardiac pacemakers; comparison of Left Ventricular Ejection Fraction (LVEF); the follow-up time of various indexes was ≥6 mo and provide full text.
The exclusion criteria: Not original research; no complete research text and meeting summary or presentation.
Data extraction and quality assessment:
Two co-authors independently extracted relevant data parameters. In the event of disagreement, rechecking of the original article followed by discussion was used to reach a consensus.
First author’s name, patient's age and gender, country of origin, year of publication, sample size, and study duration. Two independent reviewers assessed the quality of the included studies using the Cochrane Collaboration risk-of-bias tool.
Statistical analysis:
The review manager (Version 5.2, Cochrane Collaboration, 2011) was used to estimate the impact of the results in the selected report. Specifically, data for effect sizes of continuous outcomes were extracted or recalculated as Mean Difference (MD), which expresses the mean difference between the intervention and control groups in standard deviation units, with 95 % Confidence Interval (CI). Statistical heterogeneity between studies was assessed with chi-square test or Cochran’s Q test, and the I2 statistic, which measures inconsistency across study results and describes the proportion of total variation in study estimates due to heterogeneity rather than sampling error.
In detail, I2 values of 0 % indicate no heterogeneity, 25 % low heterogeneity, 25 %-50 % moderate heterogeneity, and 50 % high heterogeneity. A fixed-effect model was applied in the absence of minor heterogeneity, and a random effect model was adopted for significant heterogeneity. Funnel plots and Egger’s test were used to examine potential publication bias. Sensitivity analysis was conducted by deleting a single study each time to observe the influence of the individual outcome on the overall analysis.
Results and Discussion
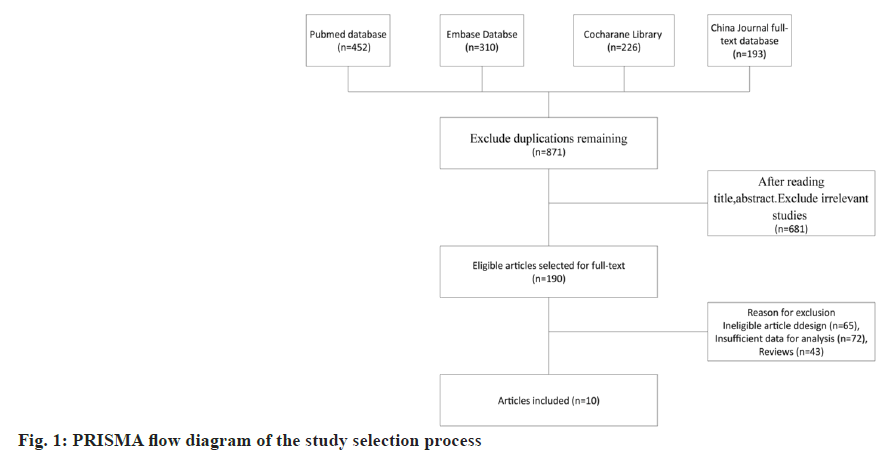
The flow diagram of the article selection process is illustrated in fig. 1, which resulted in the inclusion of 10 studies. After removing duplicates, 871 records remained. By screening the titles and abstracts, an additional 681 records were excluded because they were review articles, letters, case reports, comments, or editorials. In consideration of the study design and insufficient data presented, 180 articles were removed. Finally, 10 studies, including 1246 subjects, met the inclusion criteria and were included in this meta-analysis. A total of 10 studies were retrieved by our search that fulfilled all inclusion criteria (fig. 1).
The sample size in the individual studies ranged from 22 to 132 participants. Out of the 1246 participants, 775 (62 %) were male. Mean age ranged from 55 y to 85 y old. The primary outcome measures were LVEF, 6 Min Walking Test (6MWT), and N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) or Brain Natriuretic Peptides (BNP).
