- *Corresponding Author:
- P. Sudhakar
Department of Pharmacology,
Vinayaka Mission’s College of Pharmacy,
Vinayaka Mission’s Research Foundation (Deemed to be University),
Salem, Tamil Nadu 636008,
India
E-mail: sudhakar00pharma@gmail.com
| Date of Received | 22 April 2020 |
| Date of Revision | 16 August 2021 |
| Date of Acceptance | 11 March 2022 |
| Indian J Pharm Sci 2022;84(2):319-327 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Indigofera cassioides Rottl. Ex. DC. is a large shrub, distributed throughout the hills of India. Because of its reported robust antioxidant potential and phytoconstituents content, we were interested in investigating the in vitro thrombolytic and in vivo cardioprotective effect of methanolic leaves extract of Indigofera cassioides. An in vitro thrombolytic model was used to evaluate the clot lysis effect of different concentrations of Indigofera cassioides along with streptokinase as a positive control and distilled water as a negative control. Cardioprotective activity of methanolic leaves extract of Indigofera cassioides has been evaluated in isoproterenol-induced myocardial infarction in rats. The treatment was designed as four groups of six animals each as follows. Group I: Control, Group II: Isoproterenol alone, Group III: Methanolic leaves extract of Indigofera cassioides (200 mg/kg) and Group IV: Methanolic leaves extract of Indigofera cassioides (400 mg/kg). To evaluate the efficacy of methanolic leaves extract of Indigofera cassioides treatment against isoproterenol-induced myocardial damage, biochemical parameters and histopathological studies were carried out. High-performance thin-layer chromatography fingerprint analysis of methanolic leaves extract of Indigofera cassioides revealed the presence of terpenoid and flavonoid compounds. Methanolic leaves extract of Indigofera cassioides shows a dose-dependent clot lysis effect. Isoproterenol-induced animals exhibited significantly altered lipid profile, alanine transaminase, aspartate transaminase, alkaline phosphatase and also elevate serum cardiac activity markers creatine phosphokinase-myocardial band and lactate dehydrogenase, which were reversed near to normal levels in the pre-treatment of methanolic leaves extract of Indigofera cassioides. In conclusion, methanolic leaves extract of Indigofera cassioides exhibited significant thrombolytic activity which might be an aid to reduce the myocardial infarction. The cardioprotective effect of methanolic leaves extract of Indigofera cassioides was confirmed by the reduction in cardiac marker enzymes, altered lipid profile and protection histopathological changes on heart tissues.
Keywords
Indigofera cassioides, thrombolytic, streptokinase, isoproterenol, cardioprotection
Myocardial Infarction (MI) is one of the foremost causes of death all over the world and the prevalence of this disease approaches three million worldwide. In India, the number of patients being hospitalized for MI is increasing over the past 35 y[1]. MI is caused for MI is increasing over the past 35 y[1]. MI is caused circulation of the myocardium, which results in myocardial necrosis[2]. Consequences of MI include hyperlipidemia, peroxidation of membrane lipids and loss of plasma membrane integrity[3]. It has also been suggested that heart failure after MI may be associated with the antioxidant deficit as well as increased myocardial oxidative stress[4].
Isoproterenol-induced MI in the rat is the widely used experimental model for evaluating the cardioprotective effect of drugs[5] because pathological changes induced by isoproterenol in rats are comparable to the MI that occurs in humans[6]. Administration of isoproterenol, a beta (β)-adrenergic agonist that causes severe stress in the myocardium results in infract-like necrosis of myocytes. Few proposed mechanisms of isoproterenol- induced damage in cardiac myocytes include hypoxia due to myocardial hyperactivity, coronary hypotension, calcium overload, depletion of energy reserves and excessive production of free radicals due to oxidative metabolism of catecholamines[7]. Among the various proposed mechanisms, the generation of high cytotoxic free radicals through autooxidation of catecholamines induced by isoproterenol has been implicated as one of the major causative factors[8].
World Health Organization (WHO) recommended the use of herbal medicines as alternative medicine, particularly in developing countries. Because WHO estimated that 80 % of the World’s population are presently using herbal medicines for primary health care. Most of the current research showed that medicinal plants with antioxidant potential are also able to impart cardioprotection[9,10].
Indigofera cassioides (I. cassioides) Rottl. Ex. DC. (Syn: Indigofera pulchella Roxb.) belonging to the family Fabaceae, is a large shrub, distributed throughout the hills of India. The flowers and leaves of the plant are reported to be antiscorbutic, diuretic and alternative. A decoction of the root is given for cough and its powder is applied externally for chest pains. The leaves and roots are used for swelling of the stomach[11,12]. The leaves are used by various ethnic groups and native medical practitioners to treat many kinds of diseases such as arthritis, inflammation, tumor and liver diseases. It was also reported that it possesses potent antioxidant and anti-tumor activity[13,14].
Considering the more robust antioxidant potential and higher terpenoid, phenolic and flavonoids content of I. cassioides leaves, we were interested in investigating the effect of I. cassioides leaves on the oxidative stress- induced cardiac injury. Hence this study was aimed to investigate the in vitro thrombolytic and in vivo cardioprotective activities of Methanolic Leaf Extract of I. cassioides (MEIC) were investigated.
