- *Corresponding Author:
- Swapna Thacheril Sukumaran
Department of Botany, University of Kerala, Kariavattom, Thiruvananthapuram-695581, Kerala, India
E-mail: swapnats@yahoo.com
| Date of Received | 08 June 2021 |
| Date of Revision | 31 October 2022 |
| Date of Acceptance | 08 March 2023 |
| Indian J Pharm Sci 2023;85(2):355-360 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Inflammation is a protective mechanism offered by the body’s immune system after an injury, microbial infection, or any other physical or chemical damage. The role of cyclooxygenase and lipoxygenase enzymes in the inflammation process has been well established, and research works are focused on developing plant-based cyclooxygenase and lipoxygenase inhibitors that can provide potential anti-inflammatory drugs with no side effects. Even though there are previous reports suggesting the anti-inflammatory potential of the rhizome of the plant, no reports are available regarding the anti-inflammatory property of the leaves of Anaphyllum wightii. Hence the present study was intended to assess the anti-inflammatory activity of the leaves of Anaphyllum wightii and also to confirm the activity of rhizome in vitro. The anti-inflammatory action of the rhizome and the two morphological variants of leaves (broad and narrow leaves) of Anaphyllum wightii were determined by their inhibitory effects on cyclooxygenase and lipoxygenase enzymes. In the case of the cyclooxygenase inhibition assay, the narrow leaf extract showed the least IC50 value (43.182 µg/ml), whereas the broad leaf extract showed the least IC50 (38.174 µg/ml) for the lipoxygenase enzyme. Thus the present study revealed that the leaf extracts of Anaphyllum wightii exhibit significant anti-inflammatory potential compared to that of the rhizome extract. Hence the leaves may contain promising anti-inflammatory agents that may provide potential drugs for treating chronic inflammatory diseases. Also, this study could detect the presence of Ursolic acid, a potential anti-inflammatory agent, in Anaphyllum wightii through high resolution liquid chromatograph mass spectrometeric and high performance thin layer chromatographic analyses.
Keywords
Anaphyllum wightii, anti-inflammatory, cyclooxygenase, lipoxygenase, ursolic acid
Inflammation is a complex immune-protective response of the body against xenobiotic invasion, chemical, mechanical and thermal injury[1,2]. The usual signs of inflammation include local redness, pain, swelling, heat, and loss of function[3]. The inflammation process is mediated by several molecules that are responsible for the local effects of inflammation, such as migration of leucocytes into the target site, vasodilation, etc. Generally, this complex biological response results in the restoration of homeostasis. But, in some cases, the inflammatory response persists for a long time due to the prolonged release of inflammatory mediators, and this may be harmful to the body[4].
The biosynthesis of arachidonic acid-derived mediators of inflammation is dependent on Cyclooxygenase (COX) and Lipoxygenase (LOX) pathways. These are well-recognized pro- inflammatory enzymes essential for the production of inflammatory mediators such as leukotrienes, prostaglandins, and thromboxane from arachidonic acid[5]. Arachidonic acid is produced by the action of the enzyme phospholipase A2 on membrane phospholipids (linoleic acid)[6]. COX (Prostaglandin- endoperoxide synthase, EC 1.14.99.1) catalyze the biosynthesis of prostaglandins and thromboxanes from arachidonic acid, whereas 5- LOX (Arachidonate 5-LOX, EC 1.13.11.34) lead to the formation of leukotrienes using the substrate arachidonic acid[5]. Hence blocking of COX or LOX enzyme will reduce or inhibit the production of these inflammatory mediators that, in turn, will delay or stop the onset of an inflammatory response.
There are two major classes of anti-inflammatory drugs, namely glucocorticoids and Non-Steroidal Anti-Inflammatory Drugs (NSAIDS). Glucocorticoids possess an inhibitory action on prostaglandins and proteins involved in the inflammation process[7]. Non-steroidal drugs are most commonly used that act by inhibiting the enzyme COX and thereby affecting the production of prostaglandins and leukotrienes. However, the non-steroidal anti-inflammatory drugs possess several harmful side effects, especially related to the gastrointestinal tract and the renal system[8]. Hence researchers are focused on screening novel anti-inflammatory agents from natural sources, especially of plant origin, with no side effects.
