- *Corresponding Author:
- B. Pehlivanović
Department of Clinical Pharmacy, Faculty of Pharmacy, University of Sarajevo, Zmaja od Bosne bb, Sarajevo 71000, Bosnia and Herzegovina
E-mail: belma.pehlivanovic@ffsa.unsa.ba
| Date of Received | 10 September 2021 |
| Date of Revision | 18 June 2022 |
| Date of Acceptance | 31 March 2023 |
| Indian J Pharm Sci 2023;85(2):472-478 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Modern biomedical science has proven beneficial effects of curcumin as potential therapeutic candidate for various conditions due to its pleiotropic and pharmacological properties. The aim of the present study was to investigate antigenotoxic effects of curcumin against cisplatin induced genotoxicity as well as its antioxidative effects. In vitro cytokinesis block micronuclei assay in human lymphocytes was used for evaluation of antigenotoxic properties of curcumin. Evaluation of antioxidative properties of curcumin was determined with 2,2-Diphenyl-1-picrylhydrazyl free radical scavenging assay. Results demonstrated significant antigenotoxic and antioxidative properties of curcumin, dependent upon the tested concentrations. Consideration should be given to performing a study with broad dose interval of tested substances. Findings from the present study emphasized application of curcumin as potential antigenotoxic and antioxidative supplementary agent.
Keywords
Curcumin, antigenotoxicity, antioxidative activity, in vitro study
Over the past decades, various phytochemicals have been examined due to their antigenotoxic and antioxidative properties. It has been found that different phytochemicals can intervene in early stages of carcinogenesis and trigger numerousmolecular mechanisms that results in reducing risks of cancer formations[1]. One of the most examined and well-documented chemopreventive natural agent is curcumin[2-4]. Curcumin-(E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione is a polyphenolic, highly pleiotropic substance extracted from the rhizome powder of plant Curcuma longa L. It has been used in Asian medicine for centuries in the treatment of many disorders and now-a-days, modern biomedical science has proven beneficial effects of curcumin, due to its pleiotropic properties, as potential therapeutic candidate for various conditions[5-8]. Different in vitro and in vivo studies have demonstrated that curcumin possess wide range of pharmacological activities, such as anti-inflammatory, antimicrobial, antifungal, antiproliferative, analgetic, neuroprotective, hypoglicemic, antihyperlipemic, antioxidative and hepatoprotective activity[9-11]. All those pharmacological properties attract interests of researchers, but lately all the focus in area of biomedicine is on antigenotoxic, antioxidative and anticancer activity of curcumin. Oxidative stress and accumulation of reactive oxygen species induced by numerous chemical agents very often result in tumor formation. Many cancer related studies examined antioxidative and antigenotoxic properties of curcumin associated with prevention and tumor formation, oxidative stress and various molecular pathways that protect cells from different genotoxic agents[12-18]. Study conducted by Srinivasan et al.[19] demonstrated protective effects of curcumin in cultured human lymphocytes were γ-radiationwas applied as a genotoxic agent that induced Deoxyribonucleic Acid (DNA) damage. Another study that used mice model which was exposed to γ-radiation demonstrated significant decrease in frequencies of micronucleated polychromatic erythrocytes when treated with curcumin[20].
Cisplatin-induced genotoxicity is a frequently used model for screening of potential chemopreventive agents. Cisplatin is platinum based chemotherapeutic agent which is frequently used for treatment of numerous human cancers and sarcomas[21]. However, it has been observed that treatment with cisplatin can cause a wide range of cytotoxic processes, cell death and as other chemotherapeutics, cisplatin itself can cause a variety of effects on liver and renal impairment. Treatment with cisplatin very often leads to various side effects such as genotoxicity, neurotoxicity, infertility and nephrotoxicity[22-24]. Genotoxicity of cisplatin is explained by its ability to generate oxygen/nitrogen free radicals during chemotherapy and these effects may lead to the initiation of unrelated tumors years after the chemotherapy cessation. To overcome such side effects and unwanted processes, but yet to remain efficient, very often cisplatin has been combined with other drugs and natural substances[2,25]. Advantage was given to the natural products due to their safety, minor side effects and long consumption amongst humans. Recent studies demonstrate protective role of curcumin against cisplatin toxicity and side effects[26].