Materials and Methods
Plant collection and authentication:
Leaves of I. cassioides were collected from in and around the region of Yercaud hills, Salem, Tamil Nadu in November. The plant was authenticated by Dr. G.V.S. Murthy, Joint Director, Botanical Survey of India, Coimbatore, Tamil Nadu, India. A voucher specimen is preserved in our laboratory for future reference.
Chemicals and drugs:
Isoprenaline hydrochloride (isoproterenol) was purchased from Sigma Chemical Co. (St. Louis, Missouri, United States of America (USA)). All the chemicals and reagents used in this study were of analytical grade. A lyophilized streptokinase vial (1 500 000 International Units (IU)) was purchased from the manufacturer Cadila Pharmaceuticals, Ahmedabad.
Preparation of plant extract:
The leaves of I. cassioides were shade dried at room temperature. The dried plant materials were subjected to size reduction to a coarse powder by using a dry grinder and passed through sieve no. 40 was used for extraction. Powdered plant material (500 g) was extracted with 80 % methanol at room temperature for 72 h. The extract was filtered and concentrated to dryness under reduced pressure and controlled temperature (40° to 50°) in a rotary evaporator until all solvent was removed to give a dark-colored molten extract. The percentage yield of the MEIC was 8.16 % w/w. The extract was stored in airtight containers in a refrigerator maintained below 10° until further use.
High-Performance Thin-Layer Chromatography (HPTLC):
The 100 mg of MEIC was dissolved in 1 ml methanol and centrifuged at 3000 rpm for 3 min. A 2 µl of the prepared test solution and 2 µl standard solutions were loaded as 5 mm band length in the 3×10 Silica gel 60F254 Thin Layer Chromatography (TLC) plate using a Hamilton syringe and Linomat 5 instrument (CAMAG, Muttenz, Switzerland). The samples loaded plate was kept in TLC twin trough developing chamber (after saturated with Solvent vapor) with respective mobile phases for terpenoids n-hexane:ethyl acetate, (7.2:2.8) and for flavonoids chloroform:ethyl acetate:glacial acetic acid (60:35:5) and the plate was developed in the respective mobile phase up to 90 mm. The developed plate was dried by hot air to evaporate solvents from the plate. The plate was kept in a photo-documentation chamber (Camag Reprostar 3) and captured the images at daylight, Ultra Violet (UV) 254 nm and UV 366 nm. The developed plate was sprayed with respective reagents for terpenoids anisaldehyde sulphuric acid reagent and flavonoids 1 % ethanolic aluminium chloride reagent and dried at 100° in a hot air oven. The plate was photo-documented in daylight and at UV 366 nm mode using a photo-documentation (Camag Reprostar 3) chamber. After derivatization, the plate was fixed and scanning was done by TLC scanner 3. The peak table, peak display and peak densitogram were recorded.
In vitro thrombolytic activity:
The thrombolytic activity of I. cassioides was done by the methods of Daginawala et al. with slight modification using streptokinase as a standard reference[15,16].
The extract (5, 25, 50 and 100 mg) was suspended in 10 ml distilled water. By using a vortex mixer, the suspension was shaken vigorously. Suspension of extract and distilled water was kept overnight and gradually poured through a 0.22 μm syringe filter for the filtration. In this way, soluble supernatant was removed. Then 100 μl of this filtrated aqueous preparation was added to microcentrifuge tubes which contained the clots to check the ex vivo cardioprotective (thrombolytic) activity[16].
Streptokinase solution preparation:
Commercially available lyophilized (streptokinase) vial of 1 500 000 IU, was diluted with 5 ml of sterile distilled water. This suspension served as a stock from which 100 μl (30 000 IU) was used for in vitro thrombolysis.
Blood collection:
Whole blood (3 ml) was drawn from healthy human volunteers (n=3) by a phlebotomist without a history of oral contraceptive or anticoagulant therapy. 500 μl of blood was transferred to each of the six previously weighed microcentrifuge tubes and incubated at 37° for 45 min and was allowed to form clots.
Effect of crude leaf extract on clot lysis:
After clot formation, the serum was finely and completely aspirated without disturbing the clot and the tubes were again weighed to determine the clot weight (Clot weight=Weight of the tube containing clot–Weight of the empty tube).
Each tube containing clot was properly labeled, as a negative nonthrombolytic control, about 100 µl of distilled water was added, as a positive control, 100 µl of streptokinase solution was added and 100 µl of various concentrations of MEIC (i.e., 0.5 mg/ml, 2.5 mg/ml, 5.0 mg/ml and 10.0 mg/ml) were added separately. At last, all the tubes were incubated at 37° for 90 min. After incubation, released fluid was removed and tubes were again weighed to observe the difference in weight after clot disruption. The difference in weight before and after clot lysis was expressed as a percentage of clot lysis[17]. The thrombolytic effects of I. cassioides leaf extracts were performed by the formula below:
Percentage (%) of clot lysis=(Weight of released clot/ Clot weight)×100
The experiment was repeated 3 times with the blood samples from the 3 healthy volunteers.