Anaphyllum wightii (A. wightii) (Araceae) is an ethnomedicinally significant plant endemic to the Southern Western Ghats and is a threatened species of South India[9,10]. This plant is a tall herb with a rhizomatous stem and pinnately compound leaves.
A. wightii possesses two variations in leaves, either broad or narrow-leaved. The rhizome of this plant is used by tribal people like Kanikkars to treat various skin diseases such as eczema and scabies[11]. The tribals use the rhizome as food and also as an antidote for snake venom[12,13]. The rhizome is reported to possess various pharmacological activities, including antimicrobial, antidiabetic, antioxidant, hepatoprotective, and anti-inflammatory properties[14]. Even though there are previous studies suggesting the anti-inflammatory action of the rhizome, there are no reports regarding the anti- inflammatory property of the leaves of this plant. So the present study was intended to assess the in vitro anti-inflammatory potential of the rhizome as well as the two leaf varieties of the plant.
Also, there are no particular compounds identified as the reason for the anti-inflammatory action of this plant. Hence in this study, an effort was also made to detect the active principles of the plant that may be responsible for its anti-inflammatory action.
Materials and Methods
Plant collection:
The two morphological variants of A. wightii (broad and narrow-leaved) were collected from Ponmudi, Kallar region, Kerala. The collected plant materials were identified by a taxonomic expert from the Department of Botany, University of Kerala, and the plants were established in the department garden. Also, the voucher specimens of the broad and narrow-leaved varieties (Voucher No. KUBH 10249 and KUBH 10461, respectively) were deposited in the herbarium of the Department of Botany, University of Kerala. The leaves of both the broad and narrow varieties were selected for the in vitro anti-inflammatory assays, whereas the rhizome of only the broad-leaved variety was used.
Extraction of Plant material:
About 10 g of the powdered samples (rhizome and two leaf varieties) were used for serial soxhlet extraction with 100 ml each of the five different solvents such as petroleum ether, chloroform, acetone, methanol, and distilled water, respectively, for about 7 h. Then methanolic extracts of the two leaf varieties and both the methanolic, as well as acetone extracts of the rhizome, were selected for the in vitro anti-inflammatory assays since the acetone extract of rhizome showed more bioactivity than the methanolic rhizome extract in our own previous study[15].
In vitro anti-inflammatory studies:
RAW 264.7 cell line was purchased from National Centre For Cell Science, Pune, and was maintained in Dulbecco's modified eagles media (Himedia, India) supplemented with 10 % fetal bovine serum (Himedia, India) and grown to the confluence at 37° at 5 % CO2 in a CO2incubator.
The cells were grown to 60 % confluence, followed by activation with one μl Lipopolysaccharide (LPS) (1 μg/ml). LPS stimulated RAW cells were then exposed to different concentrations of sample solution as well as the standard Diclofenac sodium and incubated for 24 h. After incubation, the cell lysate was used for the anti-inflammatory assays.
COX inhibition assay:
The COX inhibitory potential was evaluated by the method of Walker and Gierse with slight modifications[16]. The cell lysate in Tris-HCl buffer (pH 8) was incubated with glutathione (5 mM/l) and hemoglobin (20 μg/l) for 1 min at 25°. The reaction was initiated by the addition of arachidonic acid (200 mM/l) and terminated after 20 min of incubation at 37°, by adding 10 % trichloroacetic acid in 1 N hydrochloric acid. Then the centrifugal separation was done, followed by the addition of 1 % thiobarbiturate. Finally, the absorbance at 632 nm was recorded, and the COX inhibitory potential was determined.
