- *Corresponding Author:
- D S N B K Prasanth
Department of Chemistry ,
Gudlavalleru Engineering College,
Gudlavalleru 521356,
Andhra Pradesh,
India
E-mail: dsnbkprasanth@gmail.com
| Date of Received | 20 February 2020 |
| Date of Revision | 18 June 2021 |
| Date of Acceptance | 20 July 2021 |
| Indian J Pharm Sci 2021;83(4):732-741 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This research was performed to analyze the antinociceptive task of methanolic extract of Ipomoea marginata in addition to in silico evaluation of the antinociceptive task of the separated constituents from Ipomoea marginata versus cyclooxygenase 2 enzyme together with absorption, distribution, metabolism, excretion/toxicity analysis of separated substances. In vivo antinociceptive task of methanolic extract of Ipomoea marginata was examined by acetic acid-induced agonizing, tail immersion and the hot plate on rodents. In silico activity of the isolated substances, absorption, distribution, metabolism, excretion/toxicity assessment was carried out by Autodock 4.0 and data warrior software applications. The results revealed that methanolic extract of Ipomoea marginata has the greatest possible dose-dependent antinociceptive task at all doses. Amongst the substances, Ipalbidine showed the very best docking score of -8.26, which was virtually better than standard diclofenac, i.e., -7.03, guaranteeing good binding compatibility among the ligand and the receptor than the standard and absorption, distribution, metabolism, excretion/toxicity evaluation using data warrior assures the compound has not breached Lipinski's guideline of five suggesting its safety consumption. To conclude, Ipomoea marginata can be a potent resource of antinociceptive activity and also additional simulation studies are needed to develop the performance of Ipalbidine.
Keywords
Ipomoea marginata, leucorrhoea, depression, analgesic
Pain is a beneficial tool for the body's immune system to safeguard the location harmed by various stimulations. To care for the pain, vast arrays of antinociceptive like nonsteroidal anti-inflammatory drugs (NSAIDs), steroidal medicines in addition to opioid anaesthetics are utilized, which have a different harmful effect such as hepatic damage, cardio troubles, kidney failure, erectile dysfunction, manic depression, high blood pressure, aches as well as dizziness, look of inactive diabetes mellitus, skin degeneration, reduced bone density, intestinal system, abscess, reliance, constipation and also respiratory problems. So, it is crucial to the globe to make sure a resource of cost-abusing herbal-based antinociceptive medicines with more potent and less negative results may be acquired with the medicinal plant [1-4].
Molecular docking is an essential strategy of making plans and designing new drugs, where it is expected that a tiny molecule will certainly show affinity and bind experimentally to the binding site of the target receptor. Therefore, a practical docking approach must adequately forecast the native ligand model to the receptor-binding site and the linked physico-chemical molecular communications [5-8].
Ipomoea marginata (I. marginata) Verdc. (Family Convolvulaceae) is a perennial twiner with ovate-cordate acute leaves having reddish patches; light pink (having a dark eye), funnel-shaped flowers in pedunculate subumbellate cymes and ovoid, glabrous capsules are containing 2-4 grey seeds with silky pubescence. It is prevalent near water bodies throughout India [9,10].
Traditionally, plant juice is used as a diuretic, hypotensive, deobstruent and antidote to poisoning. Tubers are used as an alternative, aphrodisiac, diuretic, uterine tonic, antidiabetic. Seeds mixed with milk taken as a vital tonic. Veterinary medicine, ground leaves applied to infected wounds [11]. In Ayurveda, it is the only plant that can cure sterility in women. This is also believed to have the power of bestowing a male child. Its root is the correct part for promoting bodily strength and also acts as a tonic [9]. The root decoction is taken internally to treat leucorrhoea and urinary complications/kidney stones [12].
Consequently, this study aimed to examine the antinociceptive task of the methanolic extract of I. marginata (MEIM) through in vivo method and in silico recognition of possible phyto-constituents as an antinociceptive.
Materials and Methods
Collection and identification of plant material:
Fresh plants of I. marginata, Convolvulaceae, were gathered from Tirumala, Tirupati, Andhra Pradesh (13° 37' 44.6340" N and also 79° 25' 28.0056" E), acknowledged and also confirmed by Prof. K. Madhava Chetty, Plant Taxonomist, Assistant Professor, Department of Botany, Sri Venkateswara University, Tirupati, Andhra Pradesh, India. They were washed after and cleaned by regular water, air-dried, ground into powder in a home appliance and preserved for pharmacognostical study.
Preparation of extracts:
The shade dried whole plant was powdered with the assistance of a miller, and a crude powder was acquired. The coarse powder (1000 g) was extracted with methanol utilizing a soxhlet device. The extract was filtered, concentrated by vaporizing the solvent in a rotating evaporator and maintained in the refrigerator.
Preliminary phytochemical Screening:
The methanolic extract of I. marginata underwent initial phytochemical testing to recognize chemical components according to the standard operating procedures [13-16].
Experimental Animals:
Male albino Wistar rats (180–200 g) and male albino mice (18–25 g) were obtained from the Animal House of V. V. Institute of Pharmaceutical Sciences, Gudlavalleru. Before and after treatment, the animals were fasted for 12 and 10 h, respectively. However, water was made available ad libitum.
Acute toxicity Studies:
Some unfavourable impacts may result within 24 h from single or multiple exposures of products, which indicate acute toxicity. Lethal dose 50 % (LD50) of the examination of the extracts is found from this research complying with Organisation for Economic Co-operation and Development (OECD) 423 guidelines. Here, an indication of any mortality found after oral management of the test was checked approximately for 1 h. For the next 5-6 h, animals were observed on an hourly basis. Nonetheless, the animals were reserved under monitoring for 2 w.
