- *Corresponding Author:
- Hongqun Qiao
School of Pharmaceutical Sciences, Nanjing Tech University, PR China
E-mail: qiaohongqun@njtech.edu.cn
| Date of Received | 09 October 2022 |
| Date of Revision | 18 July 2023 |
| Date of Acceptance | 11 September 2023 |
| Indian J Pharm Sci 2023;85(5):1429-1435 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of this study was to establish the in situ single-pass intestinal perfusion method in rats for intestinal permeability investigation of drug. Effective permeability coefficients (Peff) of 15 model drugs were investigated in rat jejunal. Krebs-Ringer buffer solution containing drug was perfused at flow rate of 0.2 ml/min and samples were taken from outlet up to 110 min, steady state was achieved after 30 min perfusion without samples taken. Gravimetric method was used to correct the net water flux. Drug concentrations in perfusion samples were determined using high-performance liquid chromatography-ultraviolet method and Peff were calculated. The Peff values obtained by in situ single-pass intestinal perfusion ranged from 0.0146±0.0026×10-4 cm/s of enalaprilat to 1.77±0.34×10-4 cm/s of antipyrine. Among the selected model drugs, the Peff values of high permeability drugs were higher than 0.2×10-4 cm/s, and less than 0.03×10-4 cm/s for low permeability drugs. The experimental rat Peff was highly correlated to the literature human Peff with correlation coefficient (R2) of 0.99. The observed experimental rat Peff values were highly correlated to the literature human Fabs (R2=0.93). All investigated model drugs have similar permeability classification compare with literature permeability classification. The predicted human Fabs were linearly correlated with literature human Fabs (R2=0.90). This method could be used for investigation of the intestinal absorption of new compounds, and for prediction the absorption fraction in human. Besides, investigation of the permeability classification of generic drug by in situ single-pass intestinal perfusion can be used for application of biowaiver for industry.
Keywords
Biopharmaceutics classification system, intestinal permeability, in situ single-pass intestinal perfusion, effective permeability coefficient
The Biopharmaceutics Classification System (BCS), first proposed by Amidon et al.[1] in 1995, is a scientific frame for classifying drugs according to solubility of the drug dose in the Gastrointestinal (GI) milieu and permeability of the drug through the GI membrane. Drugs are divided into 4 categories according to BCS: BCS class I (high solubility-high permeability), BCS class II (low solubility-high permeability), BCS class III (high solubility-low permeability) and BCS class IV (low solubility-low permeability). Before the BCS classification system was proposed, it was difficult for industry to find theoretical support to apply biowaiver from drug regulatory department. Based on this frame, when an immediate-release solid oral dosage form drug with a rapid dissolution compare to gastric emptying and the drug has high solubility, the absorption of drug is most likely to be dependent on the permeability of drug on intestine membrane. Therefore, it is not necessary for drug products containing BCS I and BCS III active ingredients to prove the in vivo bioavailability and bioequivalence, as long as the absorption of active ingredients was not significantly affected by the excipients used in the dosage form[2,3].
Solubility parameters of drugs are readily available compared to permeability parameters. The model for evaluating permeability parameters of drugs has two major categories, in vivo and in vitro. In vivo models include absorption studies in humans and animals; in vitro models include Parallel Artificial Membrane Permeability Assay, tissue model (Ussing Perfusion Chamber model), in situ model (Intestinal Perfusion model) and cell model. Among all the permeability study models, the data from in vivo model can truly reflect the absorption of drugs in the body system but it is difficulty to get. Rat in situ intestinal perfusion have become the most reliable and cost-effective model in all permeability study model. Perfusion studies in rat intestine were highly correlated with human data, even though the type of transporters and their expression levels may vary between species[4,5]. It has been reported that a high correlation was observed between human and rat small intestine permeability (R2=0.80-0.95) for drugs which permeate through the intestinal by carrier-mediated absorption and passive diffusion mechanisms[6].