A total of 1246 patients were available for the meta- analysis, including 622 patients who received RVA and 624 patients who received RVNA[17-26]. Among the studies, all studies were published in the English language; four studies were from China, the other six were from the USA, Iran, India, and Australia. Baseline characteristics of this study were presented in Table 1.
| Study | Year | Language | Country | No. of patients (male/female) | Age range (mean) | No. of RVA group | No. of RVNA group | Year of onset |
|---|---|---|---|---|---|---|---|---|
| Bai | 2016 | English | China | 60/36 | 66.7+10.7 | 46 | 50 | August 2011 to August 2014 |
| Chen | 2014 | English | China | 55/37 | 75±10 | 47 | 45 | August 2008 to August 2011 |
| Gong | 2009 | English | China | 52/38 | 70±11 | 44 | 46 | June 2006 to September 2008 |
| Hemayat | 2014 | English | Iran | 68/96 | 63.93±16.1 | 82 | 82 | October 2008 to July 2011 |
| Kaye | 2014 | English | Australia | 161/79 | 73.7±11.1 | 120 | 120 | March 2007 to January 2011 |
| Leclercq | 2016 | English | France | 191/72 | 63.8+9.5 | 132 | 131 | November 2010 to August 2013 |
| Saito | 2015 | English | Australia | 97/48 | 75+5.5 | 76 | 69 | August 2007 to December 2011 |
| Singh | 2015 | English | India | 29/22 | 57.4±10.62 | 22 | 29 | March 2010 to October 2011 |
| Schleifer | 2018 | English | America | 29/16 | 78.4±5.7 | 23 | 22 | July 2013 to August 2016 |
| Xu | 2017 | English | China | 33/27 | 67.1±7.5 | 30 | 30 | January 2012 and December 2015 |
Table 1: Characteristics of Studies Included in the Meta-Analysis
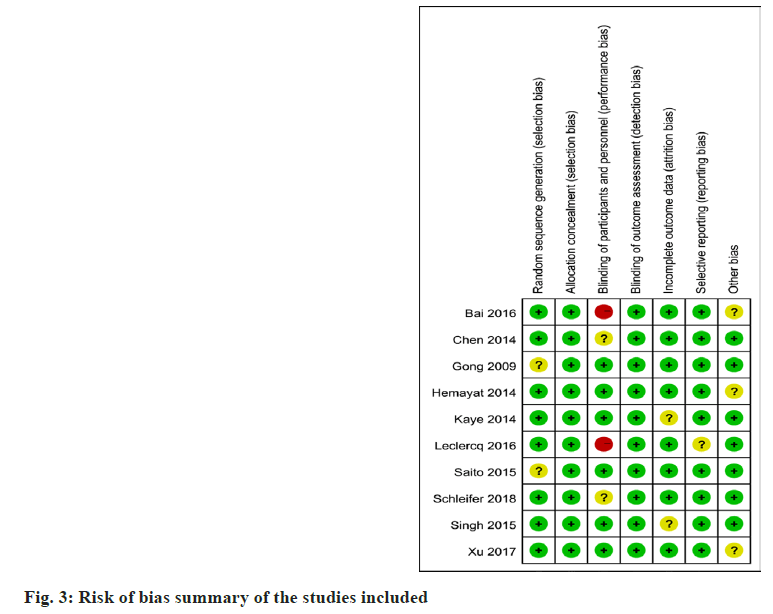
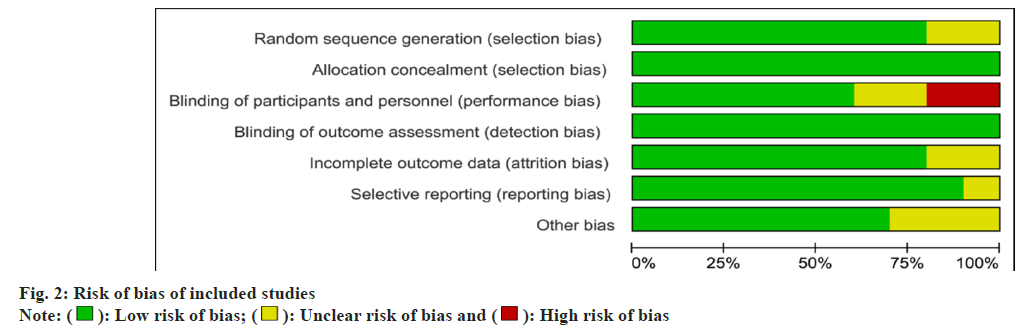
Risk of bias assessment was performed at the study level, and methodological quality assessment was performed using the Cochrane bias risk assessment tool (fig. 2).