The aim of the present study was to investigate antigenotoxic effects of curcumin as potential chemopreventive agent as well as its antioxidative potential. Genotoxic effects of cisplatin and antigenotoxic properties of curcumin were evaluated with usage of in vitro cytokinesis-block micronucleus assay in human lymphocytes, which is sensitive in vitro test very often used to asses DNA damage[27]. For evaluation of antioxidative properties of curcumin we applied commonly used in vitro test for scavenging free radicals which is based on reaction between stable 1,1-diphenyl-2-picryl hydrazyl radical (DPPH) and antioxidant[28-30].
Materials and Methods
Samples:
After obtaining written informed consent, peripheral blood samples were collected from three male volunteers (mean age: 27.3; range: 25-30). Volunteers, whose peripheral blood was examined in this study, were non-smokers and free of medication consumption. Ethical approval for the experimental testing was obtained from the Ethics Committee of Faculty of Pharmacy, University of Sarajevo (No. 0101-773/19).
Chemicals:
Curcumin was obtained from Sigma-Aldrich (St.Louis, MO, USA). Cisplatin, which was used as a damaging agent for induction of genotoxicity, was purchased from Pfizer Service Company, Belgium (Cisplatin Pfizer®) as 1 mg/ml concentrate for solution for infusion. PB-MAX Karyotyping Medium was obtained from GIBCO-Life Technologies (Grand Island, NY, USA) and Cytochalasin B from Sigma-Aldrich (St.Louis, MO, USA). DPPH and Dimethylsulphoxide (DMSO) were obtained from Semikem (BiH). All other chemicals used in study were of purest available analytical grade.
Cytokinesis-block micronucleus assay:
In vitro evaluation of antigenotoxic properties of curcumin was determined with cisplatin-induced genotoxicity and was performed using cytokinesis-block micronucleus assay in human lymphocyte cultures. The whole blood samples were collected with the help of a sterile heparinized syringe from each volunteer and were divided for five study groups:
Control group: Only peripheral blood; Cisplatin group: Cisplatin solution for infusion 1 mg/ml; Cis+Cur I: Cisplatin (1 mg/ml)+Curcumin (25 μg/ml); Cis+Cur II: Cisplatin (1 mg/ml)+Curcumin (50 μg/ml); Cis+Cur III: Cisplatin (1 mg/ml)+Curcumin (75 μg/ml).
Each group contained 400 µl of peripheral blood and 5 ml of PB-MAX Karyotyping Medium. Cultivation lasted for 72 h at temperature 37°, but in order to block cytokinesis, cultivation was shortly stopped in the 45th h and cytochalasin B was added to the cultures. When the incubation period ended, cells were collected by centrifugation for 10 min at 1000 rpm and resuspended in 0.559 g/100 ml KCl. They were then immediately treated with a fixative solution three times (methanol:acetic acid=1:3). Fixed cells were dropped on clean microscopic slides, air dried, and stained with 5 % Giemsa solution for 7 min. All slides were coded by an individual other than the scorer, and were evaluated at 400x magnification on Olympus BX51 microscope (Tokyo, Japan).
For each sample group, at least 1000 binuclear cells were scored with aim to determine the frequencies of genotoxicity markers such as micronuclei, binuclei, nucleoplasmatic bridges and nuclear buds. Those genotoxicity biomarkers were determined according to well defined protocol and criteria by Fenech[27,31]. Furthermore, Nuclear Division Index (NDI) and Nuclear Division Cytoxicity Index (NDCI) were calculated and used to assess genotoxic effects of tested substances[27].