Evaluation of Antinociceptive Activity
Acetic acid writing reflex:
This was executed, according to Gaertner et al. [17]. Male albino mice (6 per group) were infused intraperitoneally with 0.6 % acetic acid at a dosage of 10 ml/kg. The extract (200, 400 mg/kg mouth (p.o.)), morphine (5 mg/kg subcutaneously (s.c.)), and also distilled water (p.o.) were carried out 30 min before treatment with acetic acid. The writhings induced by the acid, including stomach restrictions and also back limbs stretchings, were counted for 30 min after latency duration of 5 min. The percentage of antinociceptive activity was determined as complies with:
Percentage Antinociceptive activity=N-N?×100/N
Where Nis the average number of stretchings of control per group. N? is the average number of stretchings of tests per group.
Hot Plate Method:
The rats were positioned on a warmer maintained at 55° within the restrainer. The reaction time was identified as the rats' reaction to react to the thermal pain by licking their paws or leaping. The response time was taped before (0 min) and at 15, 30, 45, and 60 min after the management of the therapies. The optimum response time was taken care of at 45 sec to stop any injury to the tissues of the paws. If the reading exceeds 45 s, it would certainly be thought about as optimal analgesia.
Tail Flick Method:
The tail-flick approach assessed the antinociceptive task of the MEIM explained [9]. Concerning 5 cm from the distal end of the tail of each rat was placed in warm water kept at 50°. The reaction time (in s) was the time taken by the rat to flip its tail due to pain. The initial analysis was omitted, and the response time was taken as the average of the next two readings. The reaction time was recorded before (0 min) and at 15, 30, 45, and 60 min after administering the therapies. The optimum response time was repaired at 15 sec to stop any tail tissue injury. If the analysis goes beyond 15 sec, it would be taken into consideration as maximum analgesia. The maximum possible analgesia (MPA) was determined as adheres to:
Percentage Antinociceptive activity=(Reaction time for treatment–Reaction time for Control)/15 s-Reaction time for control×100
Virtual screening:
A structurally based virtual screening was then performed using PyRx software. If Screening results with a binding affinity ≥10.0 kcal/mol were achieved, the corresponding compounds were used for subsequent molecular docking with AutoDock 4.0. Based on the virtual screening results, 25 identified candidate compounds were purchased from AnalytiCon Discovery GmbH (Pots-dam, Germany) to verify the in silico results.
Computational Study:
The molecular docking simulation was done utilizing on cyclooxygenase 2 (COX-2) enzyme (protein data bank ID: 4PH9 (PDB ID: 4PH9) versus the potent substances gathered from the literary works testimonial.
Preparation of ligand structures:
Structures of selected ligands (fig. 1) were downloaded in the structure data file (SDF) documents style from the PubChem Compound Database. Physicochemical abilities of the ligands satisfied the standards of Lipinski's guideline of five or else understood as Lipinski's guideline of drug-likeness [18-19].
Chemical structures in the .SDF layout was transformed into the .PDB layout making use of Discovery Studio Biovia (DSB). After that, AutoDock tools (ADT) used to check out ligand structure regarding amalgamations with nonpolar hydrogens, enhancements of Gasteiger modifications, and rotatable bonds. Structures in the ligand .PDB style was after transformed into the ligand .PDBQT style utilizing ADT, making it possible for users with AutoDock4 [20].
Preparation of COX-2 Protein:
The protein COX-2 (4PH9) was computed and installed from the Research Collaboratory for Structural Bioinformatics (RCSB) protein database and was inscribed with the PDB code 1SA0 (fig. 2). ADT software application was used to prepare the called for declaring AutoDock 4.0 by accrediting hydrogen polarities, computing Gasteiger charges to protein, and transforming protein structure .PDB documents style to .PDBQT layout [21-23] (fig. 2).
Docking Methodology:
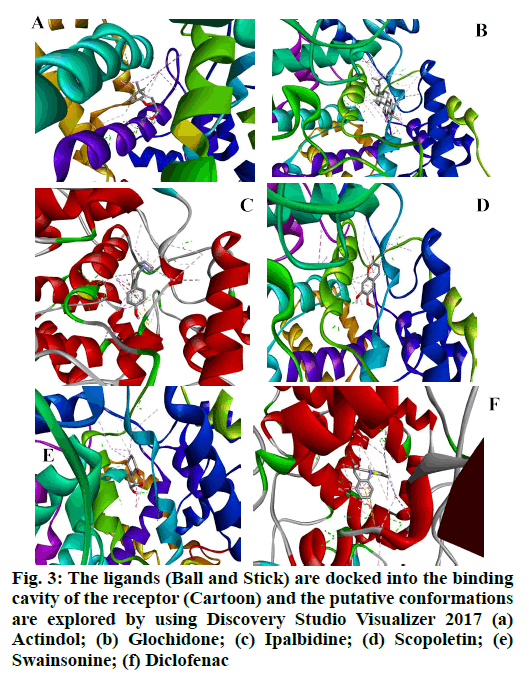
Molecular docking was carried out utilizing the AutoDock program. Ligands were anchored independently to the receptor with grid collaborates (grid facility) and grid boxes of particular dimensions for each receptor. The arrangement data was involved by opening up the note pad to run AutoDock .ADT was needed to prepare the input .PDBQT documents for beta (β)-Tubulin and also to establish the dimension and even the facility of the grid box. The grid dimension was set at 60×60×60 (x, y, as well as z) factors, and also the grid facility was assigned at x, y, as well as z measurements of 124.37, 96.859, as well as 14.124, precisely, with a grid spacing of 0.375 Å. Post-docking evaluations were pictured using DSB, which revealed the dimensions, places of binding sites, hydrogen-bond communications, hydrophobic communications, and bonding, ranging as communication distance <5 A° of from the placement of the docked ligand [20] (fig. 3).