Percentage inhibition of the enzyme was calculated as, Percentage of inhibition (%)=Absorbance of control− Absorbance of test/Absorbance of control×100
5-LOX inhibition assay:
The 5-LOX inhibitory potential was determined by the method of Axelrod et al.[17]. The reaction mixture (2 ml) contained Tris-HCl buffer (pH 7.4), 50 μl of cell lysate and sodium linoleate (200 μl; 10 mg/ml). The LOX inhibitory activity was determined as the difference in absorbance at 234 nm, which indicates the formation of 5-hydroxyeicosatetraenoic acid from linoleate. Percentage inhibition of the enzyme was calculated using the formula:
Percentage of inhibition (%)=[Absorbance of control−Absorbance of the test]/Absorbance of control×100
High resolution-liquid chromatography-mass spectroscopy analysis:
The broad and narrow leaf methanolic extracts were subjected to High Resolution-Liquid Chromatography-Mass Spectroscopy[18] using Ultra- High Performance Liquid Chromatography (UHPLC)- photodiode array-Detector Mass Spectrophotometer (High Resolution Liquid Chromatograph Mass Spectrometer (HR-LCMS) 1290 Infinity UHPLC System), Agilent Technologies, United States, to detect the anti-inflammatory compounds present in the extracts. The bioactive compounds were identified by their mass spectra and the unique mass fragmentation patterns generated.
High-performance thin-layer chromatography (HPTLC) fingerprinting[19]:
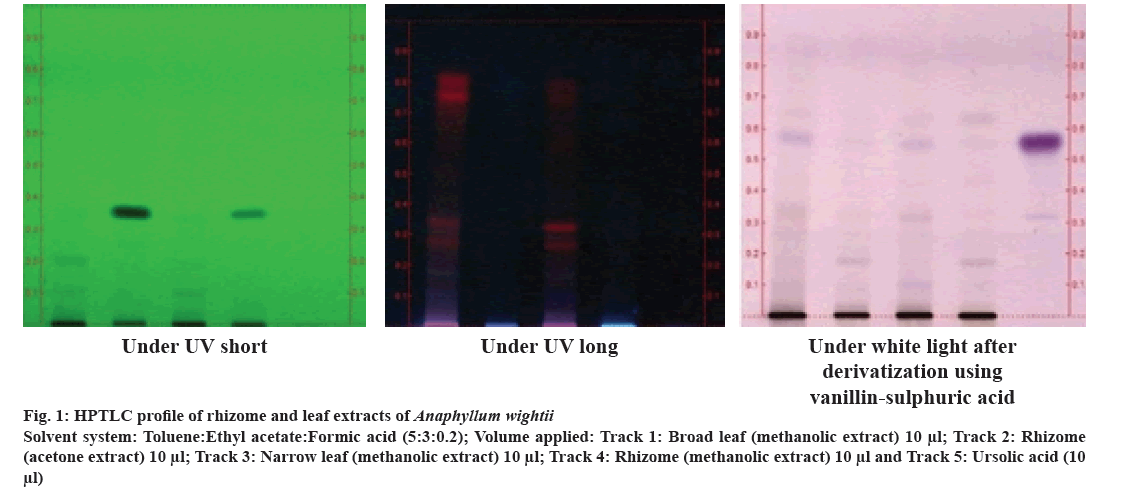
About 10 μl each of the sample extracts (100 μg/μl), as well as the standard (1.0 mg/ml), were applied as bands of width 8 mm on silica gel 60 F254 pre-coated aluminium sheets through CAMAG microliter syringe using Automatic TLC Sampler 4 (ATS4). Then, the plate was vertically introduced in a CAMAG developing chamber (10×10 cm) pre-saturated with the mobile phase. After trying a number of solvent systems, a system that gave the maximum resolution was fixed as the solvent system for the extract. The optimum separations of constituents were obtained by using toluene:ethyl acetate:formic acid (5:3:0.2) as the mobile phase. The chromatogram thus developed was air-dried to evaporate solvents from the plate. Then the plate was kept in CAMAG Visualizer, and the images were captured under Ultraviolet (UV) light at 254 and 366 nm.