Evaluation of cardioprotective effect:
Experimental animals: The colony inbred adult male albino Wistar rats (250-300 g) were obtained from the Central Animal House of Swamy Vivekanandha College of Pharmacy, Namakkal, Tamil Nadu, India. The animals were kept under standard environmental conditions of 12/12 light/dark rhythm, maintained under controlled room temperature (23±2°) and relative humidity of 60 %±10 %, in polypropylene cages. They were fed with a standard pellet diet and water ad libitum. The immature animals were acclimatized under laboratory conditions 3 d before the initiation of the experiment. The experimental protocol was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India and approved by the Institutional Animal Ethical Committee (IAEC) of Swamy Vivekanandha College of Pharmacy, Tamil Nadu, India (Approval no.889/ac/05/CPCSEA).
Experimental design: A total of 24 Wistar male rats (200-300 g) were randomly divided into four groups of six per each. The doses of MEIC were fixed based on the previous study[14]. Group I, animals received 0.5 % Carboxymethylcellulose (CMC) orally (p. o.) for 30 d and normal saline subcutaneously (s. c.) on 29th d and 30th d. Group II, animals received 0.5 % CMC p. o. for 30 d and isoproterenol (85 mg/kg, s. c.) on the 29th d and 30th d in normal saline group. Group III, animals received MEIC (200 mg/kg/d) p. o. for 30 d and were subsequently treated with isoproterenol on 29th d and 30th d. Group IV, animals received MEIC (400 mg/ kg/d) p. o. for 30 d and were subsequently treated with isoproterenol on 29th d and 30th d.
At the end of the experimental period on the 31st d rats were fasted overnight (12 h) and blood samples were collected via retro-orbital sinus puncture under mild anesthesia. Serum was obtained by centrifugation of samples at 3000 rpm for 10 min and used for further serum lipid profile and cardiac-specific injury markers estimations.
Serum lipid profile: Serum Total Cholesterol (TC), Triglycerides (TG), High-Density Lipoprotein (HDL) and Low-Density Lipoproteins (LDL) were analyzed by using commercially available laboratory kits (ARKRAY Healthcare Pvt. Ltd., Surat, India).
Serum cardiac-specific injury markers: Activity levels of Creatine Phosphokinase-Myocardial Band (CK-MB), Lactate Dehydrogenase (LDH), Alanine Transaminase (ALT), Aspartate Transaminase (AST) and Alkaline Phosphatase (ALP) in serum were estimated using commercially available kits (AGAPPE Diagnostics LTD, Kerala, India).
Histological studies:
Histological evaluation was performed on the lower portion of the heart tissue. Fresh heart tissues were excised and then fixed in 10 % formalin for 24 h. The fixative was removed by washing through running tap water overnight. After dehydration through a graded series of alcohols, the tissues were cleared in methyl benzoate, embedded in paraffin wax. The section was cut into 5 μm thickness and stained with hematoxylin and eosin. After repeated dehydration and cleaning, the sections were mounted and observed under a light microscope with 100× magnification for histological changes.
Statistical analysis:
The data represents as mean±Standard Error of the Mean (SEM) of three replicate for in vitro thrombolytic activity and six replicated determinations for cardioprotective effect. Results were analyzed statistically by one-way Analysis of Variance (ANOVA) followed by post hoc Dunnet’s test by using Statistical Package for the Social Sciences (SPSS) V.17 (Student trail version). The difference was considered significant when p<0.05.
Results and Discussion
MI is characterized by interruption of coronary blood flow to any part of the heart, which results in myocardial ischemia and necrosis[18]. MI due to thrombus formation is one of the most severe causes which increases an alarming rate in recent years[19]. In recent years, research on Cardiovascular Disease (CVD) is towards natural bioactive compounds with rich antioxidant capacity, because of their superior in the terms of efficacy and safety when compared to their synthetic agents[20]. Hence the present investigation was carried out to screen the in vitro thrombolytic and in vivo cardioprotective effect of MEIC, due to its reported potent antioxidant and free radical scavenging activity[13].
