- *Corresponding Author:
- Bixin Tang
Department of Traditional Chinese Medicine Gynecology, Shanghai Pudong New District Gongli Hospital, Second Military Medical University, Pudong, Shanghai 200135, P. R. China
E-mail: tangbixinsh@163.com
| This article was originally published in a special issue, “Drug Development in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2023:85(5) Spl Issue “158-165” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Lysyl oxidase-like 2 plays a vital role in collagen deposition and fibrosis. This study aimed to explore the preventive effect and possible mechanisms of xiaoyuningkun decoction on rats with sequelae pelvic inflammatory disease. Sequelae pelvic inflammatory disease rat model was induced by hydrochloric acid and lipopolysaccharide injection through the upper genital tract, and then xiaoyuningkun decoction was orally administered for 28 d. The histological examination, fibrosis and enzyme-linked immunosorbent assay were carried out. Immunohistochemistry, real-time qualitative polymerase chain reaction and Western blot were used to detect the expression of lysyl oxidase-like 2 and transforming growth factor-beta 1 in uterine tissues. The administration of xiaoyuningkun decoction potentially inhibited histopathological changes and inflammatory cells infiltration. Significantly decreased serum tumor necrosis factor-alpha and interleukin-6 levels suppressed fibrosis in uterine tissues of sequelae pelvic inflammatory disease rats. We also found that xiaoyuningkun decoction reduced messenger ribonucleic acid and protein expression in uterine tissues. Furthermore, serum levels of two fibrosis-related proteins, transforming growth factor-beta1 and matrix metallopeptidase 9, were decreased by xiaoyuningkun in sequelae pelvic inflammatory disease rats. Our data speculated that xiaoyuningkun has an anti-fibrosis effect in the sequelae pelvic inflammatory disease rat model, and the mechanism might be through inhibition of lysyl oxidase-like 2 and transforming growth factor-beta 1 pathways.
Keywords
Pelvic inflammatory disease, sequelae, xiaoyuningkun decoction, lysyl oxidase like 2, tumor necrosis factor-alpha
Pelvic Inflammatory Disease (PID) is a common gynecological disease, which is often seen in sexually active women. PID is an inflammatory disease caused by the colonization of microorganisms in the upper reproductive tract, such as the endometrium, fallopian tube and ovary. PID can be divided into endometritis, salpingitis, peritonitis and other types according to the location of inflammatory lesions[1]. When PID is not timely diagnosed and treated, or body immunity is declined, this disease is likely to evolve into Sequelae of PID (SPID), resulting in hyperplasia, adhesion, fibrosis of female reproductive organ, chronic pelvic pain, endometriosis and ectopic pregnancy[2]. Chronic endometritis can increase endometrial fibrosis and adhesion, thus affecting endometrial receptivity and reducing the likelihood of pregnancy[3]. Moreover, chronic infection and inflammation often involve the fallopian tube, and the healing process includes scar formation and adhesion formation, which increases the risk of tubal infertility[4]. Therefore, the prevention and treatment of SPID have become one of the most urgent problems in gynecology.
In the chronic inflammation stage of PID, there are over activation of cytokines and abnormal conduction of signal pathways, which interact in the regulation of inflammatory response and fibrosis formation. Therefore, it is a tempting target for restoring the balance of cytokine and signal pathway expression and reducing tissue damage and proliferation, and fibrosis. Transforming Growth Factor-Beta 1 (TGF-β1) and Matrix Metallopeptidase (MMP)-9 are essential cytokines to promote local tissue adhesion and hypertrophic scar formation. TGF-β1 induced Mothers Against Decapentaplegic (SMADS) signaling pathway is one of the most critical signaling pathways in wound repair and fibrosis[5], which is involved in every link from early inflammatory response to tissue damage repair and later hypertrophic scar formation. TGF-β1 signaling pathway is related to the fibrosis process, and serum TGF-β1 level in rats with chronic PID increases[6]. MMP-9 is a member of MMPs and an essential protease involved in normal matrix remodeling. Excessive production of MMPs by inflammatory cells can also promote the degradation of Extracellular Matrix (ECM). Compared with the healthy control group, the level of plasma MMP-9 in patients with PID was increased[7].
Lysyl Oxidase (LOX) belongs to copper-dependent amine oxidase and can catalyze the oxidative deamination of lysine and hydroxylysine residues of elastin and collagen molecules. LOX family includes five members with similar structures; LOX and four LOX-Like Proteins (LOXL1-4). The catalytic effect of the LOX family makes collagen and elastin fibers form covalent cross-linking and interweave into a stable network structure, thus stabilizing the ECM[8]. As a member of the LOX family, LOXL2 involves the fibrosis process of the liver, kidney, lung and other organs[9-11]. However, the expression of LOXL2 in the endometrium of PID and whether it is related to endometrial fibrosis has not been reported.