Vanillin-sulphuric acid reagent was used as the derivatizing agent for the visualization of colored bands after heating at 105° by placing it on CAMAG TLC plate heater. The plate was then visualized under white light, and the chromatograms were documented.
Statistical analysis:
Statistical analysis was carried out using the software SPSS/PC Version 22 (SPSS Inc., Chicago, IL, USA). Mean values and standard error were calculated by One Way ANOVA, and the means were compared by Duncan’s Multiple Range Test (Duncan 1955), a value of p<0.05 was considered to be significant.
Results and Discussion
Even though inflammation is a protective mechanism of the body against physical, chemical, or mechanical injuries, the persistent inflammation response may lead to several inflammatory-mediated diseases, including cancer[20]. COX and LOX are two key enzymes involved in the inflammatory pathways using arachidonic acid as the essential metabolic precursor. Arachidonic acid is a 20 carbon unsaturated fatty acid usually found in the lipid bilayer membrane in a resting position[21]. The activation of the enzyme known as phospholipase A2 by certain internal or external factors cleaves the membrane-bound arachidonic acid from the phospholipids, and the released arachidonic acid serves as the precursor for inflammatory pathways[22].
The action of COX converts the arachidonic acid into Prostaglandin H2 (PGH2), which further serves as the metabolic substrate for prostaglandin and thromboxane-associated synthases[23] for the production of inflammatory mediators such as prostaglandins and thromboxanes. Prostaglandin E2 (PGE2) is one of the most abundant prostaglandins produced in the body that increases blood flow toward the inflamed tissue through its vasodilatory activity and increases vascular permeability[24]. PGE also induces pain by sensitizing the nerve endings in both the central and peripheral nervous system[25].
COX enzyme has two major isoforms, COX-1 and COX-2. COX-1 is a fundamental enzyme present in most tissues under normal physiological conditions and is responsible for prostaglandin synthesis along with the regulation of platelet activity, gastric and renal functions[26]. Hence continuous usage of selective COX-1 inhibitors for the treatment of certain inflammatory diseases may cause renal or gastrointestinal side effects, hemorrhage, etc[27]. COX-2 is usually present only in lower amounts in the human brain, ovaries and kidneys[28], and its production is increased by external stimuli such as tissue injury leading to inflammation. Hence, selective COX-2 inhibitors reduce the production of prostaglandins and, thereby, inflammation. They also minimize the gastrointestinal side effects associated with COX-1 inhibitors but increase the likelihood of cardiovascular diseases[29].
Table 1 shows the COX enzyme inhibitory potential of the rhizome (acetone and methanolic) and leaf (methanolic) extracts of A. wightii. All four extracts showed dose-dependent inhibitory action in the COX inhibition assay, and among them, the narrow leaf methanolic extract showed the least Half-maximal inhibitory concentration (IC50) value (43.18 μg/ ml) for the COX enzyme. The lower the IC50value, the higher the inhibitory potential of the enzyme. Hence the narrow leaf extract possesses the highest inhibitory action on COX compared to the other three extracts, and the IC50shown was comparable to that of the standard anti-inflammatory drug diclofenac sodium (38.52 μg/ml).
| Concentration (µg/ml) | Percentage of inhibition | ||||
|---|---|---|---|---|---|
| Rhizome | Broad leaf | Narrow leaf | Diclofenac sodium | ||
| Acetone | Methanol | ||||
| 6.25 | 19.86±0.23 | 12.12±0.12 | 12.99±0.34 | 7.09±0.49 | 11.58±0.44 |
| 12.5 | 27.68±0.67 | 27.09±0.39 | 24.66±0.54 | 16.82±0.08 | 29.79±0.65 |
| 25 | 33.24±0.33 | 31.48±0.19 | 36.60±0.35 | 38.32±0.23 | 40.82±0.70 |
| 50 | 47.28±0.07 | 48.51±0.15 | 47.48±0.13 | 55.63±0.44 | 59.03±0.84 |
| 100 | 57.74±0.27 | 57.16±0.19 | 62.06±0.15 | 64.53±0.58 | 70.75±0.37 |
| IC50 Value | 72.08 | 72.87 | 66.61 | 43.18 | 38.52 |
Note: Values are mean±standard error; n=3
Table 1: COX Inhibitory Potential of the Rhizome and Leaf Extracts
In the case of 5-LOX inhibition assay also, all these four extracts of A. wightii showed significant inhibitory action in a dose-dependent manner. Here unlike that of the COX inhibition assay, the broad leaf extract showed the least IC50 value (38.17 µg/ml) which was lower than that of the standard diclofenac sodium (42.82 µg/ml). The lower IC50value shown by the broad leaf extract indicates its higher inhibitory potential on the 5- LOX enzyme (Table 2).