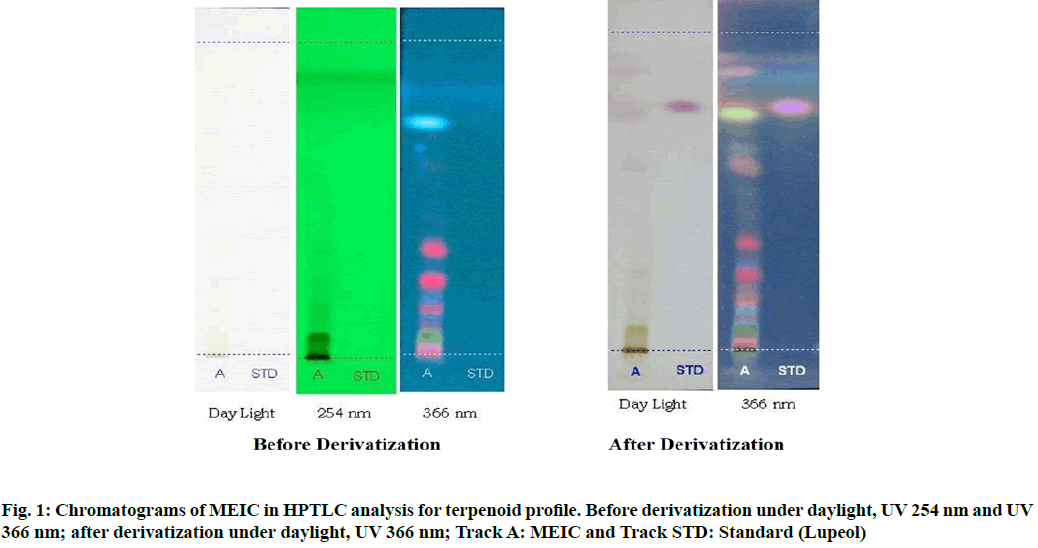
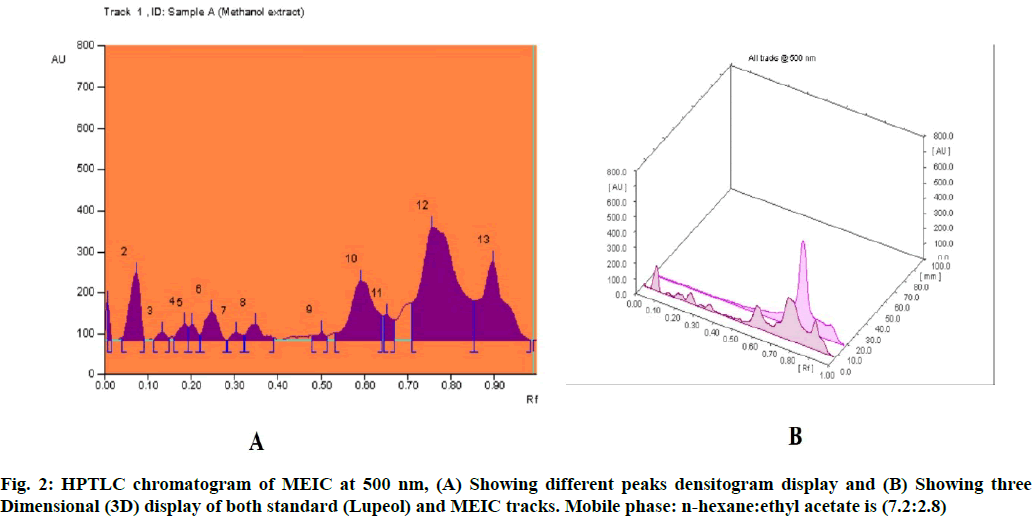
In our previous study, the primary phytochemical analysis of MEIC revealed the presence of terpenoids, alkaloids, saponins, glycosides, flavonoids and phenolic compounds[14]. In this study, the TLC chromatogram was run for MEIC along with standards for terpenoid and flavonoids profiles. Table 1 shows the presence of eight terpenoid compounds and five unknown compounds with Retention factor (Rf) value of 0.07, 0.13, 0.18, 0.25, 0.35, 0.59, 0.76, 0.90 and 0.01, 0.20, 0.30, 0.50, 0.65 respectively, which confirms the presence of terpenoids in the MEIC. Fig. 1 and fig. 2 shows the chromatogram and densitogram results for terpenoids profile, which revealed the presence of terpenoids before and after derivatization under daylight and UV 366 nm.