Two independent reviewers evaluated the quality of studies included in the review, with differences resolved by consensus or through a third reviewer if required. A summary of all kinds of bias in each study is shown in fig. 3. There are two trials with bias risk, and eight trials have no chance.
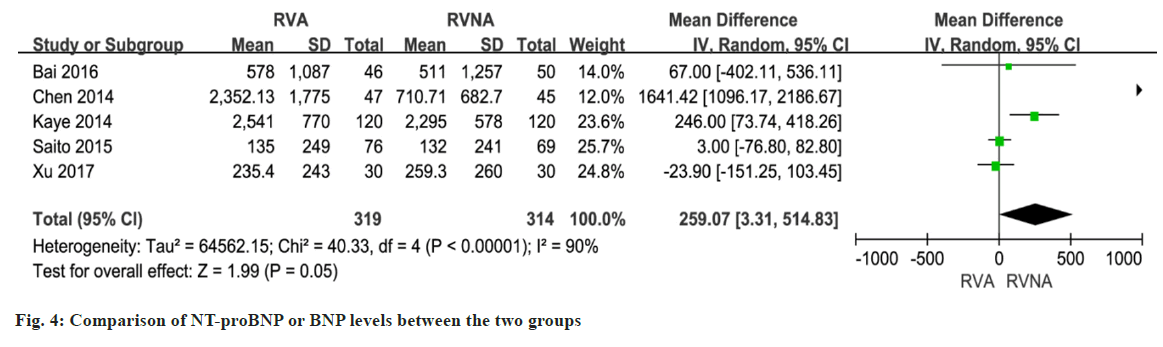
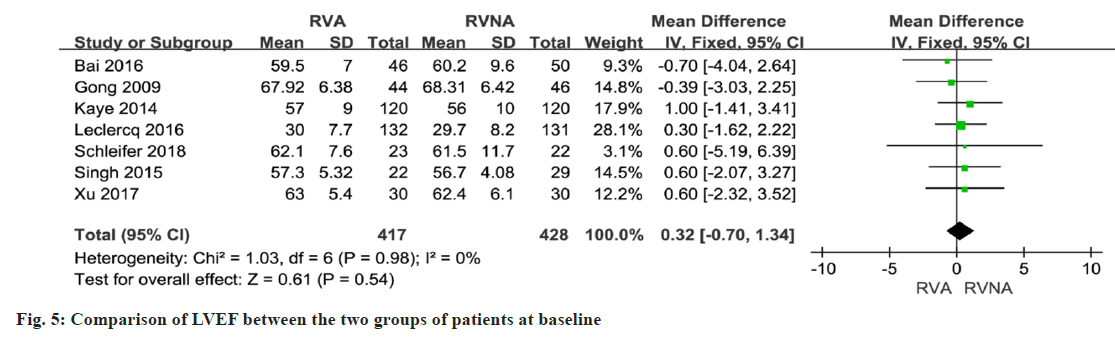
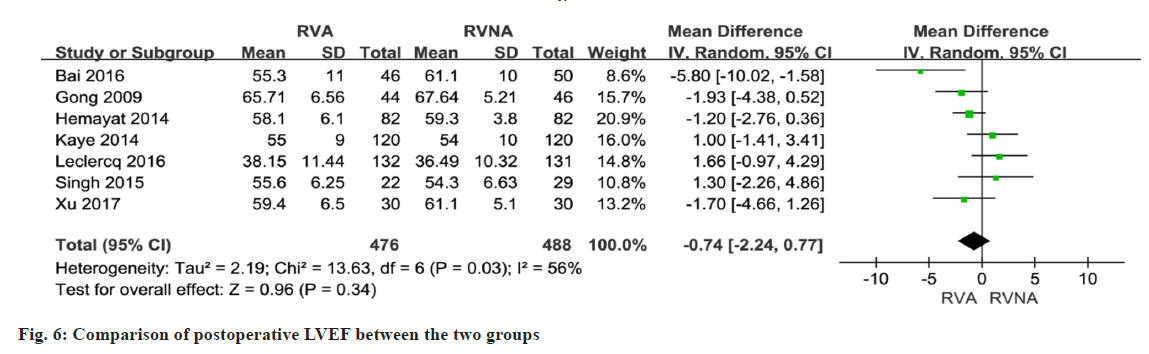
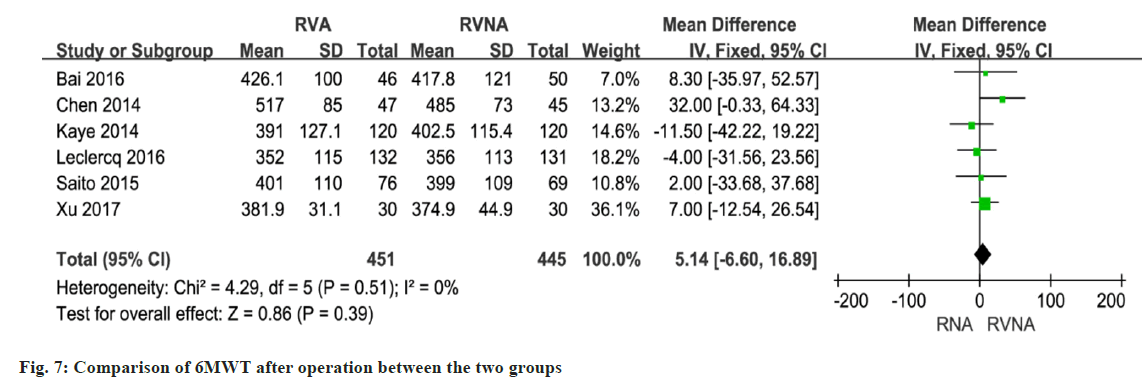
In 5 studies involving 633 patients, NT-proBNP or BNP levels were compared between the RNA and RVNA groups. The meta-analysis showed that there was a difference between the two groups (MD=259.07, 95 % CI (3.31, 514.83), p<0.01, random-effects model) (fig. 4). We performed a meta-analysis of LVEF at baseline in patients in the RVA and RVNA groups. The results showed that there was no significant difference in LVEF between the two groups (MD=0.32, 95 % CI (-0.70, 1.34), p=0.98, random