Xiaoyuningkun (XYNK) decoction is a popular Chinese medicinal compound composed of Eupolyphaga sinensis, Melia Toosendan, Angelica biserrata, and Cynanchum paniculatum (CP) Bunge) Kitagawa. XYNK decoction is used to treat acute and chronic PID and has achieved good efficacy and safety. Most of the constituents of medicinal plants in XYNK decoction have potential antimicrobial and anti-inflammatory activities and modulate inflammatory pathway proteins, such as Nuclear Factor-Kappa B (NF-κB), Tumor Necrosis Factor-Alpha (TNF-α), Interleukin-6 (IL-6) and IL-1β[12-14]. Our previous study has shown oral administration of XYNK decoction and its primary drug CP have significant analgesic and anti-inflammatory activity in acute PID rats and reduce excessive production of inflammatory cytokines[15]. Whether XYNK decoction shows an effect on chronic PID and fibrosis is unclear.
In this study, the rat model of SPID was established, and the effects of XYNK decoction on histopathology, tissue fibrosis, serum inflammatory and fibrotic cytokines, and LOXL2 and TGF-β1 proteins were investigated.
Materials and Methods
Reagents:
Lipopolysaccharide (LPS) and Pregnant Mare Serum Gonadotropin (PMSG) were purchased from Sigma-Aldrich (St. Louis, Missouri, United States of America (USA)). Hydrochloric acid was purchased from Yakuri Pure Chemicals (Kyoto, Japan). Enzyme-Linked Immunosorbent Assay (ELISA) kits for TNF-α, IL-6 and TGF-β1 were obtained from R and D Systems (Minneapolis, Minnesota, USA). LOXL2, TGF-β1 and β-actin antibodies were obtained from Abcam.
Preparation of XYNK decoction:
The XYNK decoction was composed of 12 g of Eupolyphaga sinensis, 12 g of Melia Toosendan, 12 g of Angelica biserrata and 15 g of CP. There were added with distilled water with 10 times the volume and soaked for 20 min, and then were boiled and decocted over low heat for 20 min. The decoction was homogenized and concentrated by heating until the concentration of the crude drug was 1.5 g/ml, and the concentrated solution was stored at a 4° refrigerator.
Experimental animal:
Female-specific pathogen-free Sprague–Dawley (SD) rats (8 w old; 180-220 g weight) were obtained from Shanghai SLRC Laboratory Animal Co., Ltd. (Shanghai, China). The animals were housed in standard conditions with 22°-24° temperature, 60 %-65 % relative humidity, and 12 h dark-light cycle (light on, from 7:00 to 19:00). The ethics committee approved the experimental procedures for animal experimentation of Shanghai Gongli Hospital. All rats were randomly divided into five groups; control, SPID, XYNK low (1.5 g crude drug/day), XYNK mediates (3 g crude drug/day) and XYNK high (6 g crude drug/day) groups, each with 10 rodents. The control and SPID rats received the same volume of physiological saline.
Rat SPID model construction:
A chronic SPID rat model was established as a previously described method with minor modification[16]. After acclimation for 7 d, the rats were intracervically infused with water alone (25 mg/kg, 1 N), followed by four administrations intracervically with 100 µl of PMSG (7.5 IU per mouse) 2 d before the experiment. Control rats received sterile saline. After 24 h of infection, each group of rats was orally administered the corresponding drugs for 28 d. Rats (264.2±19.3) g were sacrificed by cervical dislocation, and blood samples and uterus were collected and stored at -80° for further evaluation.
Histological evaluation:
The uterus tissues were paraffin-embedded and cut into 2 μm sections, fixed by 10 % formaldehyde for 24 h, dehydrated in alcohol, cleared in xylol, embedded in paraffin, and stained with Hematoxylin and Eosin (HE) or Masson. Five random fields of each slide were checked and photographed under microscopy (200×). The collagen volume fraction was analyzed by ImageJ software.
Immunohistochemically evaluation:
Rats were decapitated, and the parietal peritoneum was fixed with 4 % paraformaldehyde for 4 h and embedded with paraffin. Then paraffin-embedded peritoneum tissue was serially sectioned (thickness 2 μm). After deparaffinization and rehydration, antigenic recovery was performed by heat and endogenous peroxidase activity was blocked by incubation with 3 % Hydrogen peroxide (H2O2). Sections were incubated with primary antibodies against LOXL2 (1:100) overnight at 4°. On the 2nd d, sections were incubated with biotinylated Horseradish Peroxidase (HRP) conjugated secondary antibody (1:100) at room temperature for 2 h and stained with Diaminobenzidine (DAB) and hematoxylin. Five vision fields were randomly selected in each section and photographed.
