- *Corresponding Author:
- K. K. Sabu Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Pacha-Palode (Research Centre of University of Kerala), Thiruvananthapuram, Kerala 695562, India E-mail: sabu@jntbgri.res.in
| Date of Received | 29 October 2022 |
| Date of Revision | 03 May 2023 |
| Date of Acceptance | 12 March 2024 |
| Indian J Pharm Sci 2024;86(2):476-483 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The development of angiogenesis plays an essential role in the growth and survival of endometriosis. The aim was to determine the in vitro anti-angiogenic effect of Saraca asoca, Glycyrrhiza glabra, and Curcuma longa ethanolic extract in chick embryos by emphasizing its total phenolic and flavonoid contents in the sample. It was determined by Folin-Ciocalteu and aluminum chloride methods respectively. Anti-angiogenesis assay was done in chorioallantoic membrane. The high amount of total phenol and flavonoid was obtained from all three plant extracts and may be responsible for the bioactivity of the crude extract. Saraca asoca exhibits high phenolic content and anti-angiogenic activity followed by Curcuma longa with rich flavonoid and Glycyrrhiza glabra showed moderate activity for all the assays. A significant anti-angiogenic effect was observed for Saraca asoca (73.65+0.021) % and Curcuma longa (67.65+0.024) % than reference drug dienogest (62.42+0.02) % in the study. Search for new therapeutic agents with anti-angiogenic potential rich in plant secondary metabolites like phenols and flavonoids are need of the hour. The results successfully reveal that the abovementioned plants used in traditional medicines could be used for treating angiogenesis-related diseases like endometriosis.
Keywords
Total phenol, total flavonoid, chorioallantoic membrane assay, gynecological disorder, Drabkin’s reagent
Endometriosis is a gynecological disease characterized by the formation of endometrial glands and stroma seen in abnormal locations mainly outside the uterine cavity[1]. One of the key features in the initiation and progression of this disease condition is the promotion of angiogenesis resulting in inflammatory responses in the peritoneal cavity finally leading to the formation of endometriosis. Endometriotic lesions need a sufficient blood supply to be viable in their ectopic locations[2]. Some of the common characteristics of this condition can be related to cancer, including the invasion of tissues with an uncontrolled growth process of angiogenesis and its ability to avoid apoptosis[3]. The use of bioactive compounds in medicinal plants could be a promising strategy for the treatment of this disease[4].
Plant extracts with phytoconstituents such as flavonoids and phenolic compounds have proven their beneficial effects by antioxidant, anti- inflammatory and pro-apoptotic functions in endometriosis disease management[5]. As the current treatment method is limited and could produce many side effects[6], plant-based medicines could be an alternative treatment option with low cost[7]. Considering the complex pathogenesis of this disease, plants with anti-angiogenic, anti- inflammatory, anti-proliferative and antioxidant properties have been identified as promising adjuncts with better therapeutic effects. Saraca asoca (S. asoca), Glycyrrhiza glabra (G. glabra) and Curcuma longa (C. longa) are medicinal plants that are well-mentioned in Indian classical books like Bhaishajya Ratnavali and Ashtanga Hridaya emphasizing Ayurvedic formulations in treating endometriosis.
S. asoca is well known for its use in treating various gynecological disorders like menorrhagia in traditional systems of medicine. The bark of this plant is used in several Ayurvedic preparations like Asokarishta, Asoka Gritha, etc., and is considered a uterine tonic[8,9]. Phytochemical investigation of the bark revealed the presence of catechol, sterol, tannins, flavonoids and glycosides as the major bioactive compounds which are responsible for its beneficial effects like anti-estrogenic[10], anti-progestational, and anti-hemorrhagic activities[11]. G. glabra is being used as a medicinal remedy for gastritis issues, inflammatory disorders and skin-related problems[12]. The main active ingredient of this plant is glycyrrhizin. Flavonoids from the root have been investigated to have antioxidant, antitumor, anti-inflammatory and antiangiogenic activities[13]. C. longa (turmeric) contains hydrophobic polyphenol curcumin as the major bioactive compound and possesses a wide range of pharmaceutical activities such as anti-proliferative, anti-inflammatory, antioxidant and growth-suppressive properties[14]. Considering the medicinal importance of these plants, the present study was designed to understand the role of these plant extracts in inhibiting angiogenesis.