Drug Likeliness Properties:
Our research evaluated the drug likeliness of the separated compounds from I. marginata; we used OSIRIS Data warrior v 4.6.1 software [24].
Statistical Analysis:
All information was revealed as the mean±standard deviation; information went through one-way analysis of variance (ANOVA) adhered to by Tukey examination. The analytical evaluation executed with Graphpad Prism (Version 3, USA) software program. p<0.05 was taken into consideration statistically considerable.
Results and Discussion
Initial phytochemical testing of I. marginata was exposed to different phytoconstituents detailed in Table 1. The antinociceptive task of MEIM was carried out by the acetic acid method (Table 2), hot plate method (Table 3), and also tail-flick method (Table 4).
| Phytoconstituents | Method | Methanolic Extract (MEIM) |
|---|---|---|
| Flavonoids | Shinoda Test | + |
| Zn. Hydrochloride test | + | |
| Lead acetate Test | + | |
| Volatile oil | Stain test | - |
| Alkaloids | Wagner Test | + |
| Hager's Test | + | |
| Tannins & Phenols | FeCl3 Test | + |
| Potassium dichromate test | + | |
| Saponins | Foaming Test | + |
| Steroids | Salkowski test | + |
| Carbohydrates | Molish test | + |
| Acid compounds | Litmus test | - |
| Glycoside | Keller-Killani Test | + |
| Amino acids | Ninhydrin test | + |
| Proteins | Biuret test | + |
Note: "+"=Present; "-"=Absent
Table 1: Phytochemical Analysis of Methanolic Extracts of the Whole Plant of I. marginata
| Treatment | Dose (mg/kg) | N | Number of writhings within 30 min | Percentage inhibition |
|---|---|---|---|---|
| Control (acetic acid) | 0 | 6 | 25±3.45 | 0 |
| Diclofenac Sodium | 5 | 6 | 7.55±1.65* | 69.8 |
| MEIM | 50 | 6 | 22.3±2.54*a | 10.8 |
| MEIM | 100 | 6 | 20±1.25*a | 20 |
| MEIM | 200 | 6 | 18.25±3.43*a | 27 |
| MEIM | 400 | 6 | 12.32±1.86*a | 50.72 |
Note: The results are presented as a mean±standard deviation, (n=5). One-way ANOVA was used to evaluate the results, followed by Dunnett's examination. *p<0.05 vs. control; ap<0.05 vs. standard group
Table 2: Effects of Methanolic Extract of I. marginata in Acetic Acid-Induced Writhing Test.
| Treatment | Reaction Time (s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time after treatment (min) | ||||||||||
| 0 | 0.5 h | 1 h | 1.5 h | 2 h | 3 h | 4 h | 6 h | 8 h | 24 h | |