effect model) (fig. 5). We performed a meta-analysis of the postoperative LVEF of patients in the RVA and RVNA groups. The results showed that there was a significant difference in postoperative LVEF between the two groups of patients (MD=-0.74, 95 % CI (-2.24, 0.77), p=0.03, random-effects model) (fig. 6). As shown in fig. 7, included studies were involved. The results showed that there was no difference in 6MWT between the two groups (MD=5.14, 95 % CI (-6.60, 16.89), p=0.51, I2= 0 %, fig. 7).
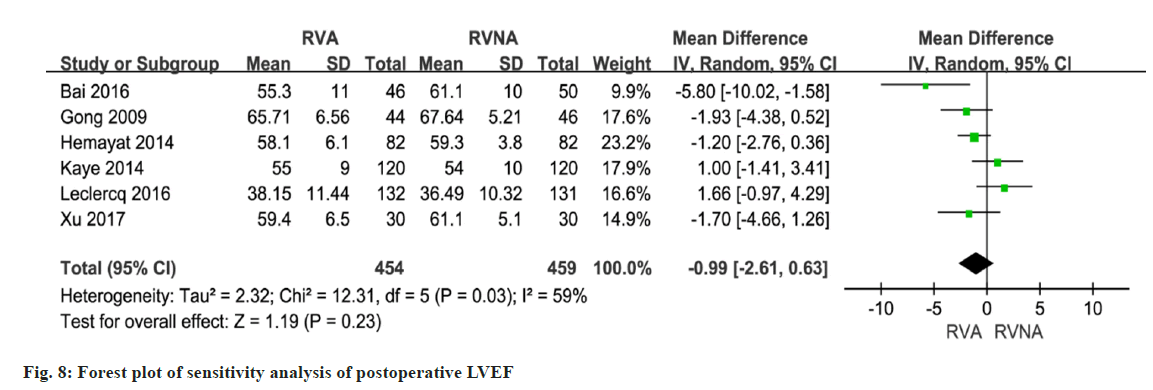
A total of 7 studies reported on postoperative LVEF. The forest plot shows that the RVNA group is better than the RVA group (MD=-0.74, 95 % CI (-2.24, 0.77), p<0.0001, I2=56 %, fig. 7). We performed a sensitivity analysis by deleting the Singh 2015 study. The results did not change much, with I2 changing from 56 % to 59 % (fig. 8), which indicates that the results of the included articles are robust.
