- *Corresponding Author:
- S. Kumar
Department of Chemistry, Iswar Saran Degree College, Allahabad - 211 004
E-mail: sanjivks77@gmail.com
| Date of Submission | 6 November 2009 |
| Date of Revision | 6 July 2010 |
| Date of Acceptance | 28 July 2010 |
| Indian J Pharm Sci 2010, 72 (4): 458-464 |
Abstract
An efficient electrochemical method for the preparation of 2-amino-5-substituted-1,3,4-oxadiazoles (4a-k) at platinum anode through the electrooxidation of semicarbazone (3a-k) at controlled potential electrolysis has been reported in the present study. The electrolysis was carried out in the acetic acid solvent and lithium perchlorate was used as supporting electrolyte. The products were characterized by IR, 1 H-NMR, 13 C-NMR, mass spectra and elemental analysis. The synthesized compounds were screened for their in vitro growth inhibiting activity against different strains of bacteria viz., Klebsilla penumoniae, Escherichia coli, Bassilus subtilis and Streptococcus aureus and antifungal activity against Aspergillus niger and Crysosporium pannical and results have been compared with the standard antibacterial streptomycin and antifungal griseofulvin. Compounds exhibits significant antibacterial activity and antifungal activity. Compounds 4a and g exhibited equal while 4c, d, i and j slightly less antibacterial activity than standard streptomycin. Compounds 4a and g exhibited equal while 4b, c, d, f and i displayed slightly less antifungal activity than standard griseofulvins.
Keywords
Acetic acid, controlled potential electrolysis, electrochemical, electrooxidation, green chemistry, oxadiazole, platinum electrode
5-substituted-2-amino-1,3,4-oxadiazoles have been found to exhibit diverse activities such as antibacterial [1,2], antiHIV [1], antifungal [3-5], antitubercular [6], virucidal [7], antimalarial [8,9], insecticidal [10], herbicidal [11], analgesic [12], antiinflammatory [13], muscle relaxant [14], anticonvulsant [15], sedative, hypnotic [16], anticancer [17] and inhibition of lipid peroxidation [18].
Literature synthesis of oxadiazoles (4) includes bromine oxidation of semicarbazide derivative and the cyclodesulfurization of acylthiosemicarbazide derivatives in solution using I2/NaOH or 1,3-dicyclohexylcarbodimide (DCC) [19-21], as well as mercury(II) acetate (Hg(OAc)2) or yellow mercury(II) oxide (HgO) [22-25]. All these methods are usually carried out in different synthetic steps that require very dangerous reagents such as bromine or a full equivalent of Hg(II) and produce undesirable mercury byproducts, which must then be removed and properly disposed off after the reaction is completed. Not only the handling of these reagents is difficult but also very hazardous to environment. From the fi rst step to the last stage of the reaction including extraction and purifi cation of the products there from demands great precautions.
In the context of green chemistry, some 5-substituted- 2-amino-1,3,4-oxadiazoles (4) have been synthesized by electrooxidative cyclization of semicarbazone (3) as a new general environmentally benign synthetic method. The development of ecofriendly synthetic methods are the need of the hour. In this respect, organic synthesis involving multi-component reactions under reagent-free conditions is a basic protocol because multistep conventional synthesis produces considerable amounts of environmentally unfavorable wastes, mainly due to a series of complex isolation procedures often involving expensive, toxic and hazardous solvents and reagents after each step. The application of electricity as a non conventional energy source for activation of reactions in suitable solvents has now gained popularity over the usual homogeneous and heterogeneous reactions, as it provides chemical processes with special attributes, such as enhanced reaction rates, better selectivity, higher yield of pure products and several eco-friendly advantages. These reactions do not require oxidizing reagents and can be performed at ordinary room temperature.
Materials and Methods
General experimental procedure
Column chromatography was carried out by using Merck silica gel 60. The purity of the synthesized compounds were ascertained by TLC on precoated silica gel plates in various solvent systems using iodine vapors and UV light as detecting agent. The melting points were recorded on an electrothermal apparatus and were uncorrected. Infra red spectra were recorded on a Shimadzu 8201 PC IR spectrophotometer in KBr pellets and reported in cm-1. 1H NMR and 13C NMR spectra were measured on Bruker DRX 300 MHz FT spectrometer instruments using DMSO-d6 as solvent with TMS and CDCl3 as internal standards (chemical shift in δ ppm). Carbon multiplities were assigned by DEPT techniques. The structures of the newly synthesized compounds were assigned on the basis of elemental analysis and were recorded on a Elementar Vario EL III. Carbon, hydrogen and nitrogen analyses were within ±0.4% of the theoretical values. Mass spectra were taken out on a Jeol SX 102/DA-6600 mass spectrometer using Argon/Xenon (6 KV, 10 mA) as the FAB gas. All the chemicals used were of synthetic and AR grade and was procured from Agros-Organics, USA, S. D. Fine Chem. Ltd., Mumbai and Merck, Mumbai, India.