DPPH free radical scavenging assay:
The reaction mixture consisted of 1 ml ethanol solution of DPPH radical of concentration 3×10-4 mol/l mixed with 2.5 ml of the tested sample whose absorbance was measured after 20 min of standing at room temperature in a dark place[29,30]. As a control solution, absorbance of the ethanol solution of DPPH radical was measured while pure ethanol was used as a blank. Antioxidant activity was presented as Radical Scavenging Capacity (RSC) and expressed in percentages (%). Following formula was used for determination of RSC:
RSC (%)=100-(Abssample×100)/(Abscontrol)
where Abssample represents the absorbance of the sample (reaction mixture of ethanol DPPH solution and test solutions) measured at 517 nm and Abscontrol the absorbance of control (ethanol solution DPPH) at 517 nm.The curve of dependence of the concentration of test solutions (mg/ml) on the free radical capacity (%) was graphically presented, on the basis of which the IC50 values for the test solutions were calculated. In this assay, the IC50 values represent the concentration of the tested solutions and standard antioxidant that inhibits 50 % of the initial concentration of DPPH radicals at 517 nm wavelength. The lower IC50 values indicated the higher antioxidative activity.
Results and Discussion
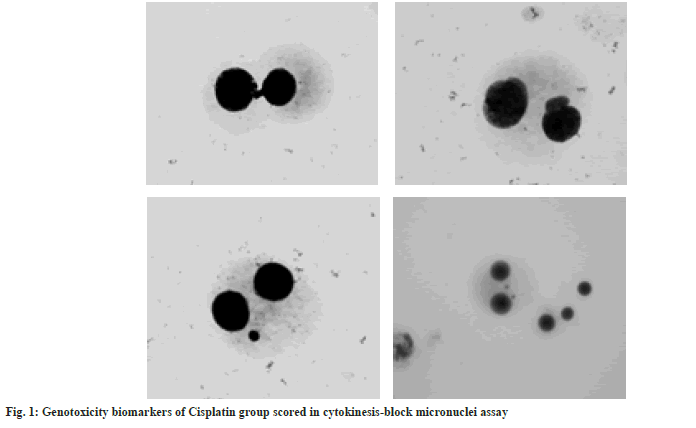
Now-a-days, in field of genetic toxicology various cytogenetic assays are used for assessment of chromosomal and DNA damage. Cytokinesis-blockmicronuclei assay in human peripheral blood lymphocytes is widely accepted for detecting damaged cytogenetic structures and yet it is very simple, cheap and convenient assay with short time needed for gathering data[27,32]. In this study, results from cytokinesis-block micronucleus assay were obtained by analyzing a total number of 1000 living cells which were counted, categorized and used for assessment of mono-nucleated, bi-nucleated, poly-nucleated cells as well as the NDI and NDCI. Results of the analysis of micronuclei, nucleoplasmatic bridges and nuclear buds in human lymphocyte culture are presented in Table 1. The frequency of binuclei and indexes of general toxicity expressed as NDI and NDCI are presented in Table 2. Values of analysed biomarkers of genotoxicity are presented as mean±standard deviation.
| Groups | Genotoxicity biomarkers | ||
|---|---|---|---|
| MN | NPB | NB | |
| Control | 10.33±2.52 | 2.33±0.58 | 1.67±0.58 |
| Cisplatin | 45.00±10.58 | 5.33±1.00 | 6.33±3.06 |
| Cis+Cur I | 26.33±5.13 | 5.00±2.08 | 5.30±1.15 |
| Cis+Cur II | 24.31±7.09 | 2.67±2.31 | 4.67±1.53 |
| Cis+Cur III | 17.33±2.52 | 0.67±1.15 | 1.67±1.15 |
Note: Values are presented as the mean±standard deviation; MN: Micronuclei; NPB: Nucleoplasmatic bridge and NB: Nuclear bud.
Table 1: Results of Cytokinesis-Block Micronuclei Assay in Human Peripheral Blood Lymphocytes.
| Groups | Genotoxicity biomarkers | ||
|---|---|---|---|
| BN | NDI | NDCI | |
| Control | 0.33±0.58 | 1.35±0.09 | 1.32±0.09 |
| Cisplatin | 6.33±3.51 | 1.22±0.02 | 1.15±0.14 |
| Cis+Cur I | 5.33±3.79 | 1.30±0.15 | 1.28±0.04 |
| Cis+Cur II | 3.67±3.76 | 1.28±0.14 | 1.26±0.18 |
| Cis+Cur III | 0.00±0.00 | 1.12±0.04 | 1.13±0.03 |
Note: Values are presented as the mean±standard deviation; BN: Binuclei; NDI: Nuclear division index and NDCI: Nuclear division cytoxicity index.