Materials and Methods
Plant materials:
The bark of S. asoca (Fabaceae) and rhizome of C. longa (Zingiberaceae) were obtained from the Ayurvedic Research Centre (ARI), Poojappura, Kerala and roots of G. glabra (Fabaceae) from Foundation for Revitalisation of Local Health Traditions (FRLHT), Bangalore respectively. All the plant materials were washed thoroughly with distilled water for removing dust particles. All these plant materials were oven dried at 40° for 1 w and then powdered using a mixer grinder and stored in the dark at room temperature for experiments.
Preparation of plant extract:
Powdered plant materials of 24 g were used for extraction by the Soxhlet method in 250 ml of ethanol and concentrated under reduced pressure with rotavapor (Buchi Labor Technik AG, Flawil, Switzerland) R-210 set at 40°. All crude extracts obtained were stored at 4° until use.
Total phenol:
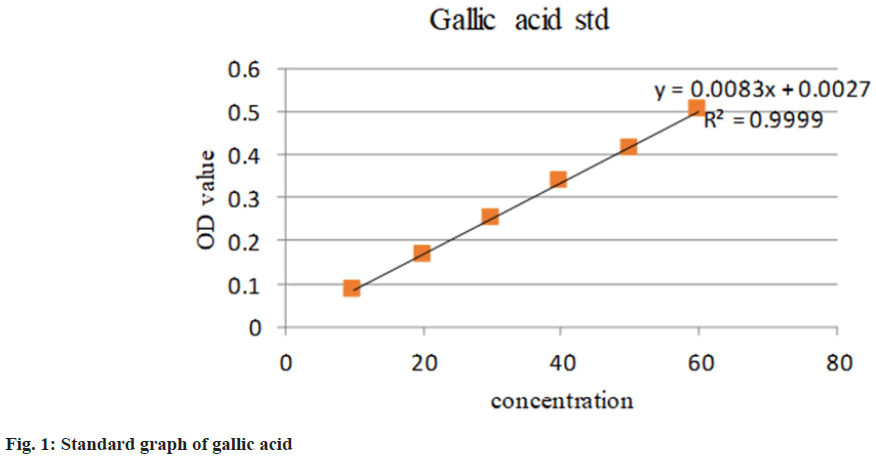
Folin-Ciocalteu (FC) method with minor modifications was used to determine the Total Phenolic Content (TPC) of different extracts using gallic acid as standard[15]. The FC reagent was mixed with test extracts, mixed thoroughly and incubated in the dark for 5 min. 20 % sodium carbonate solution was added to the solution and incubated for 30 min at room temperature. The absorbance was measured at 760 nm using a 1700 Shimadzu Ultraviolet (UV)-visible spectrophotometer. The calibration curve was prepared by employing gallic acid at a concentration of 10-60 µg/ml. The TPC was determined by using a linear regression equation from the standard gallic acid graph plot and was expressed as Gallic Acid Equivalent (GAE) per gram of the sample. The average of the triplicates was analyzed.