Synthesis of 2-amino-5-(p-bromophenyl)-1,3,4- oxadiazoles
Semicarbazide hydrochloride (1.0 g, 8.96 mmol) (2) and NaOAc (12.2 mmol) was dissolved in (10 ml) water and then an aldehyde (0.5 g, 3.04 mmol) (1) was added with continuous stirring. The mixture was left overnight, which evolved a solid product semicarbazone (3) which was used as initial compound for the electrolysis. Semicarbazone (1.0 g, 4.52 mmol) (3) and LiClO4 (0.106 g, 0.67 mmol) were dissolved in (100 ml) acetic acid.
Electrolysis
Preparative scale controlled potential electrolysis [26-30] were performed at room temperature in 250 ml three-electrode cell assembly with platinum plate as working as well as counter electrode and saturated calomel electrode as reference electrode. Magnetic stirrer was used for the proper mixing of reaction mixture. All the electrolysis experiments were carried out at their corresponding oxidation potentials and were completed in 3 to 5 h. After which no oxidation product was seen to diffuse in the bulk. All the products were solid and coloured and entirely different from the starting compound. The current potential data was recorded with the help of potentiostat at the interval of 15 min and has been depicted in Table 1. Approximately 4-6.5 Fmol-1 of electricity was passed for the electrolysis which is very small in comparison to energy used in other conventional methods. The physical and analytical data of the synthesized compounds (4a-k) has been depicted in the Table 2. The spectral (IR, 1H NMR, 13C NMR and MS) and analytical data are in good agreement with their structures.
| Entry | R | Time (h) | Applied Potential (mV) | Current (mA) | Yield (%) |
|---|---|---|---|---|---|
| 4a | o-BrC6H4 | 4 | 1540 | 110 | 88 |
| 4b | m-BrC6H4 | 5 | 2100 | 150 | 96 |
| 4c | p-BrC6H4 | 5 | 2250 | 120 | 86 |
| 4d | o-(NO2)C6H4 | 3 | 1850 | 90 | 92 |
| 4e | 3-Pyridinyl | 4 | 1800 | 70 | 79 |
| 4f | CH2Cl | 5 | 2000 | 120 | 75 |
| 4g | CHCl2 | 5 | 1900 | 80 | 81 |
| 4h | p-(CH3)C6H4 | 3 | 1450 | 90 | 85 |
| 4i | 3,4,5-(OCH3)3C6H2 | 5 | 1700 | 80 | 92 |
| 4j | 1-C10H7 | 4 | 1600 | 100 | 87 |
| 4k | 2-C10H7 | 4 | 2200 | 120 | 86 |
Table 1: Electroorganic Synthesis of 5-Substituted-2-Amino-1,3,4-Oxadiazoles (4a-K)
| Compound | R | Mol. for/ Mol. wt | Elemental analysis found (calcd) % | ||||
|---|---|---|---|---|---|---|---|
| C | H | N | Br/Cl | ||||
| 4a | o-BrC6H4 | C8H6N3OBr/240.01 | 39.52 (40.00) | 2.40 (2.50) | 17.35 (17.50 | 33.12 (33.33) | |
| 4b | m-BrC6H4 | C8H6N3OBr/240.01 | 39.56 (40.00) | 2.42 (2.50) | 17.35 (17.50) | 33.22 (33.33) | |
| 4c | p-BrC6H4 | C8H6N3OBr/240.01 | 39.53 (40.00) | 2.41 (2.50) | 17.35 (17.50) | 33.15 (33.33) | |
| 4d | o-NO2C6H4 | C8H6N5O5 /252.12 | 37.89 (38.09) | 2.40 (2.38) | 27.35 (27.77) | - | |
| 4e | 3-Pyridinyl | C7H6N4O/162.15 | 51.35 (51.85) | 3.40 (3.70) | 34.58 (34.57) | - | |
| 4f | CH2Cl | C3H4N3OCl/133.53 | 26.56 (26.96) | 2.59 (2.69) | 31.15 (31.46) | 26.60 (26.59) | |