| Track | Peak | Rf | Height | Area | Assigned substance |
|---|---|---|---|---|---|
| MEIC | 1 | 0.01 | 91.3 | 443.5 | Unknown |
| MEIC | 2 | 0.07 | 162.3 | 3564.5 | Terpenoid 1 |
| MEIC | 3 | 0.13 | 18.1 | 311.6 | Terpenoid 2 |
| MEIC | 4 | 0.18 | 39.2 | 731.8 | Terpenoid 3 |
| MEIC | 5 | 0.2 | 37.7 | 619 | Unknown |
| MEIC | 6 | 0.25 | 70.2 | 1864.9 | Terpenoid 4 |
| MEIC | 7 | 0.3 | 17.6 | 370.4 | Unknown |
| MEIC | 8 | 0.35 | 38.8 | 1147.6 | Terpenoid 5 |
| MEIC | 9 | 0.5 | 19.2 | 376.2 | Unknown |
| MEIC | 10 | 0.59 | 145 | 7120.8 | Terpenoid 6 |
| MEIC | 11 | 0.65 | 61 | 1163.7 | Unknown |
| MEIC | 12 | 0.76 | 274.7 | 20654.9 | Terpenoid 7 |
| MEIC | 13 | 0.9 | 191.9 | 9207 | Terpenoid 8 |
| STD | 1 | 0.78 | 575.5 | 30691.9 | Lupeol (standard) |
Table 1: HPTLC Fingerprint Analysis of Meic for Terpenoid Profile
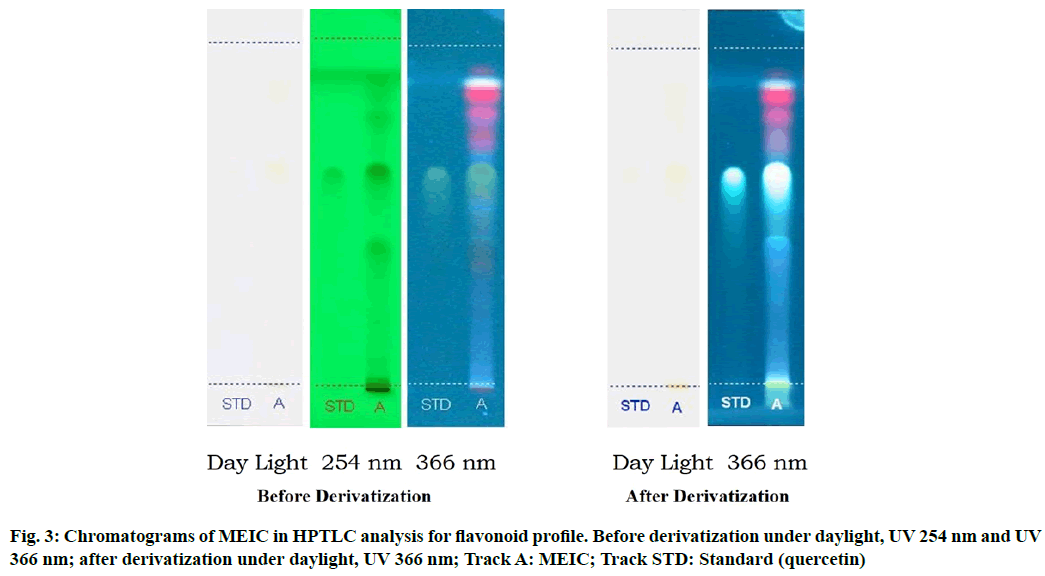
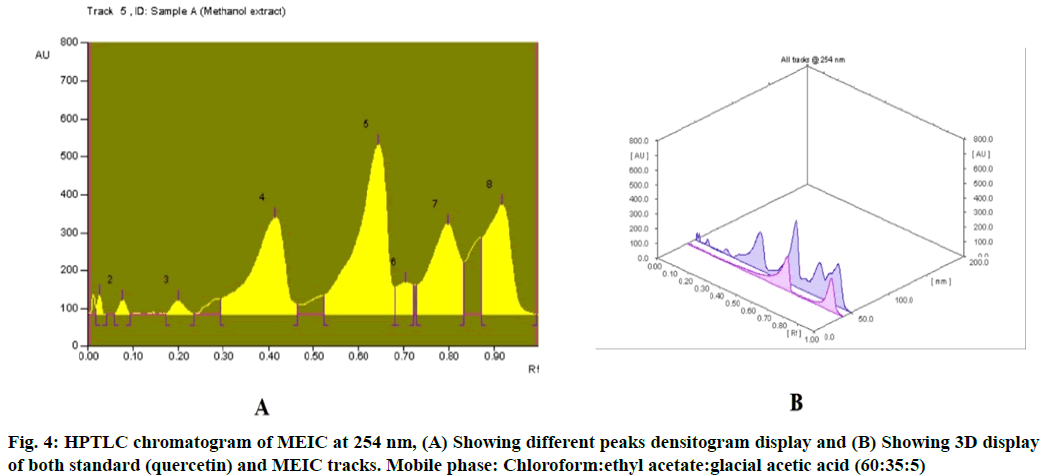
Table 2 results show the presence of three different types of flavonoids and six unknown compounds with Rf values of 0.03, 0.41, 0.64 and 0.01, 0.08, 0.20, 0.70, 0.80, 0.92 respectively, which confirms the presence of various flavonoids in I. cassioides. The standard quercetin produces a clear zone with an Rf value of 0.64. Fig. 3 and fig. 4 shows the chromatogram and densitogram results for flavonoids profile, which revealed the presence of flavonoids before and after derivatization under daylight and UV 366 nm.