Total flavonoid:
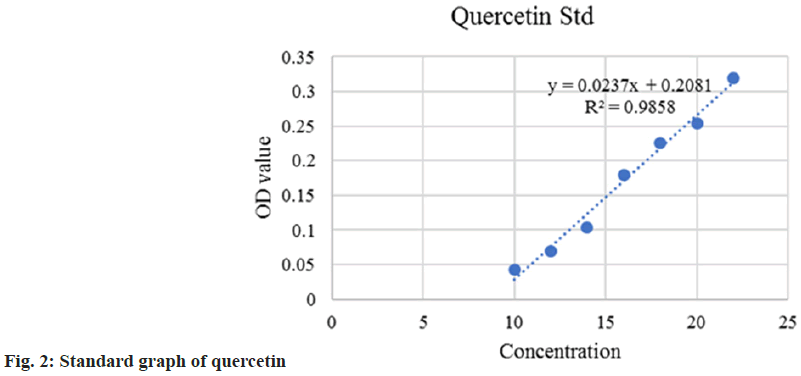
Aluminum chloride (AlCl3) method was used to determine the Total Flavonoid Content (TFC) using quercetin as standard[16,17]. Briefly, the test extract was mixed with 0.1 ml AlCl3 and 0.1 ml Potassium acetate (CH3CO2K), incubate at 30° for 10 min. The absorbance was measured at 415 nm and TFC was expressed as quercetin per gram (Quality Assurance Evaluator (QAE)) of the sample. The average of triplicates was analyzed.
Chorio-Allantoic Membrane (CAM) assay:
The chicken CAM is a highly vascularized embryonic membrane that offers clear advantages to studying vascular functions. The anti-angiogenic activity was evaluated using the CAM model according to the previously reported method with minor modifications[18]. Fertilized eggs were purchased from Kerala State Poultry Development Corporation (KEPCO), Kerala. The development of the embryo was ensured with the help of an egg candler. Eggs were carefully surface sterilized with 70 % isopropanol and incubated at 37° with 80 % relative humidity. After then the eggs were randomly divided to carry out the experiment. Positive and negative controls used were Dienogest (50 µg) and Phosphate-Buffered Saline (PBS) respectively. S. asoca, G. glabra, and C. longa ethanolic extracts (250 µg) were the test groups.
Drug administration:
On the 5th d of incubation, control and test extracts of 20 µl were inoculated into the CAM of fertilized eggs and sealed with parafilm. Tilted the egg gently and all eggs were kept in a vertical position with air sack upwards and were incubated not touching the eggshell.
Drabkin’s reagent test:
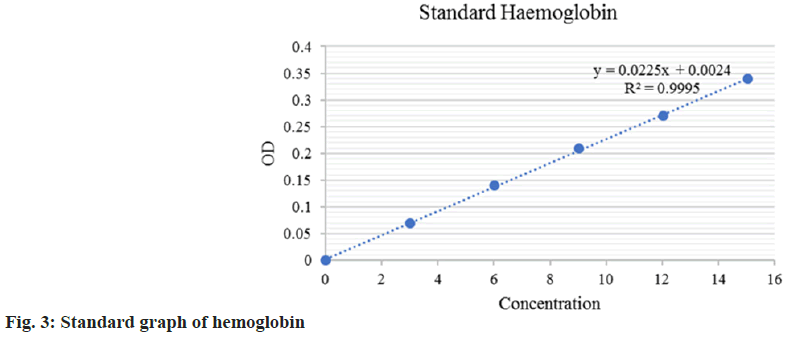
Determination of Hemoglobin (Hb) in the CAM was performed as explained by Drabkin and Austin with slight modification[19]. On the 12th d of incubation, the eggs were broken gently from the side of the air sac and the inner content was removed without disturbing the membrane. The CAM was scrapped and dispersed carefully into a tube and centrifuged at 3000 rpm for 5 min. 20 µl of supernatant was added to 5 ml of Drabkin’s reagent. The reaction was incubated at room temperature (37°) for 5 min. Hb level was measured at 546 nm in a spectrophotometer. The level of Hb in the sample is compared to a standard and results are expressed as anti-angiogenesis percentage. All the tests were performed in triplicates to ensure the reproducibility of the result.