| 4g | CHCl2 | C3H3N3OCl2/167.9 | 21.35 (21.55) | 1.68 (1.70) | 25.14 (25.14) | 41.66 (41.91) | |
| 4h | p-(CH3)C6H4 | C9H9N3O/175.19 | 61.52 (61.71) | 5.11 (5.14) | 23.85 (24.00) | - | |
| 4i | 3,4,5-(OCH3)3C6H2 | C11H13N3O4/251.24 | 52.40 (52.59) | 5.11 (5.17) | 16.52 (16.73) | - | |
| 4j | 1-C10H7 | C12H9N3O2/227.22 | 67.92 (68.24) | 4.26 (4.26) | 19.85 (19.90) | - | |
| 4k | 2-C10H7 | C12H9N3O2/227.23 | 67.95 (68.24) | 4.25 (4.26) | 19.86 (19.90) | - | |
Table 2: Physical and Analytical Data of 5-Substituted-2-Amino-1,3,4-Oxadiazole (4a-K)
2-amino-5-(o-bromophenyl)-1,3,4-oxadiazole (4a)
Brownish crystals; mp: 68-69º; IR (KBr, cm-1): 3360 (NH), 3045 (C-H aromatic), 1613 (C=N-N=C), 1470 (C=C aromatic), 1265, 1072 (C-O-C), 980, 890, 750, 595 (substituted benzene); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 6.94-7.14 (m, 4H, aromatic H); 13C NMR (DMSO-d6, δ ppm): 158.9 (C), 147.7 (C), 140.6 (C), 132.9 (CH), 131.9 (CH), 125.1 (C), 115.4 (C), 106.4 (CH); MS m/z: 240 (M+), 241 (M+ + 1, 100%) for C8H6N3OBr.
2-amino-5-(m-bromophenyl)-1,3,4-oxadiazole (4b)
Brownish crystals; mp: 75-76º; IR (KBr, cm-1): 3362 (NH), 3042 (C-H aromatic), 1620 (C=N-N=C), 1473 (C=C aromatic), 1265, 1072 (C-O-C), 980, 890, 7505, 595 (substituted benzene); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 6.94-7.24 (m, 4H, aromatic H); 13C NMR (DMSO-d6, δ ppm): 158.9 (C), 147.7 (C), 140.6 (C), 132.9 (CH), 131.9 (CH), 125.1 (C), 115.4 (C), 106.4 (CH); MS m/z: 240 (M+), 241 (M+ + 1, 100%) for C8H6N3OBr.
2-amino-5-(p-bromophenyl)-1,3,4-oxadiazole (4c)
Brownish crystals; mp: 69-70º; IR (KBr, cm-1): 3360 (NH), 3045 (C-H aromatic), 1615 (C=N-N=C), 1475 (C=C aromatic), 1275, 1075 (C-O-C), 985, 890, 755, 597 (substituted benzene); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 7.71 (d, 2H, aromatic H), 7.64 (d, 2H, aromatic H); 13C NMR (DMSO-d6, δ ppm): 158.9 (C), 147.7 (C), 140.6 (C), 132.9 (CH), 131.9 (CH), 125.1 (C), 115.4 (C), 106.4 (CH); MS m/z: 240 (M+), 241 (M+ + 1, 100%) for C8H6N3OBr.
2-amino-5-(o-nitrophenyl)-1,3,4-oxadiazole (4d)
Dark yellowish needles; mp: 71-73º; IR (KBr, cm-1): 3341 (NH), 3035 (C-H aromatic), 1607 (C=N-N=C), 1550 (N=O), 1465 (C=C aromatic),1275, 1070 (C-O-C), 985, 865, 810, 730 (substituted benzene); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 7.25-7.69 (m, 4H, aromatic H); 13C NMR (DMSO-d6, δ ppm): 171.3 (C), 147.1 (C), 146.8 (C), 140.8 (C), 136.8 (C), 134.7 (CH), 130.4 (CH), 121.2 (CH); MS m/z: 252 (M+), 253 (M+ + 1, 100%) for C8H6N5O5.
2-amino-5-(3-pyridinyl)-1,3,4-oxadiazole (4e)
Dark yellowish crystals; mp: 67-68º; IR (KBr, cm-1): 3350 (NH), 3037 (PyC-H), 1628 (C=N-N=C), 1472 (C=C aromatic), 1430-1600 (C=C and C=N str), 1070 (C-O-C); 1H NMR (DMSO-d6, δ ppm): 7.18-8.56 (m, 4H, aromatic H), 7.75 (s, 2H, NH2); 13C NMR (DMSO-d6, δ ppm): 171.2(C), 167 (C), 149.8 (CH), 135.7 (CH), 129 (C), 123.6 (CH), 122.1 (CH); MS m/z: 162 (M+), 163 (M+ + 1, 100%) for C7H6N4O.