| Concentration (µg/ml) | Percentage of inhibition | ||||
|---|---|---|---|---|---|
| Rhizome | Broad leaf | Narrow leaf | Diclofenac sodium | ||
| Acetone | Methanol | ||||
| 6.25 | 3.56±0.15 | 8.05±0.31 | 9.06±0.16 | 9.80±0.24 | 5.04±0.43 |
| 12.5 | 15.19±0.49 | 22.79±0.14 | 17.03±0.15 | 18.48±0.09 | 9.10±0.41 |
| 25 | 24.96±0.56 | 35.86±0.09 | 44.68±0.27 | 24.75±0.43 | 29.59±0.16 |
| 50 | 34.72±0.18 | 41.64±0.24 | 60.15±0.16 | 44.53±0.09 | 58.37±0.25 |
| 100 | 51.31±0.32 | 61.09±0.09 | 67.81±0.39 | 54.01±0.25 | 66.34±0.19 |
| IC50 Value | 90.99 | 71.53 | 38.17 | 81.68 | 42.82 |
Note: Values are mean±standard error; n=3
Table 2: 5- LOX Inhibitory Potential of the Rhizome and Leaf Extracts
5- LOX is an isozyme of LOX that catalyzes the production of leukotriene B4 from the precursor arachidonic acid. Leukotriene B4 is an inflammatory mediator associated with several inflammatory and allergic diseases, such as atherosclerosis, cancer, and cardiovascular diseases[30-33]. Hence 5-LOX inhibitors may reduce the production of leukotrienes and also may reduce the possibility of gastrointestinal and cardiovascular diseases that usually occur in the case of selective COX-1 and COX-2 inhibitors[34]. The disadvantage of most of the recently available synthetic anti-inflammatory drugs is their toxic effects and reappearance of symptoms after discontinuation. Hence, the development of potential anti-inflammatory drugs from medicinal plants is important[3].
Through HR-LCMS analysis, the present study could detect the compound ursolic acid in the leaf methanolic extracts of A. wightii (Table 3). Ursolic acid is a pentacyclic triterpenoid with diverse pharmacological effects, including anti-inflammatory potential[35]. According to previous reports, ursolic acid shows COX inhibitory potential with an IC50 value (59.40 μg/ml)[36] comparable to that of the leaf methanolic extracts, whereas the IC50 value of ursolic acid for LOX enzyme was reported to be much higher (137.00 μg/ml)[37]. Since this compound was not previously reported in this plant, the presence of ursolic acid in the leaf extracts was also confirmed through HPTLC analysis using the standard (fig. 1). Novel anti-inflammatory drugs of plant origin are getting more attention now-a-days since most synthetic drugs possess harmful side effects.