The percentage of inhibition of angiogenesis was calculated by the following equation:
% Anti-angiogenisis=Optical Density (OD)/OD sample×100
Results and Discussion
Being common ingredients in many Ayurvedic medicines, S. asoca, G. glabra, and C. longa have already demonstrated potent health benefits to humans. All the extracts were rich in total phenolic and flavonoid compounds. TPC and TFC methods are simple, inexpensive and quick in estimation. The content of phenol was expressed in terms of GAE (standard curve equation: Y=0.0083X–0.0027, R2= 0.9999) (fig. 1). It was observed from the analysis that the bark of S. asoca showed the highest phenolic content mg GAE/g of 483.5+0.001, G. glabra has 336+0.016 and C. longa has 164.14+0.002 in the sample (Table 1). The result of the present investigation indicates that flavonoid content was abundantly seen in C. longa when compared to G. glabra, and S. asoca. The content of flavonoid was expressed in terms of standard quercetin (Y=0.0237X–0.2081, R2=0.9895) (fig. 2). Rhizome of C. longa exhibited the highest flavonoid content of (873+0.0186) mg GAE/g than roots of G. glabra 106.56+0.0267 and bark of S. asoca 49.81+0.028 equivalence (Table 1). All data are presented as mean+Standard Deviation (SD).
| TPC (mg GAE/g) | TFC (mg QE/g) | |
|---|---|---|
| S. asoca | 483.5±0.001 | 873±0.0186 |
| G. glabra | 36±0.016 | 106.56±0.0267 |
| C. longa | 164.14±0.002 | 49.81±0.028 |
Table 1: Estimation of Phenol and Flavonoid
The rich number of natural flavonoids and phenol contents in plants have positive effects on human health as antibacterial, antiviral and anti-inflammatory agents[20]. They may differ in concentration during their growth stages and are considered to have a very vital role in disease management. The potential benefits of these secondary metabolites derived from medicinal plants may act through multiple cell signaling pathways and may exert their potential effects by healing diseased cells without affecting normal cells[21]. S. asoca bark extract is a rich source of many polyphenols such as catechin groups and the estimation of total phenol and flavonoids are also in agreement with the previous reports demonstrating the plant with a variety of pharmacological properties[22]. Epicatechin is one of the primary components of this plant. Since-epicatechin strongly inhibits the Na+/H+ exchanger, it has been hypothesized that this can alter the fluidity of the cytosolic plasma membrane and inhibits the proliferation of cancer cells[23]. It was also reported that licorice also exhibits anti- inflammatory, antiviral, gastro protective and strong estrogen-like activities due to its wide range of flavonoid and phenolic content in the plant[24]. Significant flavonoid and phenolic content in C. longa also result in different medicinal properties like anti-bacterial, anti-inflammatory, anti- carcinogenic and anti-proliferative properties[25]. Although there is little evidence to support the use of estrogen-progestins for endometriosis, the European Society of Human Reproduction and Embryology (ESHRE) guidelines on endometriosis also noted that combined hormonal contraception is frequently used as a treatment for pain associated with endometriosis[26]. Therefore, the impact of these plant extracts with reported Selective Estrogen Receptor Modulator (SERM) activity on the treatment of endometriosis can also be thoroughly investigated.