Table 2: Results of Cytokinesis-Block Micronuclei Assay in Human Peripheral Blood Lymphocytes.
As expected, the highest frequencies of cells with micronuclei, binuclei, nucleoplasmatic bridges and nuclear buds are observed in cisplatin group (fig. 1). The frequency of micronuclei represents an important indicator of DNA damage due to exposure to different genotoxic agents such as cisplatin itself[31,32]. These findings confirm genotoxic effects of cisplatin and they are similar to those previously published[25,33]. Recent studies have also demonstrated genotoxic effects of cisplatin on cultured mammalian cells and bone marrow cells[34]. With application of a comet assay on cultures of human Schwann cells, it has been observed that administration of cisplatin cause increase of cells with DNA damage for which it has been established that is dependent on dose and time of exposure[35]. Furthermore, obtained results from our study indicate reduction of micronuclei frequency in all curcumin-treated groups compared to cisplatin group (Table 1 and Table 2). These findings are in accordance to recently performed study by Shafaghati et al.[36] that used cytokinesis-block micronuclei assay in human peripheral blood lymphocytes for evaluation of antigenotoxic properties of curcumin against genotoxicity induced by 131-iodine. Their results also demonstrated reduction of micronuclei frequency in samples treated with curcumin therefore implying on potential radioprotective effects of curcumin for patients undergoing 131-iodine therapy[36]. Moreover, our results clearly demonstrate that the frequency of micronuclei, nucleoplasmatic bridges and nuclear buds in human lymphocyte culture reduces with increases of curcumin concentration. The most efficient reduction in frequency of genotoxicity biomarkers was observed in group Cis+Cur III implying that highest concentration of curcumin (75 μg/ml) provides maximal lymphocyte protection when incubated with cisplatin. Therefore, antigenotoxic effects of curcumin against cisplatin-induced genotoxicity are dose dependant. Our findings are consisted with those previously published[25,32,37]. According to similar study by Prabhu et al.[25] protective effects of curcumin against cisplatin induced genotoxicity are highly dose sensitive. Antigenotoxic properties of curcumin have been examined on genotoxicity induced by different chemical agents.
İşitez and Ciğerci shown that dietary curcumin reduces DNA damage, number of micronuclei and oxidative stress when exposed to alkylating agents. Their findings suggest that dietary curcumin shows protective antigenotoxic effects against alkylating agents due to its phenolic component which plays important role against DNA damage[38]. Various in vitro and in vivo studies outline beneficial effects of curcumin in prevention and treatment of different cancers[10]. Study performed on human hepatic cancer cells by Notarbartolo et al.[37] showed that combination of curcumin with cisplatin results with synergistic anticancer activity. According to Tomeh et al.[39]anticancer activity of curcumin is based on its involvmet in multiple mechanisms that interfere with different cell-signaling pathways.
Chemopreventive and anticancer properties of curcumin have also been associated with its antioxidative activities. Due to its conjugated structure and possession of two methoxylated phenols, curcumin exhibits antioxidative activity[40]. Free-radical scavenging antioxidative properties of curcumin are related to reduction of genotoxic damage[41]. As cisplatin stimulates production of reactive oxygen species and oxidative stress, due to inactivation of glutathione, addition of curcumin as supplement would result with beneficial effects[26]. Recently, many studies have been conducted to investigate the protective role of curcumin against oxidative damage in cells that leads to DNA damage, cancer formations and other pathological conditions[41]. In this view, we used common spectrophotometric DPPH free radical scavenging assay for determination of antioxidative activity of curcumin. Results of free RSC of curcumin solutions which were tested and compared to solutions of ascorbic acid at same concentrations are presented in Table 3.
| Concentration (mg/ml) | RSC (%) | |
|---|---|---|
| Ascorbic acid | Curcumin | |
| 1 | 68.51±0.30 | 72.86±0.21 |
| 0.75 | 65.11±0.04 | 70.02±0.05 |
| 0.5 | 60.03±1.11 | 62.11±0.12 |
| 0.25 | 55.99±0.07 | 54.98±0.17 |
| 0.1 | 50.11±0.03 | 50.87±0.23 |
Note: Values are presented as the mean±standard deviation; RSC: Radical scavaging capacity.