ELISA:
Serum in experiment rats was collected, weighed, then added to physiologic saline at the ratio of 1:5 (w/v), and then homogenized. The levels of TNF-α, IL-6, TGF-β1 and MMP-9 were measured by ELISA kits.
Real-Time quantitative Polymerase Chain Reaction (RT-qPCR):
According to the manufacturer's instructions, total Ribonucleic Acid (RNA) was isolated from Huh-7 cells in the standard control, genistein, radiation and genistein with radiation groups using a phenol-based method (TRIzol reagent, TaKaRa). The concentration and purity of the RNA were determined by assessment of the absorbance at 260 and 280 nm using a NanoDrope 1000 Spectrophotometer (Thermo Fisher Scientifice Inc., Waltham, Massachusetts), and the RNA integrity was electrophoretically verified. According to the manufacturer's instructions, reverse transcription of purified RNA was performed using a PrimeScript RT Reagent Kit (TaKaRa). Equal volumes (1 μl) of the resulting complementary Deoxyribonucleic Acid (cDNA) served as templates for the subsequent PCR. All gene transcripts were quantified using qPCR using an SYBR Premix kit (TaKaRa) and a LightCycler® 480 II apparatus (Roche Diagnostics, Mannheim, Germany). The target gene expression (2-ΔΔCt) was normalized to the endogenous b-actin gene expression. Specific primers were designed for each gene of interest.
Western blot:
Cell protein was isolated in lysis buffer and then quantified using a Bicinchoninic Acid (BCA) assay kit (Beyotime). Equal amounts of protein per lane were separated using Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane, and immunoblotted using primary and secondary antibodies against LOXL2 and TGF-β1. After being washed with a mixture of Tris-Buffered Saline with Tween-20 (TBST), membranes were incubated with HRP conjugated secondary antibody for 2 h at room temperature. Protein bands were visualized using enhanced chemiluminescence reagents (Amersham Biosciences, Buckinghamshire, United Kingdom) before digital scanning and quantitated using ImageJ software.
Statistical analysis:
The statistical data are presented as the means±Standard Error of Measurement (SEM) of the indicated numbers of samples. The differences between the two data sets were evaluated using Student’s t-test by the Statistical Package for the Social Sciences (SPSS) 18.0 statistical software (SPSS, USA). One-way Analysis of Variance (ANOVA) was used to compare the difference between more than two data sets. p<0.05 were considered as the criteria for a statistically significant difference.
Results and Discussion
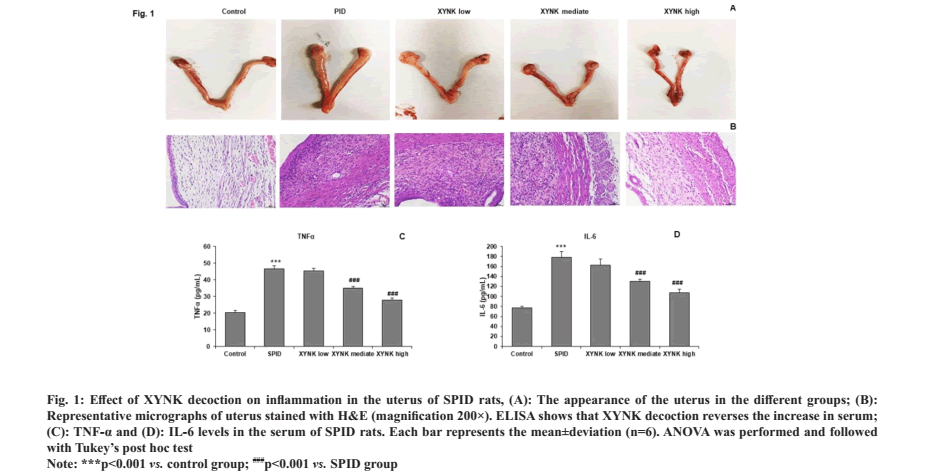
To assess the anti-inflammatory activity of XYNK decoction, uterine appearance was observed (fig. 1A). We found no apparent changes in the uterine in the control group. In the SPID group, the uteri showed different degrees of the lesion with uterine enlargement, hyperemia and uterine wall thickening. However, these lesions were significantly attenuated by the administration of XYNK decoction. The histological pathology was assessed by HE staining and observed under a light microscope (fig. 1B). The epithelium was clear and intact in the control group, and the smooth muscle was arranged regularly. In the uterine from the SPID rats, severe pathological changes were observed, and the uterine tissues were infiltrated by masses of inflammatory cells, most of which were neutrophils and lymphocytes. These results demonstrate the occurrence of inflammation in the upper genital tract. Nevertheless, compared to the SPID group, XYNK decoction administration markedly decreased histopathological changes and the infiltration of inflammatory cells.