| S. No. | Leaf variety | Name of compound | Molecular formula | m/z | RT (min) | Mass | Structure |
|---|---|---|---|---|---|---|---|
| 1 | Broad leaf | Ursolic acid | C30H48O3 | 439.4 | 8.96 | 456.371 |  |
| 2 | Narrow leaf | Ursolic acid | C30H48O3 | 439.4 | 7.4 | 456.371 |  |
Table 3: Details of the Compound Ursolic Acid Identified by HR- LCMS
Fig 1: HPTLC profile of rhizome and leaf extracts of Anaphyllum wightii
Solvent system: Toluene:Ethyl acetate:Formic acid (5:3:0.2); Volume applied: Track 1: Broad leaf (methanolic extract) 10 µl; Track 2: Rhizome (acetone extract) 10 µl; Track 3: Narrow leaf (methanolic extract) 10 µl; Track 4: Rhizome (methanolic extract) 10 µl and Track 5: Ursolic acid (10 µl)
The leaves of the ethnomedicinal plant A. wightii possess significant inhibitory action on COX and LOX enzymes and hence may provide potential anti-inflammatory agents that will be useful in the treatment of various inflammatory diseases including cancer. Ursolic acid is an efficient anti- inflammatory compound detected in the plant. However, further studies are required for the isolation and characterization of the major active anti-inflammatory agents present in the plant.
Acknowledgements:
The authors are grateful to CSIR for the financial support provided to carry out the present study.
Conflict of interest:
Authors declare no conflict of interest.
References
- Nathan C. Points of control in inflammation. Nature 2002;420(6917):846-52.
[Crossref] [Google Scholar] [PubMed]
- Barton GM. A calculated response: Control of inflammation by the innate immune system. J Clin Invest 2008;118(2):413-20.
[Crossref] [Google Scholar] [PubMed]
- Virshette SJ, Patil MK, Somkuwar AP. A review on medicinal plants used as anti-inflammatory agents. J Pharmacogn Phytochem 2019;8(4):1641-6.
- Liu CH, Abrams ND, Carrick DM, Chander P, Dwyer J, Hamlet MR, et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat Immunol 2017;18(11):1175-80.
[Crossref] [Google Scholar] [PubMed]
- Nguyen HT, Vu TY, Chandi V, Polimati H, Tatipamula VB. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci Rep 2020;10:15965.
- King TC. Inflammation, inflammatory mediators, and immune-mediated disease. Chapter-2. In: Elsevier's Integrated Pathology 2007;2:50-1.
- Lima AS, Alvim HG. Review on non-steroid antiinflammatory: Acetylsalicylic acid. Rev Inic Ciente Ext 2018;1:169-74.
- Nunes CD, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, et al. Plants as sources of anti-inflammatory agents. Molecules 2020;25(16):3726.
[Crossref] [Google Scholar] [PubMed]
- Ramasubbu R. Protecting the wild beauties. Sci Report 2010;47:19.
- Dharmapalan B, Asokhan A. Myristica swamps: Evolutionary relics. Sci Rep 2013;50:45.
- Kunjumon M, Thomas S, George RE, Thankamani VI. Phytochemical, antibacterial, and antifungal activity of rhizome from Anaphyllum wightii. Schott against clinical isolates and plant pathogens. Int J Phytomed 2016;7:459-67.
- Udayan PS, George S, Tushar KV, Indira B. Ethnomedicine of Malampandaram tribes of Achankovil forest of Kollam district, Kerala. Indian J Tradit Know 2007;6:569.
- Vijayan A, VB L, John JV, Parthipan B, Renuka C. Traditional remedies of Kani tribes of Kottoor reserve forest, Agasthyavanam, Thiruvananthapuram, Kerala. Indian J Tradit Know 2007;6:589.
- Dharsana JN, Mathew M, Premkumar N, Kuttoor DS. Preliminary screening of Anaphyllum wightiiSchott tubers for anti-inflammatory and antioxidant activity. Am J Pharm Tech Res 2014;4(4):88-97.
- Lekshmi S, Swapna TS. In vitro anticancer potential of Anaphyllum wightii Schott. against Dalton’s lymphoma ascites cell lines and molecular docking studies of β-sitosterol. Indian J Exp Biol 2020;58(08):522-6.
- Walker MC, Gierse JK. In vitro assays for cyclooxygenase activity and inhibitor characterization. Cyclooxygenases: Methods Mol Biol 2010:131-44.
[Crossref] [Google Scholar] [PubMed]
- Axelrod B, Cheesbrough TM, Laakso S. [53] Lipoxygenase from soybeans: EC 1.13. 11.12 Linoleate: oxygen oxidoreductase. In: Methods in Enzymology 1981;71: 441-51.