Table 3: Results OF DPPH Assay for tested solutions of ascorbic acid and curcumin.
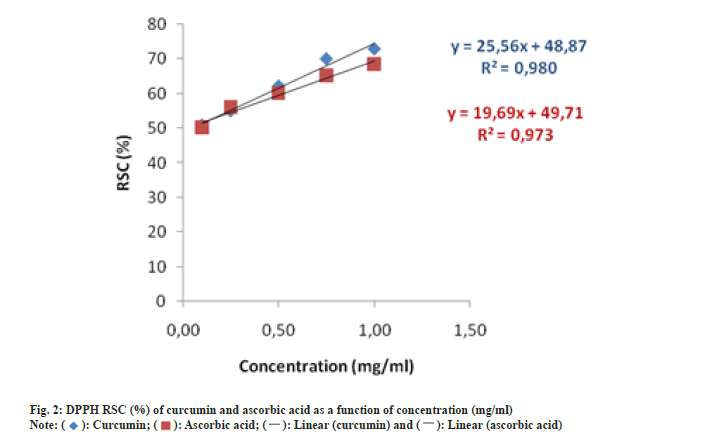
The percentage of RSC of tested solutions ranged from 50.11 % to 68.51 % and 50.87 % to 72.86 % for ascorbic acid and curcumin, respectively. Due to the fact that tested solutions of curcumin showed similar percentage of RSC compared to solution of ascorbic acid it can be stated that curcumin is as efficient antioxidant as ascorbic acid in tested concentrations. Generally, presence of phenolic group and CH2 group of the ß-diketone moiety are responsible for the free radical scavenging activity of curcumin[41]. Findings of the present study are consisted with those of Asouri et al.[42] who also found similarity between antioxidant activity of curcumin and ascorbic acid at defined concentrations. Furthermore, results from our study showed that RSC of curcumin is concentration-dependant as percentage of RSC increases with increase of curcumin concentrations.
From the liner equations response IC50 values were calculated (fig. 2). The IC50 values were found to be 0.04 mg/ml and 0.02 mg/ml for curcumin and for ascorbic acid, respectively. Lower IC50 values indicate higher antioxidative activity of tested solutions. Findings from this study support previously published research that is based on comparison of antioxidative activity and IC50 values between standard, well-known antioxidant and curcumin[41,42]. Contrary to methodology used in our study, Borra et al.[43] used different in vitro and ex vivo models to determine antioxidative and free radical scavenging activity of curcumin comparing with natural antioxidant-ascorbic acid. Their findings suggest that curcumin showed dose-dependent effect and efficiently scavenged DPPH radical and therefore can be considered as a source of natural antioxidant.
Present in vitro study assessed significant antigenotoxic and antioxidative properties of curcumin. It is noted that protective effects of curcumin against cisplatin-induced genotoxicity and free radical scavenging properties of curcumin are both dependent upon the concentration. Consideration should be given to performing a study with broader dose interval of tested substances. Furthermore, obtained data from this study are restricted to in vitro assays and there is need to conduct further research involving in vivo models. In conclusion, findings from the present study emphasized application of curcumin as potential antigenotoxic and antioxidative agent.
Conflict of Interest
Authors declare no conflict of interests.
References
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 2003;3(10):768-80.
[Crossref] [Google Scholar] [PubMed]
- Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett 2005;223(2):181-90.
[Crossref] [Google Scholar] [PubMed]
- Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, et al. Progress in cancer chemoprevention: Development of diet-derived chemopreventive agents. J Nutr 2000;130(2):467S-71S.