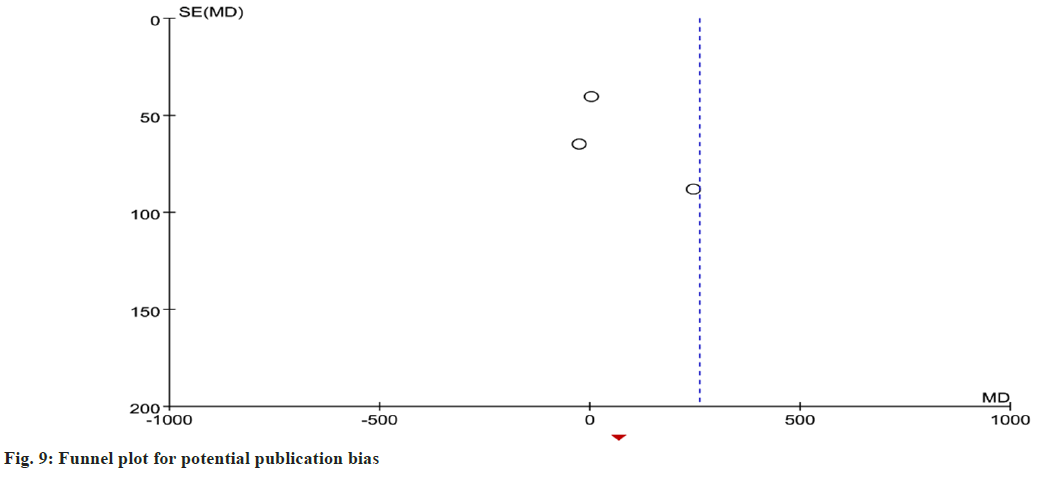
We also drew a funnel chart to assess the publication bias of NT-proBNP and BNP; the figure shows that the shape is not symmetrical. The output result suggests limited publication bias in this meta- analysis (fig. 9).
Since the last century, cardiac pacing has been successfully applied in clinical practice. Its application has brought new life to patients, improved their quality of life, and restored their working ability. RVA pacing is usually used in the conventional site[24,27]. Because the lead wire of this site is easy to locate and fix firmly, it has been widely used for a long time. However, long-term pacing can directly damage ventricular function, affect myocardial perfusion and lead to myocardial remodeling[28,29].
RVNA pacing can make the ventricular activation sequence close to physiological and obtain better hemodynamic effects. According to the anatomical characteristics of the right ventricular septum, domestic and foreign scholars have performed direct his bundle pacing, right ventricular inflow septal pacing, right ventricular outflow septal pacing, right ventricular apical septal pacing, and right ventricular septal pacing-the practice of pacing at the ventricular septum[30,31]. Most studies have confirmed that right ventricular septal pacing is technically safe, feasible, and effective. Still, different heart parts affect cardiac function, especially left ventricular function. Zhu’s research has shown that for patients with permanent pacemaker implantation whose basal state LVEF is reduced (≤40 %-45 %) or who have been followed up for >1 y, RVNA pacing has less impact on postoperative LVEF than RVA pacing. The conclusions are similar[32,33].
During RVA, pacing, electrical pulses are retrograde conducted from the apex to the ventricular septum. Most of the left ventricle is undertaken through the myocardium. The depolarization speed is slow. The abnormal movement of the posterior wall of the ventricle causes the entire heart to contract out of synchronization, loses overall coordination, and reduces ventricular compliance, which has many adverse effects on hemodynamic and cardiac function.
During RVNA pacing, the ECG is similar to its sinus rhythm. In conventional pacemaker implantation, the very easy-to-collect QRS time limit is a valuable indicator of left ventricular activity[34,35]. It has also been confirmed in the experiment that the shortening of the wave duration by changing the pacing position will lead to the improvement of left ventricular function. One of the major limitations of this systematic review study does not analyze the dynamic changes of LVEF from preoperative to postoperative. Second, no further stratified comparison was made on the specific parts of RVNA pacing[36-38].