In situ Single-Pass Intestinal Perfusion (SPIP) model provides the advantages of precise experimental control, intact blood supply, and ability to investigate regional factors influencing intestinal absorption of compounds[7-9]. It has been widely utilized to predict the extent of drug absorption and to clarify absorption mechanisms[6,10].
A list of 15 drugs, which proposed as model drugs by Food and Drug Administration (FDA) for investigation intestinal permeability, were chosen with different permeability characteristics: low, moderate and high permeability. In this work, we investigated the effective permeability coefficients (Peff) of these model drugs and compared with literature human data to establish the study model for intestinal permeability of drug.
Materials and Methods
Materials:
Enalaprilat, lisinopril, amiloride, aciclovir, phenytoin, propranolol, metoprolol, ketoprofen, chlorothiazide, ranitidine, atenolol, hydrochlorothiazide, terbutaline, metformin and antipyrine were purchased from National Institutes for Food and Drug Control (Beijing, China). Acetonitrile, methanol (High- Performance Liquid Chromatography (HPLC) grade) were obtained from TEDIA (Ohio, USA). Deionized water was generated by Milli-Q system from Millipore (Bedford, USA). All other chemicals were analytical reagent grade.
In situ single-pass intestinal perfusion in rat:
All experimental protocols were supervised and managed by Jiangsu Provincial Drug Safety Evaluation Center Agency Committee (protocol code: JA-18-009) and the experiments were conducted in accordance with the guide for the related laws and regulations and Institutional Animal Care and Use Committee (IACUC). Male Sprague Dawley rats weighing 250-350 g (Zhejiang Vital River Laboratory Animal Technology Co., Ltd., Zhejiang, China) were maintained on a 12 h light-dark cycle and had free access to water and food, six replicates for each drug. Rats were fasted for 12 h (water ad libitum) prior to each experiment, and then anaesthetized with pentobarbital solution (30 mg/kg, i.p.). Then placed on a heated slide warmer and under a heating lamp to maintain normal body temperature. The abdomen was opened with a midline incision and jejunal segment of approximately 10 cm was measured, isolated and cannulated with plastic tubing[11-13]. Cares was taken to avoid disturbance of the circulatory system and the exposed segment was kept moist with 37° saline. Initially, the intestinal segment was rinsed with isotonic saline (37°) until the outlet solution was clear. Krebs-Ringer buffer solution, consisted of 130 mM NaCl, 5mM KCl, 1.27 mM MgSO4, 0.95 mM CaCl2, 5 mM glucose and 10 mM NaH2PO4, was used as blank perfusion solution. Perfusion solution was prepared by dissolving drugs in Krebs-Ringer buffer solution and then perfused through the intestinal segment at a flow rate of 0.2 ml/min. Blank perfusion solution was perfused for 30 min without sample taken to ensure steady state conditions, followed by additional 80 min of perfusion with samples taken every 20 min. Gravimetric method was used to correct the Net Water Flux (NWF)[14-17]. All samples were centrifuged at 16 000 rpm for 2 min before analyzed by HPLC. The length of the perfused intestinal segment was measured at the endpoint of the experiment.
The Peff (cm/sec) of model drugs through the rat gut wall was determined according to the following equations:
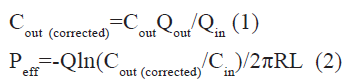
Where Cout and Cin are the outlet and the inlet concentration of drug respectively, Qout and Qin are the volume that collected at the outlet and the volume that perfusion solution reduced at the inlet respectively, Cout(corrected) is the outlet concentration of drug that has been corrected NWF by Gravimetric method, Q is the perfusion buffer flow rate (0.2 ml/ min), R is the radius of the intestinal segment, and L is the length of the perfused intestinal segment.
Analytical methods:
All in situ SPIP samples were quantified by a Essentia LC-15C HPLC (Shimadzu, Japan). More details about analytical methods of each model drug can be find in supporting information. Processing and analysis of chromatogram were performed on LabSolutions Essentia software.