[Crossref] [Google Scholar] [PubMed]
- Yin TF, Wang M, Qing Y, Lin YM, Wu D. Research progress on chemopreventive effects of phytochemicals on colorectal cancer and their mechanisms. World J Gastroenterol 2016;22(31):7058.
[Crossref] [Google Scholar] [PubMed]
- Beevers CS, Huang S. Pharmacological and clinical properties of curcumin. Botanics Targets Ther 2011;1:5-18.
- Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015;20(12):21138-56.
[Crossref] [Google Scholar] [PubMed]
- Maiti P, Dunbar GL. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int J Mol Sci 2018;19(6):1637.
[Crossref] [Google Scholar] [PubMed]
- Pehlivanović B, Bečić F. Curcumin: Phytochemical therapy in the treatment of hyperlipidemia. Bull. Chem Technol Bosnia Herzegovina 2019;52:11-16.
- Aggarwal BB, Gupta SC, Sung B. Curcumin: An orally bioavailable blocker of TNF and other pro‐inflammatory biomarkers. Br J Pharmacol 2013;169(8):1672-92.
[Crossref] [Google Scholar] [PubMed]
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett 2008;267(1):133-64.
[Crossref] [Google Scholar] [PubMed]
- Priyadarsini KI. Free radical reactions of curcumin in membrane models. Free Radic Biol Med 1997;23(6):838-43.
[Crossref] [Google Scholar] [PubMed]
- Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm 2010;343(9):489-99.
[Crossref] [Google Scholar] [PubMed]
- Hasima N, B Aggarwal B. Targeting proteasomal pathways by dietary curcumin for cancer prevention and treatment. Curr Med Chem 2014;21(14):1583-94.
[Crossref] [Google Scholar] [PubMed]
- Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: Review of the gap between basic and clinical applications. Curr Med Chem 2010;17(3):190-7.
[Crossref] [Google Scholar] [PubMed]
- Biswas J, Roy S, Mukherjee S, Sinha D, Roy M. Indian spice curcumin may be an effective strategy to combat the genotoxicity of arsenic in Swiss albino mice. Asian Pac J Cancer Prev 2010;11(1):239-47.
[Google Scholar] [PubMed]
- Pandya U, Saini MK, Jin GF, Awasthi S, Godley BF, Awasthi YC. Dietary curcumin prevents ocular toxicity of naphthalene in rats. Toxicol Lett 2000;115(3):195-204.
[Crossref] [Google Scholar] [PubMed]
- Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174(1):27-37.
[Crossref] [Google Scholar] [PubMed]
- Haverić A, Haverić S, Hadžić M, Lojo-Kadrić N, Ibrulj S. Genotoxicity and cytotoxicity analysis of curcumin and sunset yellow in human lymphocyte culture. Cell Mol Biol 2018;64(3):87-91.
[Crossref] [Google Scholar] [PubMed]
- Srinivasan M, Prasad NR, Menon VP. Protective effect of curcumin on γ-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat Res 2006;611(1-2):96-103.
[Crossref] [Google Scholar] [PubMed]
- Abraham SK, Sarma L, Kesavan PC. Protective effects of chlorogenic acid, curcumin and β-carotene against γ-radiation-induced in vivo chromosomal damage. Mutation Res Lett 1993;303(3):109-12.
[Crossref] [Google Scholar] [PubMed]
- Makovec T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol 2019;53(2):148-58.
[Crossref] [Google Scholar] [PubMed]
- Aguiar Jr PN, Tadokoro H, Da Silva GF, Landgraf MM, Noia Barreto CM, Filardi BA, et al. Definitive chemoradiotherapy for squamous head and neck cancer: Cisplatin vs. carboplatin? A meta-analysis. Future Oncol 2016;12(23):2755-64.
[Crossref] [Google Scholar] [PubMed]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol 2014;740:364-78.
[Crossref] [Google Scholar] [PubMed]
- Fuertes MA, Castilla J, Alonso C, Prez JM. Cisplatin biochemical mechanism of action: From cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 2003;10(3):257-66.