This meta-analysis identified a significant prevalence of RVNA pacing and RVA pacing in patients with a pacemaker implanted for >1 y. Our study suggests that RVNA pacing is more beneficial to LVEF than RVA. Though this finding is exciting, its beneficial clinical effect is uncertain. The data available for endpoints other than LVEF are not enough and conclusive. There is a need for large relevance references to evaluate and compare the safety and efficacy of RVNA and RVA pacing.
Author’s contributions:
Yingbiao Wu, Jie Su, and Qing Yuan Jiang participated in the conception and design of the study, library searches and assembling of relevant literature, critical review of the paper, supervising the writing of the paper, and database management; Xinpeng Cong and Luoning Zhu participated in data collection, library searches assembling relevant literature, writing the paper, and critical review. Yingbiao Wu and Jie Su have contributed equally to this work.
Funding:
This study is supported by Pudong New Area Discipline Leader Training Plan (No. PWRd2020- 20) and Pudong New Area Health Commission Clinical Summit Discipline Construction Project (PWYgf2021-04).
Conflict of interests:
The authors declared no conflict of interests.
References
- Muto C, Calvi V, Botto GL, Pecora D, Porcelli D, Costa A, et al. 176 Chronic apical and non-apical right ventricular pacing in patients with high-grade atrioventricular block: Results of the right pace study. EP Europace 2017;19(3):iii15.
[Crossref] [Google Scholar] [PubMed]
- Eng L, Hunt B, Dauber K, Hill J, Gould P, Kaye G. A Comparison of Right Ventricular Apical (RVA) and Non-Apical (RVNA) Defibrillator (ICD) lead position: A single center experience. Heart Lung Circul 2013;22:S103-4.
[Crossref] [Google Scholar] [PubMed]
- Kaye G, Hussain M, Furuya-Kanamori L, Clark J, Doi S. A meta-analysis of randomised controlled trials on the effect of right ventricular apical and non-apical pacing on left ventricular ejection fraction. Heart Lung Circ 2015;24:S234.
[Crossref] [Google Scholar] [PubMed]
- Lu J, Fan W, Yang X. Advances in non-right ventricular apical pacing sites. Clin Focus 2015;45:23-9.
- Vicent L, Fernández-Yanez J, Mateos R, Sousa-Casasnovas I, Fernández-Aviles F, Martínez-Selles M. Apical ventricular hypertrophy in the transplanted heart: A 20-year single-center experience. Rev Esp Cardiol 2021;74(2):191-4.
[Crossref] [Google Scholar] [PubMed]
- Shimony A, Eisenberg MJ, Filion KB, Amit G. Beneficial effects of right ventricular non-apical vs. apical pacing: A systematic review and meta-analysis of randomized-controlled trials. Europace 2012;14(1):81-91.
[Crossref] [Google Scholar] [PubMed]
- Muto C, Calvi V, Botto GL, Pecora D, Porcelli D, Costa A, et al. Chronic apical and nonapical right ventricular pacing in patients with high-grade atrioventricular block: Results of the right pace study. Bio Med Res Int 2018;2018:1404659.
[Crossref] [Google Scholar] [PubMed]
- Varma N. Contrasting electrical effects of apical vs. non-apical right ventricular pacing. Indian Pacing Electrophysiol J 2018;18(6):208.
- Auger D, Hoke U, Marsan NA, Tops LF, Leong DP, Bertini M, et al. Effect of induced LV dyssynchrony by right ventricular apical pacing on all-cause mortality and heart failure hospitalization rates at long-term follow-up. J Cardiovasc Electrophysiol 2014;25(6):631-7.
[Crossref] [Google Scholar] [PubMed]
- Weizong W, Zhongsu W, Yujiao Z, Mei G, Jiangrong W, Yong Z, et al. Effects of right ventricular nonapical pacing on cardiac function: A meta-analysis of randomized controlled trials. Pacing Clin Electrophysiol 2013;36(8):1032-51.