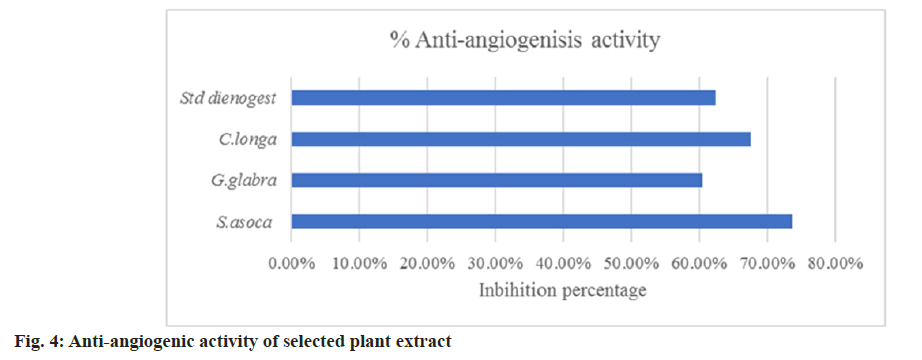
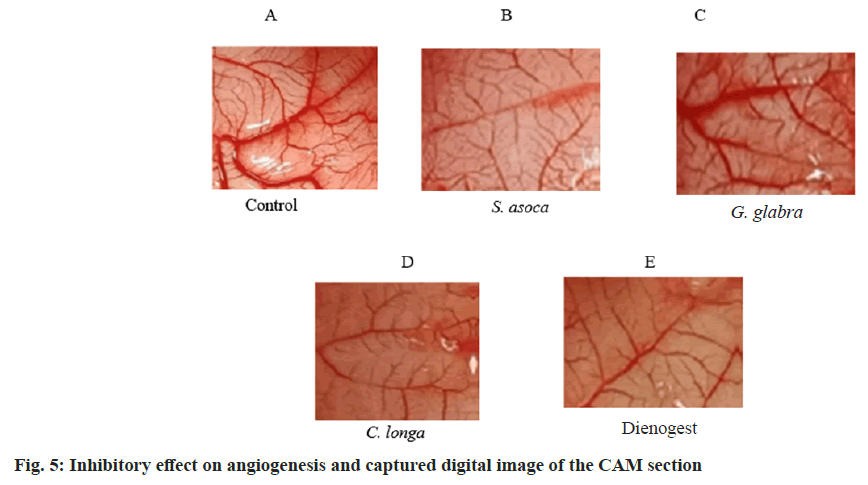
The chick embryo CAM model was used to assess anti-angiogenesis activity. In this study, we have employed the same reliable method to study the anti-angiogenic effect of the selected plant extracts. The fertilized eggs were treated with ethanolic extract of S. asoca, G. glabra, and C. longa. The anti-angiogenic potentials of the extract were evaluated on the 12th d of treatment. The in vitro quantitative determination of Hb in blood was expressed based on the cyanmethemoglobin method. The intensity of the color is proportional to the Hb concentration and is compared to known cyanmethemoglobin standard equivalence at 546 nm (standard curve equation: Y=0.0225X–0.0024, R2=0.9995) (fig. 3). From the graph, the g per decilitre (dl) concentration of control and test extracts was calculated. It is clear and evident that all the extracts show effective anti-angiogenic activity in CAM assay with values (Hb/dl) of 2 for S. asoca, 3.07 for G. glabra, 2.49 for C. longa and extract combination shows 2.7 with respect to control of 7.92. The standard drug Dienogest shows an effect of 2.91 in the assay. The inhibitory potential of extracts was expressed as a percentage anti-angiogenesis. The study reveals that S. asoca showed the highest inhibition percentage of 73.65+0.021 followed by C. longa with 67.65+0.024 and G. glabra with 60.43+0.04 in the sample (Table 2 and fig. 4). The ethanolic extracts inhibited the growth of blood vessels on CAM were photographed and can be seen in fig. 5A-fig. 5E.
| Drugs | Dose µg/ml | Tested eggs | % Anti-angiogenesis |
|---|---|---|---|
| PBS (Negative control) | - | 3 | - |
| S. asoca | 250 | 3 | (73.65±0.021) % |
| G. glabra | 250 | 3 | (60.43±0.04) % |
| C. longa | 250 | 3 | (67.65±0.024) % |
| Dienogest | 50 | 3 | (62.42±0.02) % |
Table 2: Anti-Angiogenic Effect of Extracts
Angiogenesis can be defined as the formation of new blood vessels from pre-existing vascular tissue[27]. Onishi et al.[28] have reported that the pathogenesis of endometriotic lesions includes major steps like blood vessel breakdown, membrane degradation, surrounding extracellular matrix and new blood vessel formation leading to angiogenesis. In these aspects, the long-term survival and proliferation of endometriotic lesions are critically reliant upon adequate blood supply via angiogenesis[29]. The goal of an anti-angiogenic therapy strategy for endometriosis illness is to successfully induce conception in infertile individuals while simultaneously relieving pelvic pain. Endometriotic lesions have the ability to create cytokines and growth factors that regulates their vascularization and proliferation[30]. It was also reported that the neovascularization of endometriotic lesions is significantly aided by the dominant Interleukin (IL)-1 released by active peritoneal macrophages, IL-1[31]. Estradiol promotes endometrial Vascular Endothelial Cell Growth Factor (VEGF) production, and its levels are associated with neovascularization and increased vascular permeability during the late proliferative phase[32]. As endometriosis condition is heavily reliant on estrogen, hormonal therapies aim to reduce endogenous ovarian estrogen production.