[Crossref] [Google Scholar] [PubMed]
- Prabhu YD, Ramesh N, Nishu S, Kaviyarasi R, Shalaka S, Ramgir V, Abilash VG. Protective effect of curcumin on cisplatin-induced genotoxicity in human leukocytes cultur. Int J Green Pharm 2017;11(02):S320.
- Rezaee R, Momtazi AA, Monemi A, Sahebkar A. Curcumin: A potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol Res 2017;117:218-27.
[Crossref] [Google Scholar] [PubMed]
- Fenech M. The in vitro micronucleus technique. Mutat Res 2000;455(1-2):81-95.
[Crossref] [Google Scholar] [PubMed]
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 2011;48(4):412-22.
[Crossref] [Google Scholar] [PubMed]
- Stanojević LP, Zdravković AS, Stanković MZ, Cakić MD, Nikolić VD, Ilić DP. Antioksidativna aktivnost vodeno-etanolnih ekstrakata iz lista koprive (Urtica dioica L.). Savremene Tehnol 2013;2(1):51-9.
- Stanojević L, Stanković M, Nikolić V, Nikolić L, Ristić D, Čanadanovic-Brunet J, et al. Antioxidant activity and total phenolic and flavonoid contents of Hieracium pilosella L. extracts. Sensors 2009;9(7):5702-14.
[Crossref] [Google Scholar] [PubMed]
- Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 2003;534(1-2):65-75.
[Crossref] [Google Scholar] [PubMed]
- Sinitsky MY, Druzhinin VG. The application of the cytokinesis-block micronucleus assay on peripheral blood lymphocytes for the assessment of genome damage in long-term residents of areas with high radon concentration. J Radiation Res 2014;55(1):61-6.
[Crossref] [Google Scholar] [PubMed]
- Mendonça LM, dos Santos GC, dos Santos RA, Takahashi CS, Bianchi MD, Antunes LM. Evaluation of curcumin and cisplatin-induced DNA damage in PC 12 cells by the alkaline comet assay. Hum Exp Toxicol 2010;29(8).
[Crossref] [Google Scholar] [PubMed]
- Khynriam D, Prasad SB. Cisplatin-induced genotoxic effects and endogenous glutathione levels in mice bearing ascites Dalton’s lymphoma. Mutat Res 2003;526(1-2):9-18.
[Crossref] [Google Scholar] [PubMed]
- Jirsova K, Mandys V, Gispen WH, Bär PR. Cisplatin-induced apoptosis in cultures of human Schwann cells. Neurosci Lett 2006;392(1-2):22-6.
[Crossref] [Google Scholar] [PubMed]
- Shafaghati N, Hedayati M, Hosseinimehr SJ. Protective effects of curcumin against genotoxicity induced by 131-iodine in human cultured lymphocyte cells. Pharmacogn Mag 2014;10(38):106-10.
[Crossref] [Google Scholar] [PubMed]
- Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett 2005;224(1):53-65.
[Crossref] [Google Scholar] [PubMed]
- İşitez N, CİĞERCİ İH. Effect of curcumin on the genotoxicity induced by alkylating agents. AKU J Sci Eng 2017;17(1):18-27.
- Tomeh MA, Hadianamrei R, Zhao X. A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci 2019;20(5):1033.
[Crossref] [Google Scholar] [PubMed]
- Masuda T, Maekawa T, Hidaka K, Bando H, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcumin: Analysis of oxidative coupling products from curcumin and linoleate. J Agric Food Chem 2001;49(5):2539-47.
[Crossref] [Google Scholar] [PubMed]
- Sökmen M, Akram Khan M. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacolgy 2016;24:81-6.
[Crossref] [Google Scholar] [PubMed]
- Asouri M, Ataee R, Ahmadi AA, Amini A, Moshaei MR. Antioxidant and free radical scavenging activities of curcumin. Asian J Chem 2013;25(13):7593-5.
[Crossref] [Google Scholar] [PubMed]
- Borra SK, Gurumurthy P, Mahendra J, Jayamathi KM, Cherian CN, Chand R. Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J Med Plants Res 2013;7(36):2680-90.
 .
.