| Track | Peak | Rf | Height | Area | Assigned substance |
|---|---|---|---|---|---|
| STD | 1 | 0.64 | 227.0 | 12571.7 | Quercetin (Standard) |
| MEIC | 1 | 0.01 | 51.6 | 250.1 | Unknown |
| MEIC | 2 | 0.03 | 51.4 | 365.4 | Flavonoid 1 |
| MEIC | 3 | 0.08 | 38.3 | 496.9 | Unknown |
| MEIC | 4 | 0.20 | 36.4 | 844.3 | Unknown |
| MEIC | 5 | 0.41 | 254.5 | 16634.9 | Flavonoid 2 |
| MEIC | 6 | 0.64 | 447.8 | 26036.6 | Flavonoid 3 |
| MEIC | 7 | 0.70 | 83.7 | 2515.9 | Unknown |
| MEIC | 8 | 0.80 | 237.8 | 13869.4 | Unknown |
| MEIC | 9 | 0.92 | 289.8 | 14215.7 | Unknown |
Table 2: HPTLC Fingerprint Analysis of MEIC for Flavonoid Profile
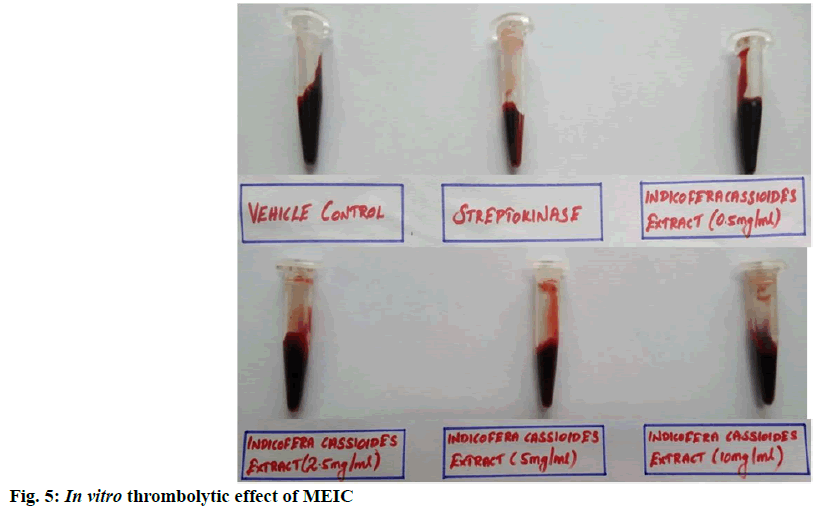
The in vitro thrombolytic activity of I. cassioides methanolic extract was determined as a part of exploring its cardioprotection effect. The results presented in Table 3 and fig. 5 indicates that the streptokinase which is known as a thrombolytic drug used as positive control shown 55 % clot lysis, 100 µl of distilled water (negative control) shown 1.94 % clot lysis. After treatment with MEIC showed significant (p<0.001) maximum 29.15 % clot lysis at the concentration 10 mg/ml when compared with negative control. And also, MEIC produces clot lysis in a concentration- dependent manner. Some of the previous studies reported that the thrombolytic activity of plant extracts is mainly due to the presence of rich secondary metabolites like alkaloids, flavonoids, tannins and terpenoids[21]. Hence the thrombolytic activity of MEIC might be due to its rich source of flavonoids and terpenoids content.
| Sample | Concentration | Percentage of clot lysis (%) |
|---|---|---|
| Negative control (Dil. Water) | 100 μl | 1.94±0.22 |
| Positive control (streptokinase) | 100 μl | 55.00±7.94*** |
| MEIC | 0.5 mg/ml | 3.72±0.94 |
| 2.5 mg/ml | 7.37±0.85 | |
| 5.0 mg/ml | 14.17±1.50*** | |
| 10.0 mg/ml | 29.15±3.00*** |
Table 3: In Vitro Thrombolytic Effect of MEIC
Isoproterenol-induced MI in rats elevates the serum TC, TG, LDL levels and decreased level of HDL[22]. Results of this study also have shown significant elevation of TC, TG, LDL and decreased level of HDL (Table 4). These changes could be attributed to enhanced lipid biosynthesis by cardiac cyclic Adenosine Monophosphate (cAMP)[23]. Also increase in serum phospholipids might be due to an increased peroxidation of membrane phospholipids released via phospholipase A2[24], which in turn decreased the phospholipid levels in heart tissue. The pre-treatment of MEIC has shown a dose-dependent beneficial effect on altered lipid profiles like elevated serum TC, TG, LDL levels and decreased HDL cholesterol levels on isoproterenol-induced MI rats.
| Treatments | TC (mg/dl) | TG (mg/dl) | HDL (mg/dl) | LDL (mg/dl) |
|---|---|---|---|---|
| Normal control | 103.17±4.98 | 93.67±4.09 | 45.33±5.38 | 96.83±4.71 |
| Isoproterenol (85 mg/kg) | 175.67±8.11a# | 146.50±5.46a# | 30.17±3.99a* | 124.83±7.85a* |
| Isoproterenol+MEIC (200 mg/kg) | 129.31±7.18a*b# | 118.83±6.98a* b$ | 31.00±4.03 | 109.66±7.01 |
| Isoproterenol+MEIC (400 mg/kg) | 105.24±6.18b# | 97.00±6.92b# | 44.67±5.00b* | 99.17±5.71b* |
Note: Values are expressed as mean±SEM, n=6; comparisons were made between: aGroup I vs. II, III and IV; bGroup II vs. III and IV; Symbols represent statistical significance: #p<0.001, $p<0.01 and *p<0.05
Table 4: Effect of MEIC on Isoproterenol-Induced Changes in Serum Lipid Profile
Administration of isoproterenol has been reported that alter the membrane permeability and cause leakage of cardiac inflammatory markers (LDH, CK-MB, AST, ALT and ALP) into the blood circulation[25]. Significant elevation of these marker enzymes indicates myocardial necrosis[7]. The result of our study also indicates that significant (p<0.001) elevated level of marker enzymes LDH, CK-MB, AST, ALT and ALP in isoproterenol alone treated group as compared to the normal control. On the contrary, MEIC treatment protected the structure and functional integrity of the myocardial membrane as evident from the significant reduction in the elevated levels of these serum marker enzymes in the rats when compared to the isoproterenol alone treated rats (Table 5). The significant reversal of these enzyme activities by pre-treatment of MEIC indicates its therapeutic potential against MI in a dose-dependent manner by preventing the isoproterenol-induced leakage of marker enzymes.