Fig. 1: Effect of XYNK decoction on inflammation in the uterus of SPID rats, (A): The appearance of the uterus in the different groups; (B):
Representative micrographs of uterus stained with H&E (magnification 200×). ELISA shows that XYNK decoction reverses the increase in serum;
(C): TNF-α and (D): IL-6 levels in the serum of SPID rats. Each bar represents the mean±deviation (n=6). ANOVA was performed and followed
with Tukey’s post hoc test.
Note: ***p<0.001 vs. control group; ###p<0.001 vs. SPID group.
XYNK decoction inhibited the excessive production of inflammatory cytokines in SPID rats to observe the changes in the inflammatory response; ELISA was performed to determine the serum concentrations of TNF-α and IL-6 in SPID rats. Compared with the control group, serum TNF-α and IL-6 levels were significantly increased in the SPID group. XYNK decoction significantly inhibited the increase of these cytokines in rats (fig. 1C and fig. 1D). These results indicated that XYNK decoction has anti-inflammatory effects on SPID rats.
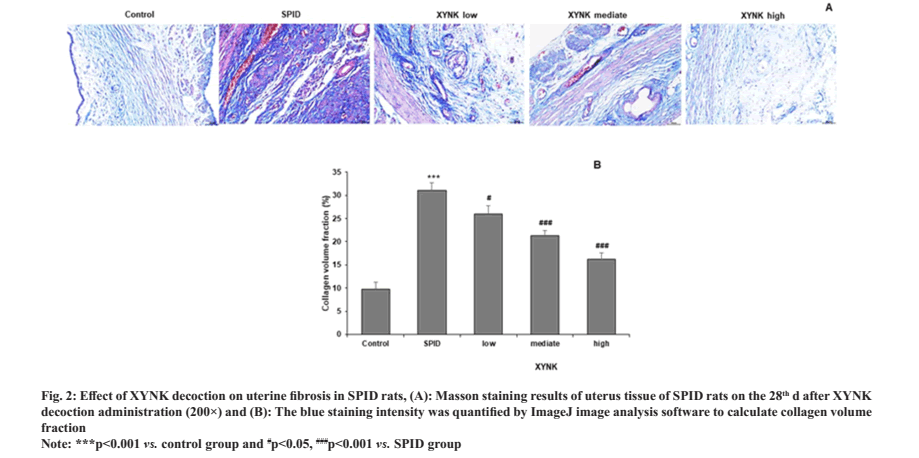
XYNK decoction suppressed uterine fibrosis in SPID rats. Masson staining was carried out in SPID rats after 28 d of XYNK decoction administration to evaluate the fibrosis in uterus tissue. The results are shown in fig. 2A. The control group showed a small number of blue collagen fibers were evenly distributed in the endometrial stroma, with clear tissue structure and regular arrangement. Where, the SPID group showed a large number of blue collagen fibers gathered, and mainly located in the stroma and muscle layer, with the close arrangement and abnormal shape; all XYNK treatment groups showed gland regeneration and decreased interstitial collagen fibers with varying degrees. Then the number of blue collagen fibers was quantified to calculate collagen volume fraction. The collagen volume fraction was increased in SPID rats and was decreased by XYNK treatment in a dose-dependent manner as shown in fig. 2B.
Fig. 2: Effect of XYNK decoction on uterine fibrosis in SPID rats, (A): Masson staining results of uterus tissue of SPID rats on the 28th d after XYNK
decoction administration (200×) and (B): The blue staining intensity was quantified by ImageJ image analysis software to calculate collagen volume
fraction.
Note: ***p<0.001 vs. control group and #p<0.05, ###p<0.001 vs. SPID group.