Statistical analysis:
Values are expressed as mean±Standard Deviation (SD). The independent t test and one-way Analysis of Variance (ANOVA) were used to assess differences comparison of permeation parameter. Differences were considered statistically significant when p<0.05. The statistical comparison was made using the statistical package SPSS, V.23.
Results and Discussion
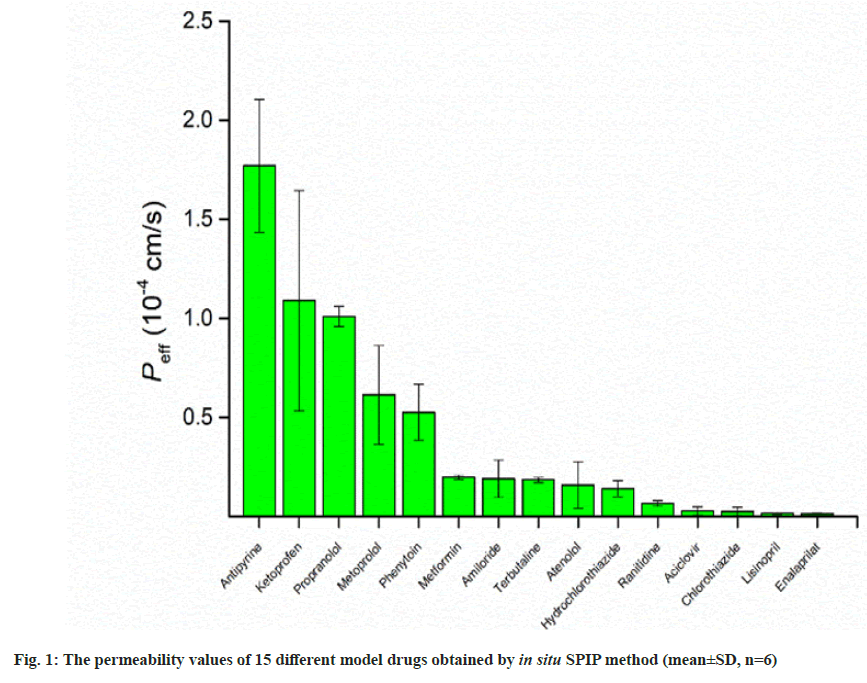
Permeability values of model drugs were investigated by in situ SPIP in rat at steady-state. During the perfusion, the collected sample were centrifuged, and then injected into the HPLC system for analysis. Gravimetric method was used to correct the NWF of rat intestine during the perfusion process. The corrected outlet concentration of each drug was obtained from equation (1), Peff value was calculated from equation (2). The permeability values were presented in fig. 1, and summarized in Table 1. The Peff of different model drugs ranged from 0.0146±0.0026×10-4 cm/s for enalaprilat and 1.77±0.34×10-4 cm/s for antipyrine, respectively.
| Drug | Concentration (μg/ml) | Peff (10-4 cm/s) |
|---|---|---|
| Antipyrine | 120 | 1.77±0.34 |
| Ketoprofen | 120 | 1.09±0.56 |
| Propranolol | 120 | 1.01±0.05 |
| Metoprolol | 120 | 0.613±0.249 |
| Phenytoin | 30 | 0.526±0.141 |
| Metformin | 120 | 0.198±0.0114 |
| Amiloride | 120 | 0.190±0.094 |
| Terbutaline | 120 | 0.185±0.014 |
| Atenolol | 120 | 0.159±0.117 |
| Hydrochlorothiazide | 120 | 0.140±0.041 |
| Ranitidine | 120 | 0.0668±0.0147 |
| Aciclovir | 120 | 0.0282±0.0208 |
| Chlorothiazide | 120 | 0.0254±0.0212 |
| Lisinopril | 120 | 0.0161±0.0028 |
| Enalaprilat | 120 | 0.0146±0.0026 |
Table 1: The Perfusion Concentrations and Permeability Values of Different Model Drugs Obtained by In Situ SPIP Method (Mean±SD, n=6)
During perfusion, the intestine will absorb and secrete water, which affects the calculation of the Peff. It is necessary to correct the NWF. NWF correction during the SPIP perfusion was usually involves the co-perfusion of a “non-absorbed” marker[14]. Since phenol red is an ionic compound, it is conventionally considered to be not absorbed in the intestine, and thus it has long been used as a correcting substance for correcting the NWF. However, it has been reported in the literature that phenol red is absorbed in the intestine[18]. For drugs with high permeability, the influence caused by phenol red has little effect on the test results, which is negligible, but for drugs with low permeability, the influence caused by phenol red cannot be ignored. Therefore, gravimetric method was used to correct the NWF during the perfusion in this study.