| Control | 2.87±0.31 | 2.96±0.19 | 2.92±0.15 | 2.88±0.32 | 2.98±0.3 | 2.86±0.05 | 2.91±0.25 | 2.84±0.22 | 3.01±0.32 | 2.92±0.18 |
| Tramadol (2 mg/kg) | 2.82±0.17 | 12.47±1.92* | 9.28±1.26* | 7.26±0.36* | 6.35±0.67* | 4.12±0.98* | 3.58±0.64* | 3.47±0.93* | 3.18±0.28* | 2.94±0.15* |
| MEIM (50 mg/Kg) | 2.67±0.04 | 2.81±0.32a | 3.32±0.21a | 3.56±0.26a | 3.62±0.12a | 3.81±0.54a | 3.57±0.45a | 2.84±0.74a | 2.8±0.21a | 2.74±0.11a |
| MEIM (100 mg/Kg) | 3.11±0.42 | 3.23±0.21 | 3.86±0.88 | 4.78±0.37 | 4.98±0.58 | 5.38±1.12 | 4.66±1.28 | 4.12±0.87 | 3.88±1.15 | 3.52±0.89 |
| MEIM (200 mg/kg) | 2.86±0.31 | 3.58±0.52a | 4.24±0.67a | 5.68±0.28a | 7.28±0.8a | 7.59±1.18a | 6.52±0.67a | 5.66±0.52a | 5.18±0.1a | 4.18±0.25a |
| MEIM (400 mg/kg) | 2.78±0.17 | 4.48±0.35* | 4.69±1.33* | 6.91±1.58* | 8.55±1.47* | 10.28±0.52* | 8.42±1.11* | 6.22±0.87* | 5.64±0.41* | 5.02±0.33* |
Note: The results are presented as a mean±standard deviation, (n=5). One-way ANOVA was used to evaluate the results, followed by Dunnett's examination. *p<0.05 vs. control; ap<0.05 vs. standard group
Table 3: Antinociceptive Profile of I. marginata Whole Plant Extract Assessed by the Hotplate Test in RATS
| Treatment | Reaction Time (s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time after treatment (min) | ||||||||||
| 0 | 0.5 h | 1 h | 1.5 h | 2 h | 3 h | 4 h | 6 h | 8 h | 24 h | |
| Control | 2.87±0.31 | 2.96±0.19 | 2.92±0.15 | 2.88±0.32 | 2.98±0.3 | 2.86±0.05 | 2.91±0.25 | 2.84±0.22 | 3.01±0.32 | 2.92±0.18 |
| Tramadol (2 mg/kg) | 2.82±0.17 | 12.47±1.92* | 9.28±1.26* | 7.26±0.36* | 6.35±0.67* | 4.12±0.98* | 3.58±0.64* | 3.47±0.93* | 3.18±0.28* | 2.94±0.15* |
| MEIM (50 mg/kg) | 2.67±0.04 | 2.81±0.32a | 3.32±0.21a | 3.56±0.26a | 3.62±0.12a | 3.81±0.54a | 3.57±0.45a | 2.84±0.74a | 2.8±0.21a | 2.74±0.11a |
| MEIM (100 mg/kg) | 3.11±0.42 | 3.23±0.21 | 3.86±0.88 | 4.78±0.37 | 4.98±0.58 | 5.38±1.12 | 4.66±1.28 | 4.12±0.87 | 3.88±1.15 | 3.52±0.89 |
| MEIM (200 mg/kg) | 2.86±0.31 | 3.58±0.52a | 4.24±0.67a | 5.68±0.28a | 7.28±0.8a | 7.59±1.18a | 6.52±0.67a | 5.66±0.52a | 5.18±0.1a | 4.18±0.25a |
| MEIM (400 mg/kg) | 2.78±0.17 | 4.48±0.35* | 4.69±1.33* | 6.91±1.58* | 8.55±1.47* | 10.28±0.52* | 8.42±1.11* | 6.22±0.87* | 5.64±0.41* | 5.02±0.33* |
Table 4: Antinociceptive Profile of I. marginata Whole Plant Extract Assessed by the Tail-Flick Test in RATS