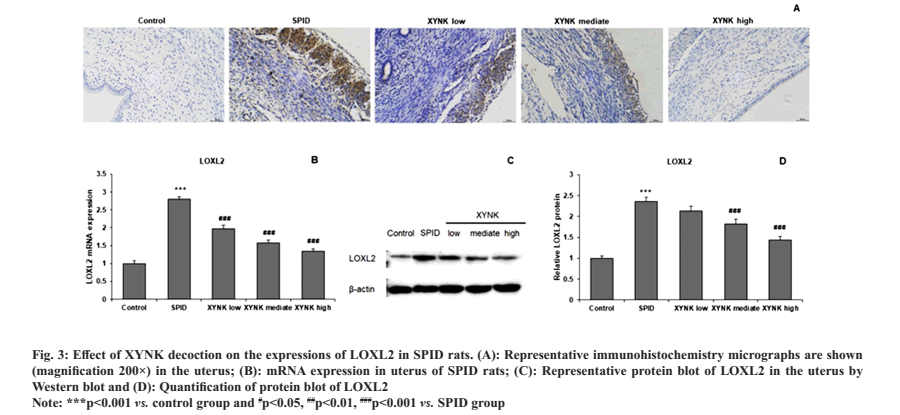
To investigate the detailed mechanisms underlying the anti-fibrosis effects by XYNK decoction, immunohistochemistry was performed to measure the protein expressions of LOXL2. Little LOXL2 expression was detected in the uterus tissue of control rats, which was markedly increased in SPID rats. However, compared with SPID rats, rats in XYNK groups showed decreased LOXL2 staining in the uterus (fig. 3A). Quantitative analysis of LOXL2 was performed in uterus tissue with RT-qPCR and Western blot. The LOXL2 mRNA expression was significantly increased in SPID rats compared to control rats and were significantly reduced by XYNK decoction at low, mediate and high groups (fig. 3B). Similarly, XYNK decoction also significantly attenuated the increase in LOXL2 protein expression in the uterus of SPID rats as shown in fig. 3C and fig. 3D.
Fig. 3: Effect of XYNK decoction on the expressions of LOXL2 in SPID rats. (A): Representative immunohistochemistry micrographs are shown
(magnification 200×) in the uterus; (B): mRNA expression in uterus of SPID rats; (C): Representative protein blot of LOXL2 in the uterus by
Western blot and (D): Quantification of protein blot of LOXL2.
Note: ***p<0.001 vs. control group and #p<0.05, ##p<0.01, ###p<0.001 vs. SPID group.
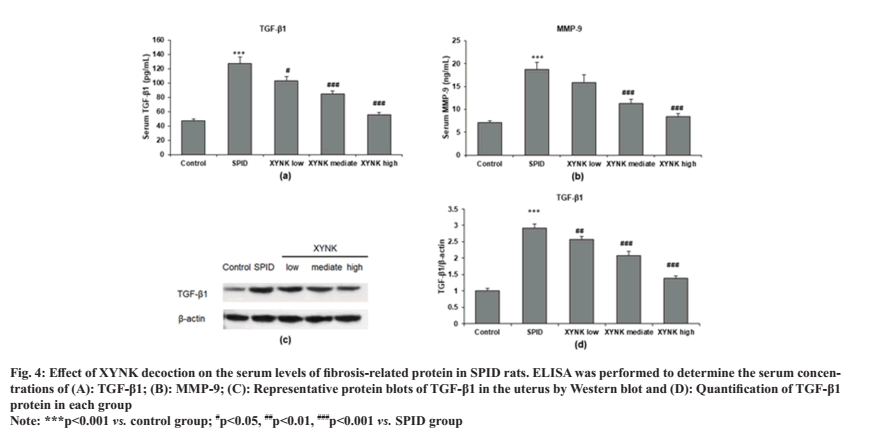
To investigate which fibrosis-related proteins are changed by XYNK decoction, ELISA was performed in the serum of SPID rats. Compared with the control group, serum TGF-β1 and MMP-9 levels were significantly increased in the SPID group. This increase was markedly attenuated by XYNK decoction (fig. 4A and fig. 4B). To confirm whether the serum TGF-β1 is produced and released from uterus tissue, Western blot was performed to measure the protein expression of TGF-β1 in the uterus (fig. 4C). Quantitative analysis showed that the TGF-β1 expression in uterus tissue was significantly increased in SPID rats and was reduced by XYNK decoction treatment as shown in fig. 4D.
Fig. 4: Effect of XYNK decoction on the serum levels of fibrosis-related protein in SPID rats. ELISA was performed to determine the serum concentrations of (A): TGF-β1; (B): MMP-9; (C): Representative protein blots of TGF-β1 in the uterus by Western blot and (D): Quantification of TGF-β1
protein in each group.
Note: ***p<0.001 vs. control group; #p<0.05, ##p<0.01, ###p<0.001 vs. SPID group.
This study investigated whether XYNK decoction mitigates fibrosis in a rat SPID model. Inflammation and fibrosis in the uterus were significantly suppressed by XYNK treatment in SPID rats. XYNK could significantly inhibit the mRNA and protein expression of LOXL2 in the uterus of SPID rats. We also found that XYNK reduced serum levels of fibrosis-related cytokine, including TGF-β1 and MMP-9, and suppressed TGF-β1 protein levels in the uterus of SPID rats.