2-amino-5-chloromethyl-1,3,4-oxadiazole (4f)
Brownish crystals; mp: 61-62º; IR (KBr, cm-1): 3360 (NH), 3062 (C-H), 1618 (C=N-N=C), 1280, 1066 (C-O-C), 680 (C-Cl); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 3.8 (s, 2H, CH2); 13C NMR (DMSO-d6, δ ppm): 170.6 (C), 167.6 (C), 24.9 (CH2); MS m/z: 133.5 (M+), 134.5 (M+ + 1, 100%) for C3H4N3OCl.
2-amino-5-dichloromethyl-1,3,4-oxadiazole (4g)
Brownish crystals; mp: 64-65º; IR (KBr, cm-1): 3360 (NH), 3065 (C-H), 1609 (C=N-N=C), 1280, 1066 (C-O-C), 690 (C-Cl); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 3.9 (s, 1H, CH); 1H NMR (DMSO-d6, δ ppm): 172.1 (C), 163.2 (C), 51.2 (CH); MS m/z: 167 (M+), 168 (M+ + 1, 100%) for C3H3N3OCl2.
2-amino-5-(p-methylphenyl)-1,3,4-oxadiazole (4h)
Light brown needles; mp: 74-75º; IR (KBr, cm-1): 3270 (NH), 3045 (C-H aromatic), 3010 (C-H), 2927, 1602 (C=N-N=C), 1473 (C=C aromatic), 1265, 1069 (C-O-C), 960, 765 (substituted benzene); H1 NMR (400 MHz, CDCl3) 7.75 (s, 2H, NH2), 7.69 (d, 2H, aromatic H), 7.62 (d, 2H, aromatic H), 1.12 (s, 3H, CH3); C13 NMR (75 MHz, CDCl3) 178.2(C), 149.9 (C), 141.3(CH), 138.6 (CH), 136.5 (CH), 129 (CH), 127.8 (CH), 126.5 (CH), 20.6 (CH3); MS m/z: 175 (M+), 176 (M+ + 1, 100%) for C9H9N3O.
2-amino-5-(3,4,5-trimethoxyphenyl)-1,3,4-oxadiazole (4i)
Dark brownish needles; mp: 84-85º; IR (KBr, cm-1): 3261 (NH), 3045 (C-H aromatic), 2815 (OCH3), 1609 (C=N-N=C), 1470 (C=C aromatic), 1270, 1069 (C-O-C), 915, 870, 790 (substituted benzene); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 6.46- 7.70 (m, 2H, aromatic H), 3.11 (s, 9H, OCH3); 13C NMR (DMSO-d6, δ ppm): 172.4 (C), 167.5 (C), 146.7 (C), 146.3 (C), 134.9 (C), 129.5 (C), 105.7 (CH), 106.5 (CH), 54.3 and 44.6 (CH3); MS m/z: 251 (M+), 252 (M+ + 1, 100%) for C11H13N3O4.
2-amino-5-(1-naphthyl)-1,3,4-oxadiazole (4j)
Dark brownish needles; mp: 94-95º; IR (KBr, cm-1): 3330 (NH), 3045 (C-H aromatic), 1612 (C=N-N=C), 1475 (C=C aromatic), 1055 (C-O-C), 775 (substituted aromatics); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 7.25-7.69 (m, 7H, aromatic H); 13C NMR (DMSO-d6, δ ppm): 171.5 (C), 149.5 (C), 133.7 (C), 128 (CH), 126 (C); MS m/z: 227 (M+), 228 (M+ + 1, 100%) for C12H9N3O2.
2-amino-5-(2-naphthyl)-1,3,4-oxadiazole (4k)
Dark brownish needles; mp: 96-97º; IR (KBr, cm-1): 3335 (NH), 3045 (C-H aromatic), 1622 (C=N-N=C), 1468 (C=C aromatic), 1045 (C-O-C), 775 (substituted aromatics); 1H NMR (DMSO-d6, δ ppm): 7.75 (s, 2H, NH2), 7.25-7.69 (m, 7H, aromatic H); 13C NMR (DMSO-d6, δ ppm): 171.6 (C), 149.5 (C), 133.7 (C), 128 (CH), 126 (C); MS m/z: 227 (M+), 228 (M+ + 1, 100%) for C12H9N3O2.