In the acetic acid approach, the diclofenac sodium was revealed 69.8 % inhibitions of analgesia. Simultaneously, 50, 100, 200 and 400 mg/kg I. marginata showed 10.8, 20, 27 and 50.72 % inhibitions of analgesia, specifically when contrasted with the acetic acid control team.
In the hot plate method, at 120 min, the mean response time for tramadol of analgesia effect revealed 19.18±1.63, while 50, 100, 200 and 400 mg/kg I. marginata showed significant analgesic effect (14.55±1.69, 16.22±2.58, 20.19±2.22 and 21.16±1.69), respectively. In the tail-flick method, at 90 min, the mean response time for tramadol of analgesia effect revealed 7.26±0.36, while 50, 100, 200 and 400 mg/kg I. marginata showed significant analgesic effect (3.56±0.26, 4.78±0.26, 5.68±0.28, 6.91±1.58) respectively. The difference was statistically significant compared with the control group.
In today's examination, to evaluate the possibility of substances being accountable for antinociceptive activity, the docking score was used to verify the prospective binding energy. The molecules were also based on intelligent learning object guide (iLOG) predictors utilizing online tools to identify their absorption, distribution, metabolism, excretion/toxicity (ADME/T) properties. 13 out of 22 substances strictly adhered to Lipinski's Rule of 5 and were picked for more analyses, and also remainder were eliminated. ADMET properties of the picked 13 substances revealed the capacity of effective compounds (Table 5).
| Compound | Total Mol weight | cLogP | H-Acceptors | H-Donors | Molar Refractivity | Rotatable Bonds | No. of Deviations |
|---|---|---|---|---|---|---|---|
| Actinidol | 194.273 | 1.509 | 2 | 1 | 56.61 | 1 | 0 |
| Agroclavine | 238.333 | 3.0185 | 2 | 1 | 79.18 | 0 | 0 |
| Arctigenin | 372.416 | 2.8465 | 6 | 1 | 100.6 | 7 | 0 |
| Beta Amyrin | 426.726 | 7.3406 | 1 | 1 | 134.88 | 0 | 1 |
| Betulinic Acid | 456.708 | 6.3706 | 3 | 2 | 136.91 | 2 | 1 |
| Caffeic Acid | 180.159 | 0.7825 | 4 | 3 | 47.16 | 2 | 0 |
| Elymoclavine | 254.332 | 2.0918 | 3 | 2 | 80.34 | 1 | 0 |
| Ergometrine | 325.411 | 0.8747 | 5 | 3 | 98.56 | 3 | 0 |
| Esculetin | 178.143 | 0.806 | 4 | 2 | 46.53 | 0 | 0 |
| Friedelin | 426.726 | 7.5888 | 1 | 0 | 134.39 | 0 | 1 |
| Glochidone | 422.694 | 7.5152 | 1 | 0 | 133.71 | 1 | 1 |
| Isoclorogenic Acid A | 516.454 | 0.7977 | 12 | 7 | 83.5 | 9 | 3 |
| Isoclorogenic Acid B | 516.454 | 0.7977 | 12 | 7 | 83.5 | 9 | 3 |