The CAM method provides a simple and rapid evaluation of the anti-angiogenic effect of the extracts in well-developed vascular tissue[33]. Paradkar et al.[21] have described it as an efficient and widely used model for determining anti or pro-angiogenic activity in a sample. CAM assay model is an informative method used to study the process of endometriotic lesion formation in order to show that a viable endometrium is necessary to form an endometriotic lesion and the results indicate that S. asoca and C. longa extracts have strong anti-angiogenic activity than positive control dienogest, a potent drug currently used in endometriosis treatment. Thus, the studies are promising and clinically relevant for the therapy. The relevant amount of flavonoids and phenolic compounds in all the studied plants may be responsible for the bioactivity of the crude extracts and serve as an inhibitory agent for angiogenesis. Since the results show a proven anti-angiogenic effect in CAM, these extracts emphasize the beneficial use of conventional drugs in endometriosis therapy. Further research on the mechanism of action of the extracts has to be worked out in future studies to substantiate these findings on the basis of lower concentrations. The involvement of angiogenesis in preventing the growth of endometriosis has been indicated by several researches. However, many of them only provide circumstantial evidence, frequently based on the downregulation of anti-inflammatory factors like VEGF levels or Matrix Metalloproteinase-9 (MMP-9) activity[34]. According to preclinical investigations, the antiangiogenic treatment may have the largest initial impact on micro metastases and in general, smaller dosages of an angiogenesis inhibitor are needed to prevent neovascularization of microscopic metastases than to regress a primary tumour[35].
Our present study reveals that medicinal plants like S. asoca, G. glabra and C. longa with their major phenolic and flavonoid compounds may have a significant role in suppressing angiogenesis. It may be presumed that the collective contribution of phenol and flavonoid compounds in the extract provides anti-angiogenic properties. The study highlighted the activity of crude extract through CAM assay. This technique was well accepted and does not need so much of financial and technical support to perform. Although many aspects are relevant to the pathophysiologic process of endometriosis, it is clear that angiogenesis is necessary for the development and maintenance of ectopic implants in the tissue. We have investigated the antiangiogenic effect through a simple method and the analyzed plant extracts seem to be a promising therapeutic source for endometriosis treatment. Further, in vivo studies and gene expression studies are needed to investigate the role of these phyto metabolites for their molecular level of action.
Acknowledgement:
We are gratefully acknowledging the facilities provided by JNTBGRI, Palode and CRDC, HLL Lifecare Limited, Trivandrum for supporting the study. This work was financially supported by ICMR for an SRF fellowship to Reshmi Nair.
Conflict of interest:
The authors declared no conflict of interests.
References
- Vallée A, Lecarpentier Y. Curcumin and endometriosis. Int J Mol Sci 2020;21(7):2440.
[Crossref] [Google Scholar] [PubMed]
- Lagana AS, Vitale SG, Granese R, Palmara V, Ban Frangez H, Vrtacnik-Bokal E, et al. Clinical dynamics of Dienogest for the treatment of endometriosis: From bench to bedside. Expert Opin Drug Metab Toxicol 2017;13(6):593-6.