- Adnan M, Patel M, Deshpande S, Alreshidi M, Siddiqui AJ, Reddy MN, et al. Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-insilico approach. Front Microbiol 2020;11:823.
[Crossref] [Google Scholar] [PubMed]
- Shailajan S, Hande H, Singh D, Tiwari B. Estimation of ursolic acid from Urtica dioica L. using validated HPTLC method. J Appl Pharm Sci 2014;4(5):092-5.
- Misra S, Hascall VC, Markwald RR, O’Brien PE, Ghatak S. “Inflammation and cancer” In: Turksen K, editor. Wound Healing: Stem Cells Repair and Restorations, Basic and Clinical Aspects: John Wiley & Sons; 2018. p. 239-74.
- Sevanian A, Kim E. Phospholiphase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med 1985;1(4):263-71.
[Crossref] [Google Scholar] [PubMed]
- Santos CM, Ribeiro D, Silva AM, Fernandes E. 2, 3-Diarylxanthones as potential inhibitors of arachidonic acid metabolic pathways. Inflammation 2017;40:956-64.
[Crossref] [Google Scholar] [PubMed]
- Sugimoto Y, Inazumi T, Tsuchiya S. Roles of prostaglandin receptors in female reproduction. J Biochem 2015;157(2):73-80.
[Crossref] [Google Scholar] [PubMed]
- Williams TJ, Peck MJ. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530-2.
[Crossref] [Google Scholar] [PubMed]
- Kojima F, Kato S, Kawai S. Prostaglandin E synthase in the pathophysiology of arthritis. Fundam Clin Pharmacol 2005;19(3):255-61.
[Crossref] [Google Scholar] [PubMed]
- Miller T. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol 1983;245(5):G601-23.
[Crossref] [Google Scholar] [PubMed]
- Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci 2013;16(5):821-47.
[Crossref] [Google Scholar] [PubMed]
- Pairet M, Engelhardt G. Distinct isoforms (COX‐1 and COX‐2) of cyclooxygenase: Possible physiological and therapeutic implications. Fundam Clin Pharmacol 1996;10(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: A systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006;296(13):1633-44.
[Crossref] [Google Scholar] [PubMed]
- Kaur G, Silakari O. Multiple target-centric strategy to tame inflammation. Future Med Chem 2017;9(12):1361-76.
[Crossref] [Google Scholar] [PubMed]
- Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med 2004;350(1):29-37.
[Crossref] [Google Scholar] [PubMed]
- Hyde CA, Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol 2009;9(6):701-15.
[Crossref] [Google Scholar] [PubMed]
- Koukoulitsa C, Hadjipavlou–Litina D, Geromichalos GD, Skaltsa H. Inhibitory effect on soybean lipoxygenase and docking studies of some secondary metabolites, isolated from Origanum vulgare L. ssp. hirtum. J Enzyme Inhib Med Chem 2007;22(1):99-104.
[Crossref] [Google Scholar] [PubMed]
- Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis 2003;62(6):501-9.
[Crossref] [Google Scholar] [PubMed]
- Kashyap D, Sharma A, S Tuli H, Punia S, K Sharma A. Ursolic acid and oleanolic acid: Pentacyclic terpenoids with promising anti-inflammatory activities. Recent Pat Inflamm Allergy Drug Discov 2016;10(1):21-33.
[Crossref] [Google Scholar] [PubMed]
- Ringbom T, Segura L, Noreen Y, Perera P, Bohlin L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J Nat Prod 1998;61(10):1212-5.
[Crossref] [Google Scholar] [PubMed]
- Simon A, Najid A, Chulia AJ, Delage C, Rigaud M. Inhibition of lipoxygenase activity and HL60 leukemic cell proliferation by ursolic acid isolated from heather flowers (Calluna vulgaris). Biochim Biophys Acta 1992;1125(1):68-72.
[Crossref] [Google Scholar] [PubMed]