[Crossref] [Google Scholar] [PubMed]
- Jastrzebski M, Kukla P, Fijorek K, Sondej T, Czarnecka D. Electrocardiographic diagnosis of biventricular pacing in patients with nonapical right ventricular leads. Pacing Clin Electrophysiol 2012;35(10):1199-208.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee M, Mercurio V, Hsu S, Mayer SA, Mathai SC, Hummers LK, et al. Assessment of right ventricular reserve utilizing exercise provocation in systemic sclerosis. Int J Cardiovasc Imaging 2021;37:2137-47.
[Crossref] [Google Scholar] [PubMed]
- Ahlberg SE, Skadsberg ND, Yue AM. Global changes of restitution dynamics during right ventricular apical and septal pacing. Heart Rhythm 2006;3:80.
- Cui L, Wang LL, Li XJ, Wang LG, Li MZ, Han B. Hypertrophic cardiomyopathy complicated with apical left ventricular aneurysm and ventricular tachycardia: A case report. Zhonghua Xin Xue Guan Bing Za Zhi 2021;49(3):276-7.
[Crossref] [Google Scholar] [PubMed]
- Bansal R, Parakh N, Gupta A, Juneja R, Naik N, Yadav R, et al. Incidence and predictors of pacemaker-induced cardiomyopathy with comparison between apical and non-apical right ventricular pacing sites. J Interv Cardiac Electrophysiol 2019;56(1):63-70.
[Crossref] [Google Scholar] [PubMed]
- Bai M, Li Q, Jiang G, Zhang L, Wang T, Zhang Z. Comparison of effectiveness of right ventricular mid-septal pacing vs. apical pacing: A randomized-controlled trials. Eur Heart J Suppl 2016;18:12-8.
[Crossref] [Google Scholar] [PubMed]
- Singh H, Patel CD, Sharma G, Naik N. Comparison of left ventricular systolic function and mechanical dyssynchrony using equilibrium radionuclide angiography in patients with right ventricular outflow tract vs. right ventricular apical pacing: A prospective single-center study. J Nucl Cardiol 2015;22:903-11.
[Crossref] [Google Scholar] [PubMed]
- Leclercq C, Sadoul N, Mont L, Defaye P, Osca J, Mouton E, et al. Comparison of right ventricular septal pacing and right ventricular apical pacing in patients receiving cardiac resynchronization therapy defibrillators: The SEPTAL CRT study. Eur Heart J 2016;37(5):473-83.
[Crossref] [Google Scholar] [PubMed]
- Hemayat S, Shafiee A, Oraii S, Roshanali F, Alaedini F, Aldoboni AS. Development of mitral and tricuspid regurgitation in right ventricular apex vs. right ventricular outflow tract pacing. J Interv Cardiac Electrophysiol 2014;40:81-6.
[Crossref] [Google Scholar] [PubMed]
- Saito M, Kaye G, Negishi K, Linker N, Gammage M, Kosmala W, et al. Dyssynchrony, contraction efficiency and regional function with apical and non-apical RV pacing. Heart 2015;101(8):600-8.
[Crossref] [Google Scholar] [PubMed]
- Xu H, Xie X, Li J, Zhang Y, Xu C, Yang J. Early right ventricular apical pacing-induced gene expression alterations are associated with deterioration of left ventricular systolic function. Dis Markers 2017;2017:8405196.
[Crossref] [Google Scholar] [PubMed]
- Kaye GC, Linker NJ, Marwick TH, Pollock L, Graham L, Pouliot E, et al. Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: Results of the protect-pace study. Eur Heart J 2015;36(14):856-62.
[Crossref] [Google Scholar] [PubMed]
- Schleifer JW, Pislaru SV, Lin G, Powell BD, Espinosa R, Koestler C, et al. Effect of ventricular pacing lead position on tricuspid regurgitation: A randomized prospective trial. Heart Rhythm 2018;15(7):1009-16.
[Crossref] [Google Scholar] [PubMed]
- Chen K, Mao Y, Liu SH, Wu Q, Luo QZ, Pan WQ, et al. Is right ventricular mid-septal pacing superior to apical pacing in patients with high degree atrio-ventricular block and moderately depressed left ventricular function? J Zhejiang Univ Sci B 2014;15:507-14.