Screening for Antimicrobial activity
All the synthesized compounds were tested for antimicrobial activity by adopting the experimental method of Benson [31]. Whatman No.1 filter paper discs of 6 mm diameter, placed in a Petri dish, were autoclaved. The test compounds in measured quantities (1.0 mg, 0.5 mg) were dissolved in 5 ml dimethylformamide to produce 200 ppm and 100 ppm solutions, respectively. The filter paper discs were allowed to dry and the amount of the substance per disc was taken as 500 and 250 μg. The bacterial (24 h) and fungal (48 h) cultures from the slants were diluted with sterile water and mixed thoroughly to prepare a clear homogeneous suspension. These suspensions were uniformly spread on solidifi ed agar (nutrient and potato dextrose agar) medium. The fi lter paper discs prepared from dimethylformamide medium were carefully placed over the spread cultures and incubated at 37º for 24 h for bacteria and at 28-30º for 48 h for fungi. Paper discs treated with dimethylformamide alone served as control. After the incubation period the plates were examined for inhibition zones. The diameters of inhibition zones (including the diameter of the disc) were measured. All determinations were made in triplicate for each of the compounds and the average value was taken. The antibacterial and antifungal screening results were presented in Tables 3 and 4.
| Compound | Zone of inhibition (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli (ATCC 25922) | K. pneumonia (CIP 53153) | B. subtilis (ATCC 6633) | S. aureus (ATCC 25923) | ||||||||
| 25 ppm | 50 ppm | 25 ppm | 50 ppm | 25 ppm | 50 ppm | 25 ppm | 50 ppm | ||||
| 4a | 18 | 21 | 17 | 23 | 18 | 24 | 17 | 22 | |||
| 4b | 4 | 6 | 4 | 7 | 5 | 7 | 4 | 6 | |||
| 4c | 15 | 18 | 3 | 20 | 14 | 19 | 13 | 19 | |||
| 4d | 12 | 17 | 9 | 13 | 12 | 15 | 11 | 15 | |||
| 4f | 16 | 20 | 17 | 21 | 15 | 20 | 14 | 19 | |||
| 4g | 17 | 21 | 14 | 20 | 16 | 20 | 16 | 20 | |||
| 4i | 15 | 18 | 14 | 18 | 13 | 17 | 17 | 19 | |||
| Streptomycin | 20 | 23 | 19 | 24 | 19 | 24 | 19 | 23 | |||
Table 3: Antibacterial Screening Results of Compounds (4a-K)
| Compound | A. niger (ATCC 16404) | C. pannical (ATCC 10231) | ||||
|---|---|---|---|---|---|---|
| 10 ppm | 100 ppm | 1000 ppm | 10 ppm | 100 ppm | 1000 ppm | |
| 4a | 18 | 43 | 76 | 19 | 43 | 78 |
| 4b | 15 | 38 | 65 | 16 | 36 | 67 |
| 4c | 44 | 58 | 98 | 45 | 57 | 98 |
| 4d | 21 | 46 | 75 | 24 | 43 | 78 |
| 4f | 38 | 56 | 97 | 40 | 51 | 96 |
| 4g | 40 | 53 | 96 | 42 | 53 | 97 |
| 4i | 21 | 44 | 70 | 20 | 43 | 68 |
| Griseofulvin | 66 | 86 | 100 | 65 | 83 | 100 |
Table 4: Antifungal Screening Results of Compounds (4a-K)*
Antiinflammatory activity against carrageenaninduced rat paws oedema
Antiinflammatory activity was determined by carrageenan-induced rat paw method of Winter [32]. Male Wistar rats (125-150 g) were used for the experiment. They were fed with standard pellet diet and water was given ad libitum. The animals were acclimatized for one week under laboratory conditions before performing the test. They were housed in polypropylene cages under standard conditions (30±1º, 12/12 h light/dark cycles on 60-70% RH). The standard groups received phenylbutazone 50 mg/kg body weight po, suspended in 1% w/v of carboxymethylcellulose (CMC) in distilled water. The test group received synthesized compounds (4a-k) (50 mg/kg body weight po, suspended in 1% w/v of CMC in water). The control group received corresponding amount of vehicle (1% w/v of CMC). All the test compounds and standard drug were administered 30 min prior to carrageenan injection.
Acute edema was induced in the right hind paw of rats by injecting 0.1 ml of freshly prepared 1% w/v of aqueous solution of carrageenan (Sigma, USA) in the subplanter region of right hind paw. After the carrageenan injection the paw volume was measured before and after 1, 2 and 3 h by plethysmometer (UGO-Basile, Italy). The difference between the left and right paw was taken as a measure of oedema. Any signifi cant reduction in the volume of the paw compared to the control group was considered as anti-inflammatory response. Percent inhibition of infl ammation after 3 h was calculated by applying Newbould formula. % inhibition = 100×1–(a – x)/ (b – y), where, x = mean paw volume of rats before the administration of carrageenan injection in the test and the standard groups, y = mean paw volume of rats before the administration of carrageenan injection in the control group, a = mean paw volume of rats after the administration of carrageenan and test compound or standard compound, b = mean paw volume of rats after the administration of carrageenan injection in control group. The results are presented in Table 5.