| Treatments | CK-MB (IU/l) | LDH (IU/l) | AST (IU/l) | ALT (IU/l) | ALP (IU/l) |
|---|---|---|---|---|---|
| Normal control | 196.83±14.95 | 49.50±3.03 | 45.33±3.12 | 45.67±2.43 | 32.00±3.34 |
| Isoproterenol (85 mg/kg) | 477.33±24.15a# | 103.67±4.63a# | 92.00±8.47a# | 82.00±6.70a# | 49.33±2.53a# |
| Isoproterenol+MEIC (200 mg/kg) | 293.00±16.01a#b# | 65.17±5.74a*b# | 62.67±6.62a*b$ | 58.17±4.86b# | 42.83±3.68a* |
| Isoproterenol+MEIC (400 mg/kg) | 214.33±8.45b# | 52.50±3.87b# | 51.33±3.42b# | 45.67±2.86b# | 30.50±2.59b# |
Note: Values are expressed as mean±SEM, n=6; comparisons were made between: aGroup I vs. II, III and IV; bGroup II vs. III and IV; Symbols represent statistical significance: #p<0.001, $p<0.01 and *p<0.05
Table 5: Effect of MEIC on Isoproterenol-Induced Changes in Serum Cardiac-Specific Injury Markers
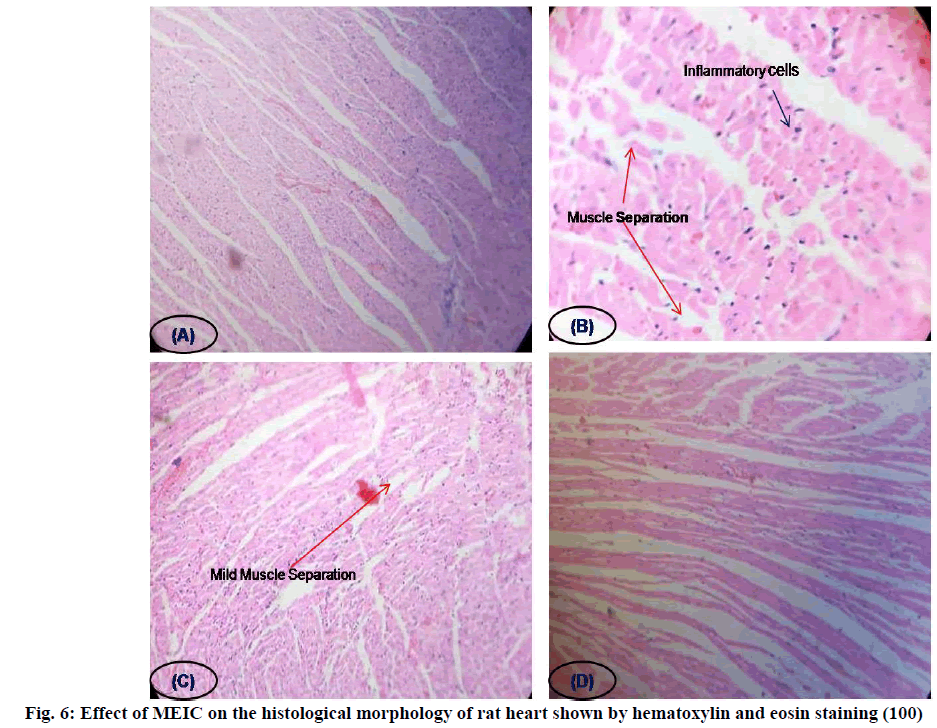
Histopathological examination of myocardial tissue in control illustrated clear integrity of the myocardial cell membrane and no inflammatory cell infiltration was observed. Isoproterenol injected rats showed coagulative necrosis, separation of cardiac muscle fibers and infiltration of inflammatory cells. The reduced inflammatory cell infiltration and normal cardiac muscle fiber architecture further confirmed the cardioprotective effect of MEIC in a dose-dependent manner (fig. 6).
Based on the result of the present study it was concluded that subcutaneous administration of isoproterenol- induced MI in rats altered the plasma lipid profile and induce the cellular leakage of myocardial inflammatory markers. Oral administration of MEIC exhibited a significant reduction in cardiac inflammatory marker enzymes and altered lipid profile and also protects the histopathological changes induced by isoproterenol. The reported thrombolytic potential of I. cassioides also aids to reduce MI. Hence this cardioprotective potential of MEIC was due to its potent antioxidant activity may be attributed to its phytoconstituents terpenoid and flavonoid contents. Further investigations are needed to explore the active principles and exact mechanism of action of I. cassioides in the prophylaxis and treatment of cardiovascular disorders.