[Crossref] [Google Scholar] [PubMed]
- Gong X, Su Y, Pan W, Cui J, Liu S, Shu X. Is right ventricular outflow tract pacing superior to right ventricular apex pacing in patients with normal cardiac function? Clin Cardiol 2009;32(12):695-9.
[Crossref] [Google Scholar] [PubMed]
- Małecka B, Ząbek A, Maziarz A, Lelakowski J. Influence of heart failure etiology on the effect of upgrading from right ventricular apical to biventricular or bifocal pacing in patients with permanent atrial fibrillation and advanced heart failure. Pol Arch Med Wewn 2012;122(3):89-97.
[Crossref] [Google Scholar] [PubMed]
- Yamashita K, Tanaka H, Komatsu M, Hirata KI. Isolated right ventricular apical hypertrophy. Intern Med 2021;60(11):1793-4.
- Horiuchi Y, Yahagi K, Tanaka T, Yokozuka M, Miura S, Tanabe K. Left ventricular apical pseudoaneurysm with shunt to the right ventricle following transapical aortic valve replacement. Cardiovasc Interv Ther 2021;37(2):412-3.
[Crossref] [Google Scholar] [PubMed]
- Flevari P, Leftheriotis D, Fountoulaki K, Panou F, Rigopoulos AG, Paraskevaidis I, et al. Long-term nonoutflow septal vs. apical right ventricular pacing: Relation to left ventricular dyssynchrony. Pacing Clin Electrophysiol 2009;32(3):354-62.
[Crossref] [Google Scholar] [PubMed]
- Qadir F, Shahid M, Saeed HY, Mohyudin MT, Saad AB, Iqbal Z. New onset heart failure after right ventricular pacing in patients with normal left ventricular function. J Islamabad Med Dental Coll 2021;10(1):51-5.
- Botto GL, Maglia G, Calvi V, Pecora D, Porcelli D, Costa A, et al. P1665 Chronic apical and non-apical right ventricular pacing in patients with high-grade atrioventricular block: Results of the right pace study. Eur Heart J 2017;38(1):ehx502-P1665.
- Yu Juan Y, Chen Y, Wu M, Zhen Z, Yao Q. P6550 Right ventricular apical pacing vs. non-right ventricular apical pacing induced tricuspid regurgitation: Implication of 3D echocardiographic location of leads. Eur Heart J 2019;40(1):ehz746-1140.
- Changwe GJ, Hongxin L, Zhang HZ, Wenbin G, Liang F, Cao XX, et al. Percardiac closure of large apical ventricular septal defects in infants: Novel modifications and mid-term results. J Card Surg 2021;36(3):928-38.
[Crossref] [Google Scholar] [PubMed]
- Choudhary D, Chaurasia AK, Kumar SM, Arulkumar A, Thajudeen A, Namboodiri N, et al. Radial left ventricular dyssynchrony by speckle tracking in apical vs. non apical right ventricular pacing-evidence of dyssynchrony on medium term follow up. J Cardiovasc Thorac Res 2016;8(1):20-5.
[Crossref] [Google Scholar] [PubMed]
- Mumin G, Celiker C. Right ventricular apical and septal pacing: Long term impacts on ventricular function. Eur Heart J Cardiovasc Imag 2021;22(1):jeaa356-367.
- Hussain MA, Furuya-Kanamori Lu, Kaye G, Clark J, Doi SA. The effect of right ventricular apical and nonapical pacing on the short- and long-term changes in left ventricular ejection fraction: A systematic review and meta-analysis of randomized-controlled trials. Pacing Clin Electrophysiol 2015;38(9):1121-36.
[Crossref] [Google Scholar] [PubMed]
- Khoo C, Tung SK. Utilisation of a right ventricular apical lead for pacing backup in patients with a his-bundle lead. Can J Cardiol 2013;29(10):191.
- Unger P, Paesmans M, Amzulescu M, David-Cojocariu A. Value of TAPSE normalized to right ventricular length as a substitute to speckle tracking-derived right ventricular free wall longitudinal strain assessment. Eur Heart J Cardiovasc Imag 2021;22(1):jeaa356-124.
 High risk of bias
High risk of bias