| Compounds | Normal paw volume (x) | Paw oedema 3h after carrageenan injected (a) | % inhibition of oedema (1-(a-x)/(b-y))×100 |
|---|---|---|---|
| 4a | 0.66 ± 0.04 | 0.85 ± 0.03 | 46.41 |
| 4b | 0.70 ± 0.03 | 0.91 ± 0.03 | 24.12 |
| 4c | 0.68 ± 0.03 | 0.88 ± 0.04 | 35.62 |
| 4d | 0.69 ± 0.04 | 0.89 ± 0.03 | 32.38 |
| 4e | 0.72 ± 0.03 | 0.93 ± 0.03 | 21.12 |
| 4f | 0.66 ± 0.04 | 0.91 ± 0.04 | 35.78 |
| 4g | 0.65 ± 0.03 | 0.90 ± 0.03 | 42.21 |
| 4h | 0.67 ± 0.02 | 0.87 ± 0.02 | 44.12 |
| 4i | 0.715 ± 0.05 | 0.945 ± 0.04 | 20.78 |
| 4j | 0.70 ± 0.04 | 0.93 ± 0.05 | 25.00 |
| 4k | 0.71 ± 0.03 | 0.92 ± 0.05 | 24.85 |
| Control | 0.68 ± 0.03 | 0.98 ± 0.04 (b) | - |
| (y) | |||
| Phenylbutazone | 0.68 ± 0.04 | 0.84 ± 0.03 | 54.12 |
Table 5: Antiinflammatory Activity of Compounds (4a-K)
Results and Discussion
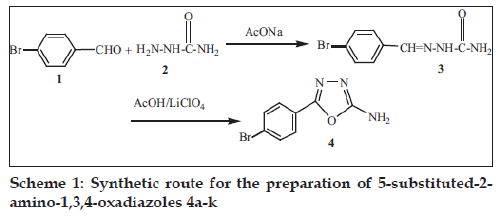
It is obvious that an environmentally benign synthetic method for the synthesis of 2-amino-5-substituted- 1,3,4-oxadiazoles would be highly attractive. Keeping this objective in the mind, we have synthesized 2-amino-5-substituted-1,3,4-oxadiazole 4 derivatives through the electrooxidation of semicarbazone 3 at a platinum electrode in controlled potential electrolysis in acetic acid (Scheme 1). This electrochemical cyclization evolves the oxadiazoles without requirement of any hazardous reagents. We have used acetic acid as a solvent and lithium perchlorate (LiClO4) as an electrolyte that can be handled very easily without major precautions.
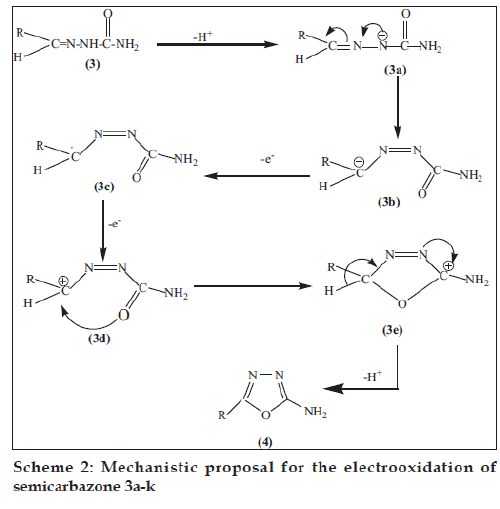
The fi rst step of the mechanism (Scheme 2) represents the deprotonation of 3 to form an anion 3a, which rearranges itself into 3b and evolves a free radical 3c after one electron oxidation. Subsequent second electron oxidation from the free radical 3c gives a carbocation 3d. The formation of carbon oxygen bond completes the ring. On loosing a proton in the last step 2-amino-5-substituted-1,3,4-oxadiazole 4 was obtained.
The antimicrobial activity indicated that compounds 4a, b, c, d, f, g and i were found to be active against Klebsilla penumoniae, Escherichia coli, Basillus subtilis, Streptococus aureus organisms at the two concentrations (25 and 50 ppm) taking Streptomycin as the standard. The majority of the compounds exhibited significant antibacterial activity against E. coli, K. pneumonia, B. subtilis and S. aureus as compared to standard streptomycin. The screening results of antibacterial activity revealed that compound 4a and g exhibited approximately similar activity to the standard Streptomycin. Compounds 4c, d, i and j exhibited slightly less antibacterial activity. Compound 4b exhibited weak while other compounds have zero or negligible antibacterial activity against all bacterial strains used for our evaluation. The screening results showed that compounds 4b, c d, f and i displayed better antifungal activity against Aspergillus niger and C. pannical along with the standard fungicide Griseofulvins. The screening results revealed that compounds 4a and g showed equal antifungal activity when compared with the griseofulvins.