Conflict of interests:
The authors declared no conflict of interest.
References
- Krishnaswami S. Observations on serial changes in coronary artery disease in Indians. Curr Sci 1998;74(12):1064-8.
- Whellan DJ. Heart failure disease management: Implementation and outcomes. Cardiol Rev 2005;13(5):231-9.
[Crossref] [Google Scholar] [PubMed]
- Krushna G, Kareem MA, Devi KL. Antidyslipidaemic effect of Aegle marmelos Linn. fruit on isoproterenol induced myocardial injury in rats. Internet J Pharmacol 2009;6(2).
- Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol 1996;148(1):291-300.
[Google Scholar] [PubMed]
- Naik SR, Panda VS. Cardioprotective activity of polyherbal extracts in experimental myocardial necrosis in rodents: An evidence of antioxidant activity. J Complement Integr Med 2008;5(1):35.
- Nirmala C, Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 1996;159(2):85-93.
[Crossref ] [Google Scholar] [PubMed]
- Mohanty I, Arya DS, Dinda A, Talwar KK, Joshi S, Gupta SK. Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin Pharmacol Toxicol 2004;94(4):184-90.
- Singal PK, Kapur N, Dhillon KS, Beamish RE, Dhalla NS. Role of free radicals in catecholamine-induced cardiomyopathy. Can J Physiol Pharmacol 1982;60(11):1390-7.
[Crossref] [Google Scholar] [PubMed]
- Ai AL, Bolling SF. The use of complementary and alternative therapies among middle-aged and older cardiac patients. Am J Med Qual 2002;17(1):21-7.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization. WHO launches the first global strategy on traditional and alternative medicine. WHO Press release; 2002.
- Nadkarni K, Nadkarni AK. Indian Materia Medica. Bombay: Popular Prakashan Private Ltd.; 1976. p. 799.
- Kirtikar KR, Basu BD. Indian Medicinal Plants. Dehradun: International Book Publishers; 1993. p. 714-5.
- Kumar RS, Rajkapoor B, Perumal P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac J Trop Biomed 2012;2(4):256-61.
[Crossref] [Google Scholar] [PubMed]
- Kumar RS, Rajkapoor B, Perumal P. In vitro and in vivo anticancer activity of Indigofera cassioides Rottl. Ex. DC. Asian Pac J Trop Med 2011;4(5):379-85.
[Crossref] [Google Scholar] [PubMed]
- Kam PC, Liew S. Traditional Chinese herbal medicine and anaesthesia. Anaesthesia 2002;57(11):1083-9.
[Crossref] [Google Scholar] [PubMed]
- Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb J 2006;4(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Alam MN, Biozid MS, Islam MR, Abedin MJ, Chowdhury S. In vitrocomparative study of cytotoxic and thrombolytic effects of methanolic extract of Cissus pentagonaand Thunbergia grandiflora (Roxb.) leaves. J Coast Life Med 2015;3(9):724-7.
- Kloner RA. New observations regarding post-ischemia/reperfusion myocardial swelling. J Am Coll Cardiol 2015;65(4):324-6.
[Crossref] [Google Scholar] [PubMed]
- Sai Sandeep Y, Panigrahi M, Divya GC, Beena DB. Evaluation of in vitro thrombolytic activity of phytochemicals in Bacopa monnieri Linn. J Pharm Res 2012;5(1):100-1.
- Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH. High catechin concentrations detected in Withania somnifera (Ashwagandha) by high performance liquid chromatography analysis. BMC Complement Altern Med 2011;11(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Dwivedi S. Terminalia arjuna Wight & Arn.-a useful drug for cardiovascular disorders. J Ethnopharmacol 2007;114(2):114-29.
[Crossref] [Google Scholar] [PubMed]
- Prince PS, Suman S, Devika PT, Vaithianathan M. Cardioprotective effect of ‘Marutham’ a polyherbal formulation on isoproterenol induced myocardial infarction in Wistar rats. Fitoterapia 2008;79(6):433-8.
[Crossref] [Google Scholar] [PubMed]
- Paritha IA, Shyamala DC. Effect of alpha-tocopherol on isoproterenol-induced changes in lipid and lipoprotein profile in rats. Indian J Pharmacol 1997;29(6):399.
[Google Scholar] [PubMed]
- Karthikeyan K, Bai BS, Devaraj SN. Efficacy of grape seed proanthocyanidins on serum and heart tissue lipids in rats subjected to isoproterenol-induced myocardial injury. Vascul Pharmacol 2007;47(5-6):295-301.
[Crossref] [Google Scholar] [PubMed]
- Deodato B, Altavilla D, Squadrito G, Campo GM, Arlotta M, Minutoli L, et al. Cardioprotection by the phytoestrogen genistein in experimental myocardial ischaemia‐reperfusion injury. Br J Clin Pharmacol 1999;128(8):1683-90.