[Crossref] [Google Scholar] [PubMed]
- Swiersz LM. Role of endometriosis in cancer and tumor development. Ann New York Acad Sci 2002;955(1):281-92.
[Crossref] [Google Scholar] [PubMed]
- Balan A, Moga MA, Dima L, Dinu CG, Martinescu CC, Panait DE, et al. An overview on the conservative management of endometriosis from a naturopathic perspective: Phytochemicals and medicinal plants. Plants 2021;10(3):587.
[Crossref] [Google Scholar] [PubMed]
- Della Corte L, Noventa M, Ciebiera M, Magliarditi M, Sleiman Z, Karaman E, et al. Phytotherapy in endometriosis: An up-to-date review. J Complement Integr Med 2020;17(3):20190084.
[Crossref] [Google Scholar] [PubMed]
- Becker CM, Gattrell WT, Gude K, Singh SS. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil Steril 2017;108(1):125-36.
[Crossref] [Google Scholar] [PubMed]
- Ashrafizaveh A, Sabouri Fard H, Azmoudeh E. Application of medicinal plants, acupuncture, massage therapy and transcutaneous electric nerve stimulation in treatment of endometriosis: Review study. Iran J Obstetr Gynecol Infertil 2019;22(5):90-100.
- Verma AB, Saroj A, Gautam B, Dubey C, Tripathi S. Review on ethnobotanical importance of Saraca indica. IJPRS 2014;3:313-21.
- Balekundri A, Mannur V. Quality control of the traditional herbs and herbal products: A review. Future J Pharm Sci 2020;6:1-9.
- Yadav NK, Saini KS, Hossain Z, Omer A, Sharma C, Gayen JR, et al. Saraca indica bark extract shows in vitro antioxidant, antibreast cancer activity and does not exhibit toxicological effects. Oxid Med Cell Longev 2015;2015:205360.
[Crossref] [Google Scholar] [PubMed]
- Shahid AP, Salini S, Sasidharan N, Padikkala J, Raghavamenon AC, Babu TD. Effect of Saraca asoca (Asoka) on estradiol-induced keratinizing metaplasia in rat uterus. J Basic Clin Physiol Pharmacol 2015;26(5):509-15.
[Crossref] [Google Scholar] [PubMed]
- Sheela ML, Ramakrishna MK, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol 2006;6(3):494-8.
[Crossref] [Google Scholar] [PubMed]
- Nagaraj SR, Lingaraj SM, Balaraju Y, Kumar A, Salimath BP. MTA1 induced angiogenesis, migration and tumor growth is inhibited by Glycyrrhiza glabra. IOSR J Pharm 2012;2:34-43.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharm 2007;4(6):807-18.
[Crossref] [Google Scholar] [PubMed]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. InMethods in enzymology 1999;299:152-78.
- Cedric SO, Louis-Clement OE, Joseph-Privat O, Cheikna Z, Edouard NE, Alfred TS. Ethnotherapy study, phytochemical screening and antioxidant activity of Antrocaryon klaineanum Pierre and Anthocleista nobilis G. Don. Med Plants Gabon. Int J Adv Res 2015;3(5):812-9.
- Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol 2000;72(1-2):35-42.
[Crossref] [Google Scholar] [PubMed]
- Ngoua-Meye-Misso RL, Ndong JD, Sima-Obiang C, Ondo JP, Ndong-Atome GR, Ovono Abessolo F, et al. Phytochemical studies, antiangiogenic, anti-inflammatory and antioxidant activities of Scyphocephalium ochocoa Warb.(Myristicaceae), medicinal plant from Gabon. Clin Phytosci 2018;4:1-3.
- Drabkin DL, Austin JH. Spectrophotometric studies: II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem 1935;112(1):51-65.
- Yuliani S, Mustofa, Partadiredja G. The neuroprotective effects of an ethanolic turmeric (Curcuma longa L.) extract against trimethyltin-induced oxidative stress in rats. Nutr Neurosci 2019;22(11):797-804.