| Isoclorogenic Acid C | 516.454 | 0.7977 | 12 | 7 | 83.5 | 9 | 3 |
| Lysergol | 254.332 | 1.4317 | 3 | 2 | 81.14 | 1 | 0 |
| Penniclavine | 270.331 | 0.6083 | 4 | 3 | 82.34 | 1 | 0 |
| Swainsonine | 173.211 | -1.0292 | 4 | 3 | 46.64 | 0 | 0 |
| Taraxerol | 426.726 | 7.3406 | 1 | 1 | 134.88 | 0 | 1 |
| Umbelliferone | 162.144 | 1.1517 | 3 | 1 | 44.51 | 0 | 0 |
| Ipalbidine | 231.338 | 2.0733 | 2 | 1 | 74.43 | 0 | 0 |
| Scopoletin | 192.17 | 1.0817 | 4 | 1 | 51 | 1 | 0 |
Table 5: Drug Likeliness Analysis of Separated Phytoconstituents from I. marginata
| Ligands | Highest to the Lowest mode of conformation with corresponding RMS binding affinities in ΔG (Kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Actinidol | -6.99 | -6.99 | -6.97 | -6.97 | -6.96 | -6.96 | -6.93 | -6.62 | -6.4 |
| Arctigenin | -5.79 | -3.31 | -1.8 | -1.12 | -0.37 | - | - | - | - |
| Glochidone | -2.91 | -2.5 | -2.33 | 31.58 | 31.59 | 31.71 | 32.33 | 33.54 | 33.61 |
| Swainsonine | -6.16 | -6.1 | -6.1 | -6.07 | -6.03 | -5.96 | -4.92 | -4.86 | -4.77 |
| Chlorogenic acid | -6.07 | -3.87 | -3.45 | -2.62 | -1.28 | -0.62 | 4.96 | - | - |
| Ipalbidine | -8.26 | -8.23 | -7.52 | -7.43 | -7.03 | -6.98 | -6.94 | -6.13 | -6.11 |
| Scopoletin | -6.91 | -6.91 | -6.86 | -6.81 | -5.85 | -5.82 | -5.8 | -5.8 | -5.78 |
| Diclofenac | -7.03 | -6.94 | -6.91 | -6.9 | -6.49 | -6.41 | -5.16 | -5.14 | -4.6 |
Table 6: Binding Affinities of Isolated Compounds at the Active Site of COX-2
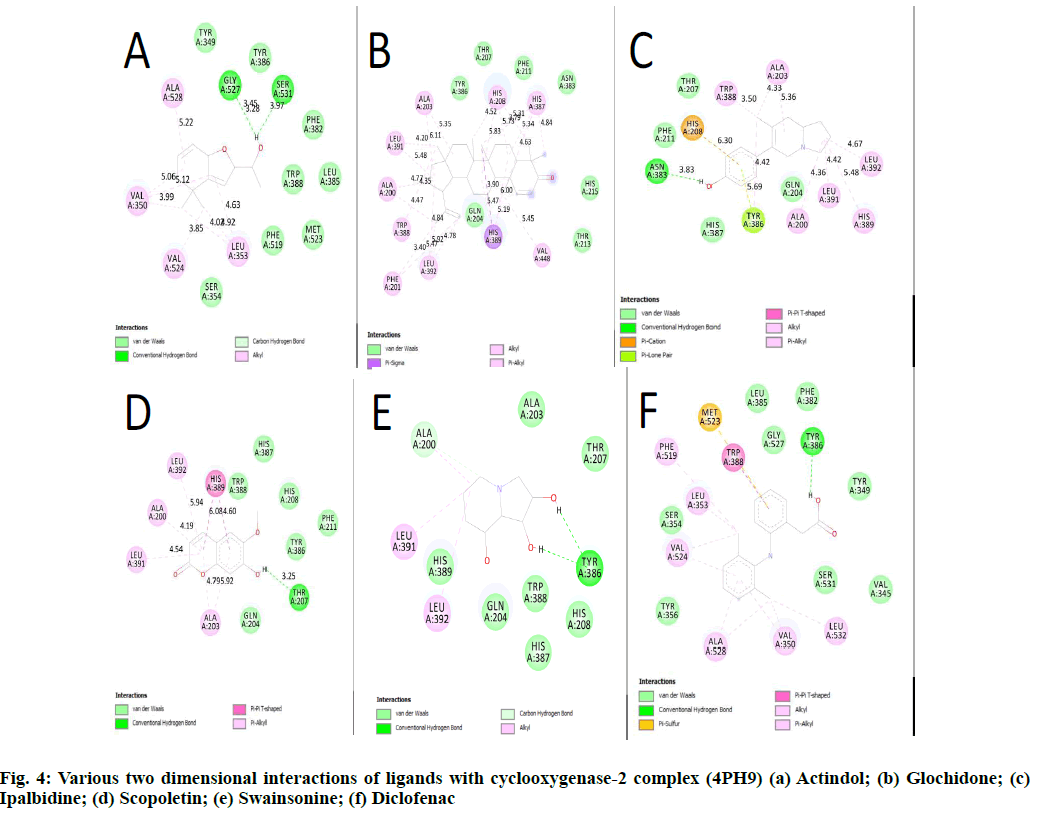
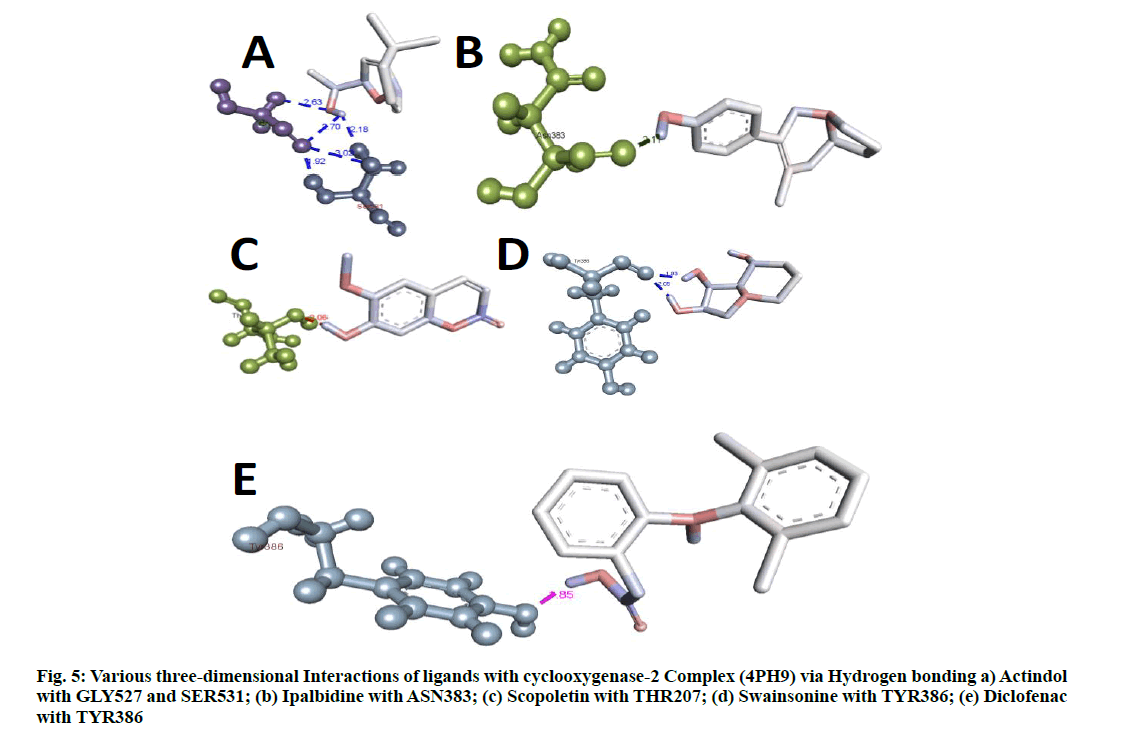
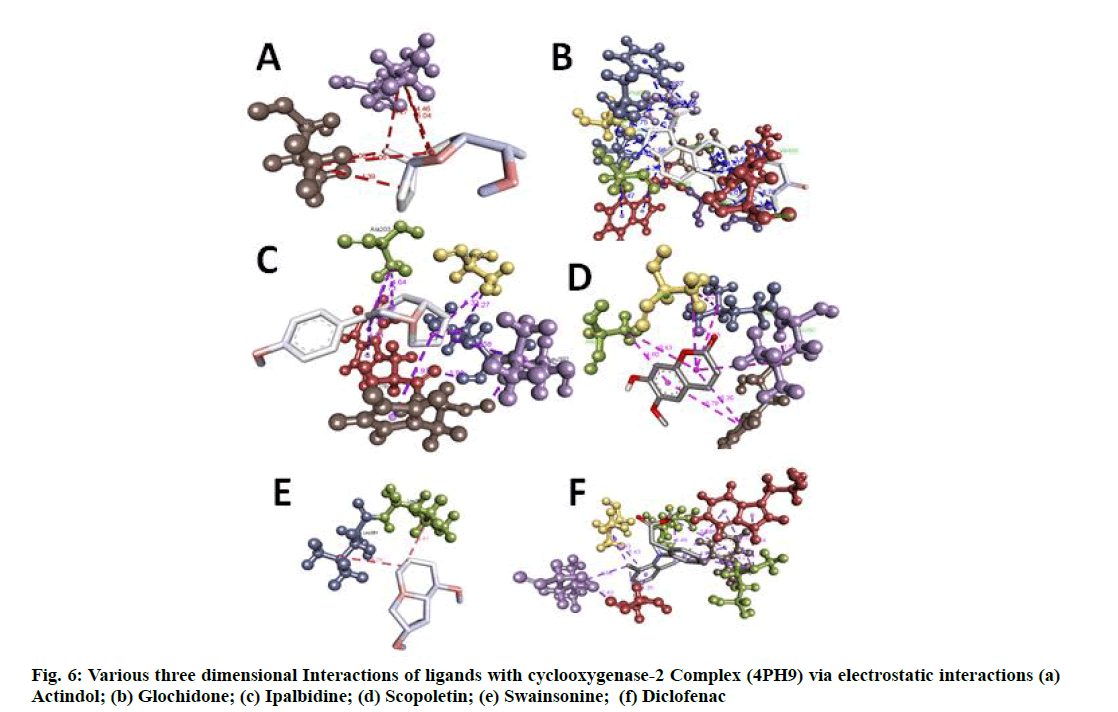
Docking researches revealed that out of all isolated substances consisted of in the study; Ipalbidine had the most excellent docking rating of -8.26 kcal/mol, which showed both hydrogen bond interactions (ASN383) and also electrostatic interactions (ALA200, LEU39, HIS389, LEU392, ALA203, TRP388, HIS208 and TYR386) with the COX-2 enzyme. Except for swainsonine, no other compounds form hydrophobic interactions. However, all compounds had significant electrostatic communications. The standard diclofenac revealed a possible docking rating of -7.03, which was virtually lesser than ipalbidine. The outcomes obtained by the autodock 4.0 are shown in Table 6 and Table 7, and the protein-ligand interactions revealing hydrogen bonding and binding settings, which are also published in fig. 4- fig. 6.