The antimicrobial activity of the compounds varied upon the type and position of the substituents at 5-substituted-2-amino-1,3,4-oxadiazole moiety. It can be concluded from the antimicrobial screening results that when 5-substituted-2-amino-1,3,4-oxadiazoles were substituted with aryl halide the antimicrobial activity was altered to an appreciable extent.
The results of antiinflammatory activity (Table 5) shows that the compounds 4d and 4h were active (p<0.01) with the standard. Moreover, compounds 4c, d, f and g showed signifi cant antiinfl ammatory activity (p<0.01). Carrageenan-induced paw edema was taken as a prototype of exudative phase of infl ammation. The development of edema has been described as biphasic. The initial phase was due to the release of histamine, serotonins, 5-hydroxy tryptamine and kinins in the fi rst hour after injection of carrageenan. The second phase was related to the release of prostaglandin [33-35] like substances in 2-3 h. Hence, the signifi cant anti-infl ammatory effect may be due to an inhibitory effect exerted predominantly on the mediators of inflammation induced by phlogogenic stimuli.
Acknowledgements
The author thank UGC, New Delhi, for fi nancial assistance, Sophisticated Analytical Instrumentation Facility (SAIF) a division of CDRI Lucknow, for providing spectral and analytical data, and the Principal, SMM Tawn PG College, Ballia, for providing the facilities of screening of antiinfl ammatory activity.
References
- El-Emam AA, Al-Deeb OA, Al-Omar M. Synthesis, antibacterial and anti-HIV activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazolin-2-thiones. Bioorg Med Chem 2004;12:5107-13.
- Holla BS, Gonaslaves R, Shenoy S. Synthesis and antibacterial studies of a new series of 1,2-bis(1,3,4-oxadiazol-2-yl)ethanes and 1,2-bis(4-amino-1,2,4-triazol-3-yl)ethanes. Eur J Med Chem 2000;35:267-71.
- Maslat AO, Abussaud M, Tashtoush H, Al-Talib M. Synthesis, antibacterial, antifungal and genotoxic activity of bis-1,3,4-oxadiazole derivatives. Pol J Pharmacol 2002;54:55-9.
- Xiajuan Z, Zhang Z, Jin G. Synthesis and biological activity of 1,3,4-oxadiazole-substituted pyridazinones. J Chem Res 2002;5:228-30.
- Zou XJ, Lai LH, Jin GY, Zhang ZX. Synthesis, Fungicidal Activity, and 3D-QSAR of Pyridazinone-Substituted 1,3,4-Oxadiazoles and 1,3,4-Thiadiazoles. J Agric Food Chem 2002;50:3757-60.
- Kucukguzel SG, Oruc EE, Rollas S, Sahin F, Ozbek A. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur J Med Chem 2002;37:197-206.
- Chauhan D, Chauhan JS, Singh J, Bajpai SK, Joshi MN. Synthesis and bioevaluation of some novel nucleosides as antiherptic agents. Indian J Chem 2003;42B:215-8.
- Kagthara PR, Shah NS, Doshi RK, Parekh HH. Synthesis of 2,5-disubstituted-1,3,4-oxadiazoles as biologically active heterocycles. Indian J Chem 1999;30B:572-76.
- Preethi RK, Niraj SS, Rajeev KD, Parekh HH. Synthesis of 2,5-disubstituted-1,3,4-oxadiazolesas biologically active heterocycles. Heterocycl Comm 1998;4:561.
- Mohan TP, Vishalakshi B, Bhat KS, Kendappa GN. Synthesis and insecticidal activity of some 1,3,4-oxadiazole derivatives containing phenoxyfluoro group. Indian J Chem 2004;43B:1798-801.
- Kennedy DA, Summers LA. Chemical constitution and activity of Herbicides: Part XIV: Reduction potential and herbicidal activity of 4,4-(1,3,4-thiadiazoly-2,5-diyl) and 4,4-(1,3,4-oxadiazol-2,5-diyl) bis (1-methylpyridinium) diiodides. J Heterocycl Chem 1981;18:401-10.
- Santagati M, Modica M, Santagati A, Russo F, Caruso A, Cutuli V. Synthesis and pharmacological properties of benzothiazole, 1,3,4-oxadiazole and 1,2,4-thiadiazole derivatives. Pharmazie 1994;49:880-4.