In the present study, chronic pathogen infections promoted fibrosis in the endometrium, as evidenced by increased blue collagen fibers and elevated serum. XYNK showed promising activity in attenuating the fibrosis in the uterine of SPID rats. The occurrence, development, outcome and prognosis of SPID are associated with inflammatory cytokines. Infection initiates both the inflammatory reaction and damage repair mechanism simultaneously, thus stimulating fibroblasts' abnormal proliferation and activation. In addition to repairing the damaged tissue, the repair mechanism also promotes the aggregation and synthesis of inflammatory cells and intensifies the immune-inflammatory reaction[17]. This vicious cycle makes the disease gradually develop into a chronic process, leading to tissue destruction, extensive adhesion, proliferation and fibrosis, and scar formation. There are no or only a small number of pathogens in this sequelae stage. The essential reason lies in the excessive production of cytokines caused by an imbalance between immune response and damage repair, so antibiotic treatment is often ineffective[3,18]. Therefore, the key therapeutic strategy for SPID is regulating the balance between immunity and repair mechanisms.
In recent years, the mechanism of action of Traditional Chinese Medicine (TCM) on SPID has been extensively explored, and it has become a research hotspot[19,6], which shows that TCM can affect the occurrence development and prognosis of SPID with multiple targets. Our previous study demonstrated the anti-inflammatory activity of XYNK decoction in acute PID rats and reduced excessive production of Intercellular Adhesion Molecule 1 (ICAM-1)[15], and adhesion molecules associated with organ fibrosis[20]. Therefore, we speculated whether XYNK decoction is also effective in chronic PID and tissue fibrosis, and validated it in the present study. Taken together, we add XYNK decoction as a new TCM formula in the treatment of chronic PID, with fibrosis as its main therapeutic target.
Our study showed that XYNK decoction markedly decreased the expressions of LOXL2 in the uterus of SPID rats. LOXL2 is a member of the LOX family. LOXL2 is involved in forming fibrosis in the lungs and atria[21,22]. The expression and role of LOXL2 in the reproductive organs of PID are unclear. As another member of the LOX family, LOX was highly expressed in the endometrium of infertility patients caused by endometriosis, and LOX gene overexpression increased the expression of genes related to fibrosis and ECM remodeling[23]. This indicates that LOXL2 might also involve fibrosis in SPID, thus acting as a target for antifibrogenic therapy[24]. This hypothesis was validated in this study, with LOXL2 expression being higher in SPID rats' uterus than in control rats.
Moreover, fibrosis could enhance tissue adhesion in chronic endometritis, one type of PID[3]. Therefore, fibrosis is the key pathological feature of adhesion through promoting ECM deposition and replacing the endometrium with fibrous connective tissue[25]. Our study found fibrosis and LOXL2 can be suppressed by XYNK decoction in the uterus of SPID rats and indicates LOXL2 is a promising target protein in antifibrogenic therapy of SPID which needs more studies for validation.
TGF-β1 is the most active promoter of fibrosis through inducing the synthesis and secretion of collagen I, collagen III and other ECM[26]. Studies have shown that TGF-β1 is mainly expressed in endometrial glandular epithelial cells. The expression of TGF-β1 in the endometrium of patients with Intrauterine Adhesion (IUA) was increased and was positively correlated with the degree of endometrial fibrosis[27]. In a chronic PID rat model treated with Man-Pen-Fang, a Chinese herbal compound, TGF-β1 levels in serum and uterine were decreased[6], which is in accordance with our results by XYNK decoction. This indicates that the TGF-β1 signaling pathway played an essential role in the process of endometrial fibrosis in SPID. MMP-9 is a crucial proteinase involved in normal matrix remodeling, and its excessive production by inflammatory cells can promote ECM degradation[28]. High plasma MMP-9 level was observed in PID patients compared with healthy controls[7]. Our study also showed reduced serum MMP-9 in SPID rats after XYNK decoction treatment.
Moreover, LOXL2 is a potential activator of the TGF-β/Smad signaling pathway. Pulmonary fibrosis compared with control mice. Furthermore, silencing LOXL2 significantly decreased TGF β downstream protein pSmad2/3 levels in bleomycin-induced pulmonary fibrosis animals[29], and inhibited TGF-β1-mediated induction of EndMT marker in endothelial cells[30]. Whether LOXL2 regulate TGF-β1, related pathway in SPID is unknown and needs further investigation.