| Ligands | Binding Affinity, ΔG (Kcal/mol) | Amino acids involved and Distance (A°) | ||
|---|---|---|---|---|
| Hydrogen Binding Interactions | Hydrophobic Interactions | Electrostatic Interactions | ||
| Actinidol | -6.99 | GLY527 (3.28; 3.45), SER531 (3.97) | VAL350 (3.99, 5.06, 5.12), LEU353 (3.85, 4.63, 4.02) | |
| Glochidone | -2.91 | ALA203 (5.35, 6.11), LEU391 (4.20, 5.48), ALA200 (4.47, 4.77), TRP388 (4.35), PHE201 (3.40; 5.47), LEU392 (4.78, 5.92), HIS389 (3.90), VAL448 (5.45), HIS387 (4.84, 4.63; 5.73) | ||
| Swainsonine | -6.16 | TYR386 (3.68; 4.73) | ALA200 (4.29) | ALA200 (4.29), LEU391 (4.28), LEU392 (5.29) |
| Chlorogenic acid | -6.07 | PHE530 (5.60) | PHE210 (6.19), PHE382 (6.44), LEU535 (4.28) | |
| Ipalbidine | -8.26 | ASN383 (3.83) | ALA200 (4.36), LEU391 (4.42), HIS389 (5.48), LEU392 (4.67), ALA203 (4.33; 5.36), TRP388 (3.50), HIS208 (6.30), TYR386 (4.42, 5.69) | |
| Scopoletin | -6.91 | THR207 (3.25) | - | LEU392 (5.94), ALA200 (4.19), LEU391 (4.54), ALA203 (4.79; 5.92), HIS389 (4.60, 6.08) |
| Diclofenac | -7.03 | TYR386 (5.16) | TRP388 (6.61), MET523 (7.88), PHE519 (5.12), LEU353 (4.28), VAL524 (4.27; 4.88), ALA528 (3.73; 4.91), VAL350 (4.45, 5.12), LEU532 (5.16) | |
Table 7: Interactions of COX-2 Amino Acid Residues with Ligands at Receptor Sites
The significant addition of therapeutic herbs in medicine growth and commercial medications resources has been incredible. However, pharmaceuticals currently used in therapy and swelling like narcotics and non-steroidal anti-inflammatory drugs possess a higher risk of toxicity [25]. Conversely, natural medicines used, given that the old times keep lower toxicity, have much better absorption and are abundant [26]. Therefore, scientists worldwide remain searching for plants that possess such biological activities, which will undoubtedly help develop existing clinical strategies providing more affordable and efficient therapy.
In the writhing induced model, the stomach constriction approach helps to review the outer antinociceptive task. As soon as acetic acid is infused, intraperitoneally (i.p), the body raises the generation of Prostaglandin E2 and Prostaglandin F2α (PG E2 and PG F2α), accountable for the swelling effect and agony understanding through the COX pathway [27]. The outcome mentioned above suggests that the I. marginata extract significantly prevented the writing, which can be discussed by disrupting the path, causing analgesia.
The hot plate and tail immersion techniques are strategies for establishing central antinociceptive task [28]. These two techniques better review centrally acting analgesics and opioid receptor agonists. Table 2 and Table 3 for the antinociceptive activity of I. marginata in tail immersion and hot plate taste.
The docking research disclosed an intriguing perspective regarding the communication between the provided ligands and the COX-2 enzyme. Protein-ligand communication showed that actindol, swainsonine, ipalbidine, scopoletin and diclofenac created hydrogen bonds to GLY527, SER531; TYR-386; ASN383; THR207and TYR386, respectively. Lesser negative energy shows a stable system, leading to the most likely binding synergy that describes why ipalbidine has the most effective docking score.
Medicine exploration greatly relies on bioinformatics that entails molecular docking related to hit recognition and lead optimization. Unfortunately, numerous medication candidates stop working on getting to the marketplace despite investing substantial funds and time due to their unsafe details and even failing short in crucial tests [29]. Such failings might be avoided by the computational approach, which anticipates the inclination of a substance before it is synthesized. It has accelerated medicine exploration to reduce the overall number of repetitions, time and expenditure [30]. Virtual docking also requires ligand-protein docking to assess a vast collection of the substances to determine the structures that probably crises to a protein [31].
In the current research study, we anchored six ligands of I. marginata right into the energetic site of the COX-2 enzyme and also, the docking score was gauged, making use of Autodock 4.0. As a result, the ipalbidine had the highest docking rating of -8.26 kcal/mol, recommending a strong communication between ipalbidine and the COX-2 enzyme. It is comprehended that the evaluation of ADMET in the exploration phase considerably lowering the faltering connected to pharmacokinetics in the scientific stage [32]. Therefore, the computer-based prognosis of ADME/T from the molecular structure is a legitimate choice for experiential methods [33]. In this research, ipalbidine has the greatest "drug-likeness" for oral delivery due to its low molecular weight, which enhances the permeation of medicine [34], reduced lipophilicity (log Po/w) showing far better oral absorption as well as bioavailability [35], as well as reduced hydrogen-bonding ability connecting to more significant permeability in the structure and also absorption [18]. For this reason, ipalbidine is an ideal choice for antinociceptive medicine.
The research demonstrated that MEIM possesses a considerable analgesic task, as verified by three designs in this research study. First, high-throughput Screening using molecular docking and ADME/T evaluation resulted in the verdict that ipalbidine revealed the best fitness score and appropriates human intake. Even more, a complicated measurable SAR model is needed to guarantee its safety and security and bioefficacy. Finally, the outcomes confirm the ethnomedicinal use of I. marginata to pain condition, which recommend this plant, might be a prospective resource for advancing a new analgesic agent.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Craig CR, Stitzel RE. Modern pharmacology with clinical applications: Lippincott Williams & Wilkins; 2004.