[Crossref] [Google Scholar] [PubMed]
- Paradkar P, Dandekar S, Joshi J, Amonkar A, Vaidya A. Assessment of in vitro-in vivo antimigratory and anti-angiogenic activity of Curcuma longa linn., and Tinospora cordifolia willd. extracts in cervical cancer. Int J Pharm Sci Rev Res 2017;42(2):87-93.
- Kuo WL, Huang YL, Wang ST, Ni CL, Shien BJ, Chen CC. Chemical constituents of Trema orientalis. J Chin Med 2007;18(1):27-36.
- Matteucci E, Rizvi SI, Giampietro O. Erythrocyte sodium/hydrogen exchange inhibition by (−) epicatechin. Cell Biol Int 2001;25(8):771-6.
[Crossref] [Google Scholar] [PubMed]
- Velvizhi S, Annapurani S. Estimation of total flavonoid, phenolic content, and free radical scavenging potential of Glycyrrhiza glabra root extract. Asian J Pharm Clin Res 2018;11(4):231-5.
- Sharma M, Thakur P, Saini RV, Kumar R, Torino E. Unveiling antimicrobial and anticancerous behavior of AuNPs and AgNPs moderated by rhizome extracts of Curcuma longa from diverse altitudes of Himalaya. Sci Rep 2020;10(1):10934.
[Crossref] [Google Scholar] [PubMed]
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: Management of women with endometriosis. Hum Reprod 2014;29(3):400-12.
[Crossref] [Google Scholar] [PubMed]
- Dai ZJ, Lu WF, Gao J, Kang HF, Ma YG, Zhang SQ, et al. Anti-angiogenic effect of the total flavonoids in Scutellaria barbata D. Don. BMC Complement Altern Med 2013;13:150.
[Crossref] [Google Scholar] [PubMed]
- Onishi M, Ichikawa T, Kurozumi K, Date I. Angiogenesis and invasion in glioma. Brain Tumor Pathol 2011;28(1):13-24.
[Crossref] [Google Scholar] [PubMed]
- Arablou T, Kolahdouz-Mohammadi R. Curcumin and endometriosis: Review on potential roles and molecular mechanisms. Biomed Pharmacother 2018;97:91-7.
[Crossref] [Google Scholar] [PubMed]
- Lebovic DI, Shifren JL, Ryan IP, Mueller MD, Korn AP, Darney PD, et al. Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum Reprod 2000;15(suppl_3):67-77.
[Crossref] [Google Scholar] [PubMed]
- Lebovic DI, Bentzien F, Chao VA, Garrett EN, Meng YG, Taylor RN. Induction of an angiogenic phenotype in endometriotic stromal cell cultures by interleukin-1β. Mol Hum Reprod 2000;6(3):269-75.
- Charnock-Jones DS, Macpherson AM, Archer DF, Leslie S, Makkink WK, Sharkey AM, et al. The effect of progestins on vascular endothelial growth factor, oestrogen receptor and progesterone receptor immunoreactivity and endothelial cell density in human endometrium. Hum Reprod 2000;15(suppl_3):85-95.
[Crossref] [Google Scholar] [PubMed]
- Kue CS, Tan KY, LaM ML, Lee HB. Chick embryo chorioallantoic membrane (CAM): An alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp Anim 2015;64(2):129-38.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Zhao F, Lin F, Chen J, Huang Y. Lipoxin A 4 inhibits the development of endometriosis in mice: The role of anti-inflammation and anti-angiogenesis. Am J Reprod Immunol 2012;67(6):491-7.
[Crossref] [Google Scholar] [PubMed]
- DeMoraes ED, Fogler WE, Grant D, Wahl ML, Leeper DB, Zrada S, et al. Recombinant human angiostatin (rhA): A phase I clinical trial assessing safety, pharmacokinetics (PK) and pharmacodynamics (PD). Proc Am Soc Clin Oncol 2001;20:3a.