- Mullican MD, Wilsonj MW, Connor DT, Kostlan CR, Schrier DJ, Dyer RD. Design of 5-(3,5-Di-ter-butyl-4-hydroxyphenyl)-1,3,4-thiadiazol-1-yl-1,3,4-oxadiazoles and 1,2,4-triazoles as orally active, non ulcerogenic anti-inflammatory agents. J Med Chem 1993;36:1090-9.
- Yale HI, Losee K. 2-Amino-5-substituted-1,3,4-oxadiazoles and 5-iminosubstituted-2-1,3,4-oxadiazolines: A group of novel muscle relaxants. J Med Chem 1966;9:478-83.
- Khan MS, Khan RM, Drabu S. Anticonvulsant and antibacterial activity of some new 1,3,4-oxadiazole derivatives. Indian J Heterocycl Chem 2001;11:119-22.
- Maillard J, Vincent M, Morin R, Bernard M. Hypnotic and sedative drug, 2-(o-hydroxyphenyl)-1,3,4-oxadiazole: French Pat M379. Chem Abstr 1962;57:15251g.
- Jessen KA, English NM, Wang JY, Maliartenou SK, Archer SP, Qiu L. The discovery and mechanism of action of novel tumour-selective and apoptosis-inducing 3,5-diaryl-1,2,4-oxadiazole series using a chemical genetics approach. Mol Cancer Ther 2005;4:761-71.
- Farghaly AA, Bekhit AA, Park JY. Design and synthesis of some oxadiazolyl, thiazolidinyl and thiazolyl derivatives of 1H-pyrazole as anti-inflammatory and antimicrobial agents. Arch Pharm 2000;333:53-7.
- Omar FA, Mahfouz NM, Rahman MA. Design, synthesis and anti-inflammatory activity of some 1,3,4-oxadiazole derivatives. Eur J Med Chem 1996;31:819-25.
- Golovlyova SM, MoskvichevYA, Alov EM, Kobylinsky DB, Ermolaeva VV. Synthesis of novel five membered nitrogen containing heterocyclic compounds from derivatives of arylsulfonyl and arylthioacetic and propionic acids. Chem Heterocycl Compd 2001;37:1102-6.
- Liu F, Wang B, Zhang Z. Studies on 1-(2’-phenyl-1’,2’,3’-triazol-4’-formyl)-4-arylthiosemicarbazides and related heterocyclic compounds. Youji Huaxue 2001;21:1126-31.
- Hetzheim A, Moeckel K. Recent advances in 1,3,4-oxadiazole chemistry. AdvHeterocycl Chem 1967;7:183-224.
- Hill J. Comprehensive Heterocyclic Chemistry. In: Potts KT. editors. Vol. 6. Oxford: Pergamon Press; 1984. p. 427.
- Wang X, Li Z, Yang J. Synthesis of 2-(4-methoxylphenyloxy-acetylamido)-5-aryloxymethyl-1,3,4-oxadiazoles under microwave irradiation. Synth Commun 2002;32:1097-103.
- Cappo FT, Evans KA, Graybill TL, Burton G. Efficient one pot preparation of 5-substituted-2-amino-1,3,4- oxadiazoles using resin bound reagents. Tetrahedron Lett 2004;45:3257-60.
- Mann CK, Barnes KK. Electrochemical Reactions. Non aqueous Systems. New York: Marcel Dekker, Inc; 1970. p. 13-8.
- Fry AJ. Synthetic Organic Electrochemistry. New York: Wiley-Interscience Publication; 1989. p. 71-8.
- Shono T. Electroorganic Synthesis. London: Academic Press Ltd; 1991. p. 11-9.
- Sharma LK, Kumar S, Yadav P, Singh RK. Electrochemical nuclear acetamidation of aromatic compounds at the platinum anode. Indian J Chem 2008;47B:1277-80.
- Kumar S, Sharma LK, Singh RK. Electroinducedaldol condensation at platinum electrode. J Indian Chem Soc 2006;83:1160-2.
- Benson HJ. Microbiological Applications. 5th ed. Boston: W. C. Brown Publications; 1990. p. 134.
- Winter CA, Risley EA, Nuss GW. Carrageenan induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 1962;111:544-7.
- Brooks PM, Day RO. Nonsteroidal anti-inflammatory drugs: Difference and similarities. N Engl J Med 1991;324:1716-25.
- Larsen GL, Hanson PM. Mediators of inflammation. Annu Rev Immunol 1983;1:335-9.
- Vane J, Booting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J 1987;1:89-96.