- Boursinos L, Karachalios T, Poultsides L, Malizos K. Do steroids, conventional non-steroidal anti-inflammatory drugs, and selective Cox-2 inhibitors adversely affect fracture healing. J Musculoskelet Neuronal Interact 2009;9(1):44-52.
- Gunn-Moore D. NSAIDs and Cats—it's been a Long Journey. SAGE Publications Sage UK: London, England; 2010.
- Koech SC, Ouko RO, Michael NM, Ireri MM, Ngugi MP, Njagi NM. Analgesic activity of dichloromethanolic root extract of Clutia abyssinica in swiss albino mice. Nat Prod Chem Res 2017;5(255):2.
- Yasmen N, Aziz M, Tajmim A, Akter M, Hazra AK, Rahman SM. Analgesic and anti-inflammatory activities of diethyl ether and n-hexane extract of Polyalthia suberosa leaves. Evid Based Complement Alternat Med 2018;2018.
- Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol 2001;15(5):691-703.
- Shih SC, Chang CW. Nonsteroidal anti-inflammatory drug-related gastrointestinal bleeding in the elderly. Int J Gerontol 2007;1(1):40-5.
- Aziz MA, Mehedi M, Akter MI, Sajon SR, Mazumder K, Rana MS. In vivo and In silico evaluation of analgesic activity of Lippia alba. Clin Phytosci 2019;5(1):1-9.
- Daniel M. Medicinal plants: chemistry and properties: CRC press; 2016.
- Roxburgh W. Flora Indica: Or, Descriptions of Indian Plants. Reprinted Literatim from Carey's Edition of 1832: Thacker, Spink; 1874.
- Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology (5 Volume Set): CRC press; 2016.
- Silja V, Varma KS, Mohanan K. Ethnomedicinal plant knowledge of the Mullu kuruma tribe of Wayanad district, Kerala; 2008.
- Kokate C. Practical pharmacognosy. Vallabh Prakashan, New Delhi; 1986. p. 111.
- Raaman N. Phytochemical techniques. Pitam Pura, New Delhi: New India Publ. Agency; 2006.
- Wallis TE. Textbook of pharmacognosy; 1955. p. 3.
- Khandelwal KR. Practical pharmacognosy : techniques and experiments. Maharashtra: Niral Prakashan; 2008.
- Gaertner M, Müller L, Roos J, Cani G, Santos A, Niero R, et al. Analgesic triterpenes from Sebastiania schottiana roots. Phytomedicine 1999;6(1):41-4.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Del Rev 1997;23(1-3):3-25.
- Zhang MQ, Wilkinson B. Drug discovery beyond the 'rule-of-five'. Curr Opin Biotechnol 2007;18(6):478-88.
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31(2):455-61.
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 2009;30(16):2785-91.
- Lim SV, Rahman MBA, Tejo BA. Structure-based and ligand-based virtual Screening of novel methyltransferase inhibitors of the dengue virus. BMC Bioinform 2011;12(13):1-12
- Jaghoori MM, Bleijlevens B, Olabarriaga SD. 1001 Ways to run AutoDock Vina for virtual Screening. J Comput Aided Mol Des 2016;30(3):237-49.
- Sander T, Freyss J, von Korff M, Rufener C. DataWarrior: an open-source program for chemistry aware data visualization and analysis. J Chem Inf Model 2015;55(2):460-73.
- Park JH, Son KH, Kim SW, Chang HW, Bae K, Kang SS, et al. Antiin?ammatory activity of Synurus deltoides. Phytother Res 2004;18(11):930-3.
- Li RW, Myers SP, Leach DN, Lin GD, Leach G. A cross-cultural study: anti-inflammatory activity of Australian and Chinese plants. J Ethnopharmacol 2003;85(1):25-32.
- Ahmed F, Hossain MH, Rahman AA, Shahid IZ. Antinociceptive and sedative effects of the bark of Cerbera odollam Gaertn. Adv Tradit Med 2006;6(4):344-8.
- Singh P, Bast F. In silico molecular docking study of natural compounds on wild and mutated epidermal growth factor receptor. Med Chem Res 2014;23(12):5074-85.
- Chen YZ, Zhi DG. Ligand–protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins 2001;43(2):217-26.
- Singh J, Chuaqui CE, Boriack-Sjodin PA, Lee WC, Pontz T, Corbley MJ, et al. Successful shape-based virtual screening: the discovery of a potent inhibitor of the type I TGFβ receptor kinase (TβRI). Bioorg Med Chem Lett 2003;13(24):4355-9.
- Rester U. From virtuality to reality-Virtual Screening in lead discovery and lead optimization: a medicinal chemistry perspective. Curr Opin Drug Discov Devel 2008;11(4):559-68.
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol 2014;32(1):40-51.
- Dahlin JL, Inglese J, Walters MA. Mitigating risk in academic preclinical drug discovery. Nat Rev Drug Discov 2015;14(4):279-94.
- Duffy FJ, Devocelle M, Shields DC. Computational approaches to developing short cyclic peptide modulators of protein–protein interactions. Computational Peptidology; 2015. p. 241-71.
- Daina A, Michielin O, Zoete V. iLOGP: a simple, robust and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J Chem Inf Model 2014;54(12):3284-301.