In this work, the Peff values investigated by in situ SPIP in rat were ranged from 0.0146±0.0026×10-4 cm/s for enalaprilat to 1.77±0.34×10-4 cm/s for antipyrine. Drug with Peff<0.03×10-4 cm/s in the rat small intestine is classified as low permeability, whereas drug with Peff>0.2×10-4 cm/s is completely absorbed (classified as high permeability)[4]. Among the selected model drugs, the Peff of high permeability drugs obtained in this experiment were higher than 0.2×10-4 cm/s and Peff of low permeability drugs were less than 0.03×10-4 cm/s, indicating a high correlation of in situ SPIP model to permeability classification of drug. For industry with desire in generic drug development, the investigation in permeability of drugs by a appropriate method is crucial. The FDA has approved the biowaiver for immediate-release solid oral dosage forms based on a BCS system, which divided drugs in different categories according to solubility and permeability. The in situ SPIP method could be suitable for investigation of permeability.
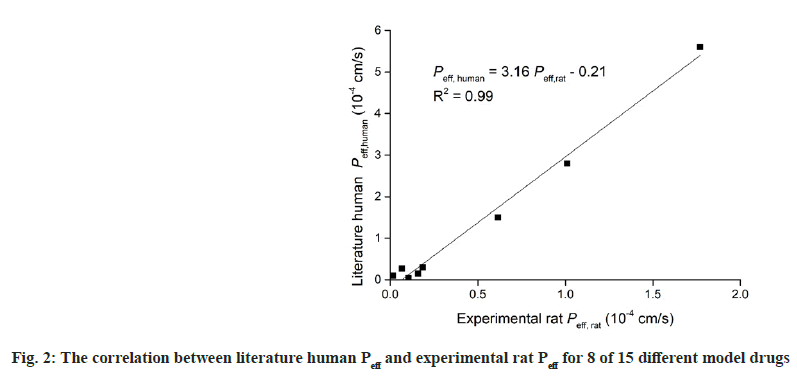
Table 2 summarizes the permeability values for 8 different model drugs obtained by in-situ SPIP and literature human Peff data[19-22]. The correlation between the two sets of data, the in situ SPIP vs. human Peff value, was presented in fig. 2. It can be seen that an excellent correlation was obtained between the experimental rat Peff and literature human Peff, as evident by correlation coefficient (R2) of 0.99, which indicating the validity of this work. These results indicated that in situ SPIP method could be used for investigation of the intestinal absorption of new compounds, and for predicting the absorption fraction in human, which is important in early drug development.