In conclusion, the endometrium in SPID rats is accompanied by prominent fibrosis in the process of natural healing without intervention. XYNK decoction can reduce tissue fibrosis injury in SPID rats and may play an anti-fibrosis effect by regulating the levels of LOXL2 and TGF-β 1 in serum and uterine tissue. LOXL2 may coordinate with TGF-β1/Smad signaling pathway and participate in the pathological process of endometrial fibrosis in SPID. Our results also suggest that LOXL2 may be an essential target for the treatment of fibrosis and adhesions of SPID and provide a necessary basis for developing therapeutic drugs.
Funding:
This study was supported by; leading talents of health system in Pudong New Area (No. PWR12019-04); the fifth batch of national excellent clinical talents of TCM (grant no. 2022 [1]) and Shanghai Municipal Health Commission, general project, clinical study on the treatment of sequelae of PID with xiaoyuningkun decoction, 202040118.
Ethical approval:
All methods and procedures are used in this study were in agreement with the ethical principles and standards of the institutional and national bioethical commission and with the Helsinki of Declaration 1964 and its later revisions. The ethics committee of Shanghai Pudong New Area Gongli hospital approved the animal study. All methods carried out in this study were in accordance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Authors’ contributions:
Bixin Tang designed the study, supervised and wrote the manuscript; Chan Xie and Yiyun Chen performed the experiment, collected data and analyzed; Hairun Zhua and Hanlin Ma performed the experiment and analyzed the data. Fang Wang performed the statistical analysis and revised the manuscript. Chan Xie and Yiyun Chen have contributed equally to this work. All authors have read and approved the manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Das Breanne B, Ronda J, Trent M. Pelvic inflammatory disease: Improving awareness, prevention and treatment. Infect Drug Resist 2016;9:191-7.
[Crossref] [Google Scholar] [PubMed]
- Chayachinda C, Rekhawasin T. Reproductive outcomes of patients being hospitalised with pelvic inflammatory disease. J Obstetr Gynaecol 2017;37(2):228-32.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Yang H, Guo Y, Yang G, Chen Y. The impact of chronic endometritis on endometrial fibrosis and reproductive prognosis in patients with moderate and severe intrauterine adhesions: A prospective cohort study. Fertil Steril 2019;111(5):1002-10.
[Crossref] [Google Scholar] [PubMed]
- Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: A population-based nested controlled study. Clinics 2018;73:e364.
[Crossref] [Google Scholar] [PubMed]
- Guo LP, Chen LM, Chen F, Jiang NH, Sui L. Smad signaling coincides with epithelial-mesenchymal transition in a rat model of intrauterine adhesion. Am J Transl Res 2019;11(8):4726.
[Google Scholar] [PubMed]
- Zhang LJ, Zhu JY, Sun MY, Song YN, Rahman K, Peng C, et al. Anti-inflammatory effect of Man-Pen-Fang, a Chinese herbal compound, on chronic pelvic inflammation in rats. J Ethnopharmacol 2017;208:57-65.
[Crossref] [Google Scholar] [PubMed]
- Wang PH, Tsai HT, Tee YT, Lin LY, Yang SF, Hsieh YS. Significant elevation of plasma matrix metalloproteinase-9 level and its ratio to matrix metalloproteinase-2 in patients with pelvic inflammatory disease. Fertil Steril 2009;92(5):1679-84.
[Crossref] [Google Scholar] [PubMed]
- Trackman PC. Lysyl oxidase isoforms and potential therapeutic opportunities for fibrosis and cancer. Expert Opin Ther Targets 2016;20(8):935-45.
[Crossref] [Google Scholar] [PubMed]
- Cosgrove D, Dufek B, Meehan DT, Delimont D, Hartnett M, Samuelson G, et al. Lysyl oxidase like-2 contributes to renal fibrosis in Col4α3/Alport mice. Kidney Int 2018;94(2):303-14.
[Crossref] [Google Scholar] [PubMed]
- Sayed N, Khurana A, Saifi MA, Singh M, Godugu C. Withaferin A reverses bile duct ligation-induced liver fibrosis by modulating extracellular matrix deposition: Role of LOXL2/Snail1, vimentin, and NFκB signaling. Biofactors 2019;45(6):959-74.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Li S, Li W. LOX/LOXL in pulmonary fibrosis: Potential therapeutic targets. J Drug Targeting 2019;27(7):790-6.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zhan Y, Zhang D, Dai B, Ma W, Qi J, et al. Eupolyphaga sinensis walker displays inhibition on hepatocellular carcinoma through regulating cell growth and metastasis signaling. Sci Rep 2014;4(1):5518.
[Crossref] [Google Scholar] [PubMed]
- Zhu GY, Bai LP, Liu L, Jiang ZH. Limonoids from the fruits of Melia Toosendan and their NF-κB modulating activities. Phytochemistry 2014;107:175-81.