| Drug | Peff (10-4 cm/s) | |
|---|---|---|
| Human, literature | Rat, experimental | |
| Antipyrine | 5.6±1.6 | 1.77±0.34 |
| Propranolol | 2.8±1.3 | 1.01±0.05 |
| Metoprolol | 1.5±0.9 | 0.613±0.249 |
| Terbutaline | 0.3±0.3 | 0.185±0.014 |
| Atenolol | 0.15±0.2 | 0.159±0.117 |
| Hydrochlorothiazide | 0.04±0.05 | 0.140±0.041 |
| Ranitidine | 0.273±0.247 | 0.0668±0.0147 |
| Enalaprilat | 0.1±0.3 | 0.0146±0.0026 |
Table 2: Permeability Values for 8 of 15 Different Model Drugs Obtained by In Situ SPIP and Literature Human Peff data. Data presented as Mean±SD
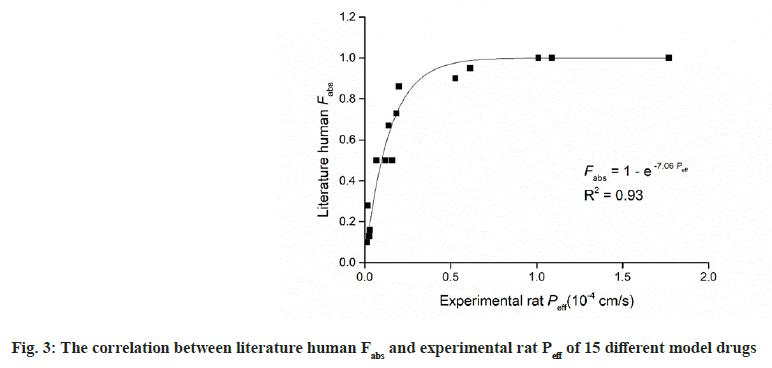
A strong correlation was observed between experimental rat Peff data and literature human Fabs (R2=0.93). Fig. 3 fitting to the follow equation;
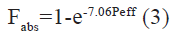
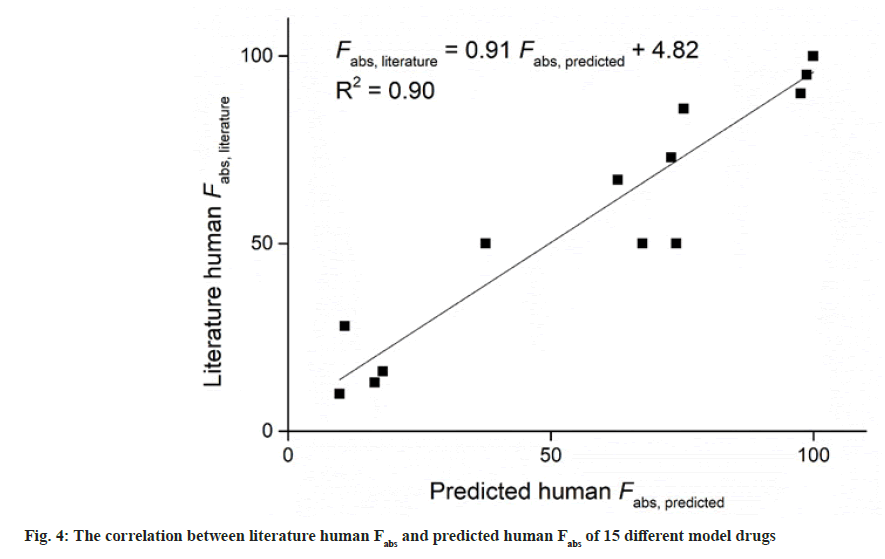
Table 3 summarizes the literature human Fabs data, and the corresponding predicted Fabs by the experimental model using above-mentioned equation, and BCS permeability class[19,23]. According to FDA, when there is no evidence to suggest the instability in gastrointestinal tract, drugs can be considered as high permeability drug when the fraction absorbed in human gastrointestinal tract is investigated to be 85 % or more[2]. This means that drugs with Fabs higher than 85 % can be considered as high permeability drug. As shown in Table 3, Fabs of 5 different model drugs obtained by equation (3) were higher than 85 %, indicating a high correlation of absorption fraction between this work and literature. It can be seen in Table 3 that the Fabs of metformin was 86 %, which was very nearly to the boundary between high permeability and moderate permeability. It was classed as moderate permeability by FDA. Similar situation have occurred in ranitidine, which literature Fabs was 50 %, nearly to the boundary between moderate permeability and low permeability. Similar finding also have been found in other literature[6]. In this work, the predicted Fabs of metformin and ranitidine using above-mentioned equation was 75 % and 38 %, respectively. When drugs with Fabs nearly to the boundary between different permeability classification, the application of the in situ SPIP model may not distinguish them well, which may be a limitation of the model. In this study, all the investigated model drugs by in situ SPIP have similar permeability classification compare with literature permeability classification. The predicted human Fabs by in situ SPIP model were linearly correlated with literature human Fabs (R2=0.90) (fig. 4).
| Drug | Literature human data | Predicted data | ||
|---|---|---|---|---|
| Fabs (%) | Permeability class | Fabs (%) | Permeability class | |
| Antipyrine | 100 | H | 100 | H |
| Ketoprofen | 100 | H | 100 | H |
| Propranolol | 100 | H | 100 | H |
| Metoprolol | 95 | H | 99 | H |
| Phenytoin | 90 | H | 98 | H |
| Metformin | 86 | M | 75 | M |
| Amiloride | 50 | M | 74 | M |
| Terbutaline | 73 | M | 73 | M |
| Atenolol | 50 | M | 67 | M |
| Hydrochlorothiazide | 67 | M | 63 | M |
| Ranitidine | 50 | M | 38 | M |
| Aciclovir | 16 | L | 18 | L |
| Chlorothiazide | 13 | L | 16 | L |
| Lisinopril | 28 | L | 11 | L |
| Enalaprilat | 10 | L | 10 | L |
Note: H: High; M: Moderate and L: Low
Table 3: Literature Human Fabs Data and Corresponding Predicted Fabs and BCS Permeability Class of 15 Different Model Drugs
In this work, we investigated the Peff of 15 model drugs with different permeability classification by in situ SPIP method. The Peff of high permeability drugs were higher than 0.2×10-4 cm/s and Peff of low permeability drugs were less than 0.03×10-4 cm/s. The Peff values obtained in this work showed a high correlation with those in human, and a strong correlation was observed between human Fabs and experimental rat Peff. Besides, the literature human Fabs and predicted human Fabs was strongly correlated. The results indicating that in situ SPIP method could be used for investigation of the intestinal absorption of new compounds and for prediction the absorption fraction in human, which is crucial in drug development.
Acknowledgements:
This study was financially supported by the Association Fund Project of the Science and Technology Department of Bijie Prefecture (No. [2023] 61).
Conflict of interests:
The authors declare that they have no competing interest.
References
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 1995;12:413-20.
[Crossref] [Google Scholar] [PubMed]
- Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system guidance for industry. Food and Drug Administration. 2017.
- Morais JA, Lobato MD. The new European Medicines Agency guideline on the investigation of bioequivalence. Basic Clin Pharmacol Toxicol 2010;106(3):221-5.
[Crossref] [Google Scholar] [PubMed]
- Fagerholm U, Johansson M, Lennernäs H. Comparison between permeability coefficients in rat and human jejunum. Pharm Res 1996;13:1336-42.
[Crossref] [Google Scholar] [PubMed]
- Kim JS, Mitchell S, Kijek P, Tsume Y, Hilfinger J, Amidon GL. The suitability of an in situ perfusion model for permeability determinations: Utility for BCS class I biowaiver requests. Mol Pharm 2006;3(6):686-94.
[Crossref] [Google Scholar] [PubMed]
- Lozoya-Agullo I, Zur M, Wolk O, Beig A, González-Álvarez I, González-Álvarez M, et al. In situ intestinal rat perfusions for human Fabs prediction and BCS permeability class determination: Investigation of the single-pass vs. the Doluisio experimental approaches. Int J Pharm 2015;480(1-2):1-7.
[Crossref] [Google Scholar] [PubMed]
- Kuang G, Yi H, Zhu M, Zhou J, Shang X, Zhao Z, et al. Study of absorption characteristics of the total saponins from Radix Ilicis Pubescentis in an in situ single-pass intestinal perfusion (SPIP) rat model by using ultra performance liquid chromatography (UPLC). Molecules 2017;22(11):1867-84.
[Crossref] [Google Scholar] [PubMed]
- Jagabalan JY, Murugaiyah V, Zainal H, Mansor SM, Ramanathan S. Intestinal permeability of mitragynine in rats using in situ absorption model. J Asian Nat Prod Res 2019;21(4):351-63.