[Crossref] [Google Scholar] [PubMed]
- Wei P, Zhang T, Dong H, Chen Q, Mu X, Hu G. Anti-inflammatory and antiviral activities of cynanversicoside A and cynanversicoside C isolated from Cynanchun paniculatum in influenza a virus-infected mice pulmonary microvascular endothelial cells. Phytomedicine 2017;36:18-25.
[Crossref] [Google Scholar] [PubMed]
- Tang B, Wu K, Meng Q, Wang F. Comparison of the analgesic and anti-inflammatory effects of xiaoyuningkun decoction with Cynanchum paniculatum and fukeqianjin in a mouse model of pelvic inflammatory disease. Med Sci Monit 2019;25:9094-102.
[Crossref] [Google Scholar] [PubMed]
- Oh Y, Lee J, Kim HC, Hahn TW, Yoon BI, Han JH, et al. Establishment of hydrochloric acid/lipopolysaccharide-induced pelvic inflammatory disease model. J Vet Sci 2016;17(3):413-9.
[Crossref] [Google Scholar] [PubMed]
- Zaiss DM, Minutti CM, Knipper JA. Immune and non-immune mediated roles of regulatory T-cells during wound healing. Immunology 2019;157(3):190-7.
[Crossref] [Google Scholar] [PubMed]
- Curry A, Williams T, Penny ML. Pelvic inflammatory disease: Diagnosis, management and prevention. Am Family Phys 2019;100(6):357-64.
[Google Scholar] [PubMed]
- Bu X, Liu Y, Lu Q, Jin Z. Effects of “danzhi decoction” on chronic pelvic pain, hemodynamics and proinflammatory factors in the murine model of sequelae of pelvic inflammatory disease. Evid Based Complement Alternat Med 2015;2015:547251.
[Crossref] [Google Scholar] [PubMed]
- Li X, Zhou Y, Zou R, Chen H, Liu X, Qiu X, et al. Associations of serological biomarkers of sICAM-1, IL-1β, MIF and su-PAR with 3 mo mortality in acute exacerbation of idiopathic pulmonary fibrosis. Mediators Inflamm 2020;2020:4534272.
[Crossref] [Google Scholar] [PubMed]
- Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J 2014;43(5):1430-8.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Tang K, Tianbao X, Wang J, Yang J, Li D. Increased serum lysyl oxidase-like 2 levels correlate with the degree of left atrial fibrosis in patients with atrial fibrillation. Biosci Rep 2017;37(6):BSR20171332.
[Crossref] [Google Scholar] [PubMed]
- Ruiz LA, Báez-Vega PM, Ruiz A, Peterse DP, Monteiro JB, Bracero N, et al. Dysregulation of lysyl oxidase expression in lesions and endometrium of women with endometriosis. Reprod Sci 2015;22(12):1496-508.
[Crossref] [Google Scholar] [PubMed]
- Puente A, Fortea JI, Cabezas J, Arias Loste MT, Iruzubieta P, Llerena S, et al. LOXL2—A new target in antifibrogenic therapy? Int J Mol Sci 2019;20(7):1634.
[Crossref] [Google Scholar] [PubMed]
- Torres-De La Roche LA, Campo R, Devassy R, Sardo AD, Hooker A, Koninckx P, et al. Adhesions and anti-adhesion systems highlights. Facts Views Vis Obgyn 2019;11(2):137-49.
[Google Scholar] [PubMed]
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: The master regulator of fibrosis. Nat Rev Nephrol 2016;12(6):325-38.
[Crossref] [Google Scholar] [PubMed]
- Hu J, Zeng B, Jiang X, Meng Y, Zhu Y, Mao M. The expression of marker for endometrial stem cell and fibrosis was increased in intrauterine adhesious. Int J Clin Exp Pathol 2015;8(2):1525.
[Google Scholar] [PubMed]
- Robert S, Gicquel T, Victoni T, Valença S, Barreto E, Bailly-Maître B, et al. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci Rep 2016;36(4):e00360.
[Crossref] [Google Scholar] [PubMed]
- Wen X, Liu Y, Bai Y, Li M, Fu Q, Zheng Y. LOXL2, a copper-dependent monoamine oxidase, activates lung fibroblasts through the TGF-β/Smad pathway. Int J Mol Med 2018;42(6):3530-41.
[Crossref] [Google Scholar] [PubMed]
- de Jong OG, vander Waals LM, Kools FR, Verhaar MC, van Balkom BW. Lysyl oxidase-like 2 is a regulator of angiogenesis through modulation of endothelial-to-mesenchymal transition. J Cell Physiol 2019;234(7):10260-9.
[Crossref] [Google Scholar] [PubMed]