[Crossref] [Google Scholar] [PubMed]
- Al Shaker HA, Qinna NA, Badr M, Al Omari MM, Idkaidek N, Matalka KZ, et al. Glucosamine modulates propranolol pharmacokinetics via intestinal permeability in rats. Eur J Pharm Sci 2017;105:137-43.
[Crossref] [Google Scholar] [PubMed]
- Patel JR, Barve KH. Intestinal permeability of lamivudine using single pass intestinal perfusion. Indian J Pharm Sci 2012;74(5):478-81.
[Crossref] [Google Scholar] [PubMed]
- Athukuri BL, Neerati P. Enhanced oral bioavailability of domperidone with piperine in male wistar rats: Involvement of CYP3A1 and P-gp inhibition. J Pharm Pharm Sci 2017;20:28-37.
[Crossref] [Google Scholar] [PubMed]
- Sirisha K, Achaiah G, Prasad N, Bhasker S, Umachander L, Reddy VM. Multidrug resistance reversal activity of some new dihydropyridines studied by in situ single-pass intestinal perfusion (SPIP) method in rat. Pharm Chem J 2018;52:8-14.
- Wu L, Bi Y, Wu H. Formulation optimization and the absorption mechanisms of nanoemulsion in improving baicalin oral exposure. Drug Dev Ind Pharm 2018;44(2):266-75.
[Crossref] [Google Scholar] [PubMed]
- Sutton SC, Rinaldi MT, Vukovinsky KE. Comparison of the gravimetric, phenol red, and 14C-PEG-3350 methods to determine water absorption in the rat single-pass intestinal perfusion model. Aaps Pharmsci 2001;3:93-7.
[Crossref] [Google Scholar] [PubMed]
- Reis JM, Dezani AB, Pereira TM, Avdeef A, Serra CH. Lamivudine permeability study: A comparison between PAMPA, ex vivo and in situ Single-Pass Intestinal Perfusion (SPIP) in rat jejunum. Eur J Pharm Sci 2013;48(4-5):781-9.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Zhang B, Liu M, Zhang X, Shi D, Guo L, et al. Further study of influence of Panax notoginseng on intestinal absorption characteristics of triptolide and tripterine in rats with Tripterygium wilfordii. Pharmacogn Mag 2018;14(53):95-102.
[Crossref] [Google Scholar] [PubMed]
- Ates M, Kaynak MS, Sahin S. Effect of permeability enhancers on paracellular permeability of acyclovir. J Pharm Pharmacol 2016;68(6):781-90.
[Crossref] [Google Scholar] [PubMed]
- Cui SM, Zhao CS, He ZG. Study on absorption mechanism of puerarin using rat everted gut sac. Lishizhen Med Mater Med Res 2008;19:1715-6.
- Salphati L, Childers K, Pan L, Tsutsui K, Takahashi L. Evaluation of a single-pass intestinal-perfusion method in rat for the prediction of absorption in man. J Pharm Pharmacol 2001;53(7):1007-13.
[Crossref] [Google Scholar] [PubMed]
- Lennernas H, Nylander S, Ungell AL. Jejunal permeability: A comparison between the ussing chamber technique and the single-pass perfusion in humans. Pharm Res 1997;14(5):667-71.
[Crossref] [Google Scholar] [PubMed]
- Winiwarter S, Bonham NM, Ax F, Hallberg A, Lennernäs H, Karlén A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J Med Chem 1998;41(25):4939-49.
[Crossref] [Google Scholar] [PubMed]
- Takamatsu N, Kim ON, Welage LS, Idkaidek NM, Hayashi Y, Barnett J, et al. Human jejunal permeability of two polar drugs: Cimetidine and ranitidine. Pharm Res 2001;18:742-4.
[Crossref] [Google Scholar] [PubMed]
- Skolnik S, Lin X, Wang J, Chen XH, He T, Zhang B. Towards prediction of in vivo intestinal absorption using a 96-well Caco-2 assay. J Pharm Sci 2010;99(7):3246-65.
[Crossref] [Google Scholar] [PubMed]