- *Corresponding Author:
- Deying Li
Department of Oncology, Shanghai Pudong New Area Lianyang Community Health Service Center, Pudong, Shanghai 201204, China
E-mail: 13524797250@163.co
| Date of Received | 18 December 2021 |
| Date of Revision | 05 October 2022 |
| Date of Acceptance | 12 August 2023 |
| Indian J Pharm Sci 2023;85(4):1144-1149 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the influence and possible mechanism of extracts from Phellinus igniarius (expanded program on immunization) on oral squamous cell carcinoma cell proliferation, migration and invasion. CAL27 cells were treated with expanded program on immunization or transfected with microRNA- 655-3p. Furthermore, transfected CAL27 cells were exposed to 600 μg/ml expanded program on immunization. Proliferation, migration and invasion were assessed using 3-(4,5-dimethylthiazole-2-yl)- 2,5-diphenyltetrazolium bromide and Transwell. Matrix metallopeptidase 2, matrix metallopeptidase 9 and proliferating cell nuclear antigen protein levels were monitored using Western blot. After various doses of expanded program on immunization, cell viability, migration, invasion, proliferating cell nuclear antigen, matrix metallopeptidase 2 and matrix metallopeptidase 9 protein levels were reduced, but microRNA-655-3p content was enhanced in a dose-dependent way. Meanwhile, increased microRNA-655- 3p might diminish cell proliferation, migration and invasion. Beyond that, microRNA-655-3p knockdown overturned high-dose expanded program on immunization-mediated tumor development repression. Expanded program on immunization could hinder oral squamous cell carcinoma cell development via modulating microRNA-655-3p.
Keywords
Oral squamous cell carcinoma, immunization, microRNA-655-3p, proliferation, metastasis
As a prevalent clinical malignancy, Oral Squamous Cell Carcinoma (OSCC) has a low 5 y survival rate due to local invasion and cervical lymph node metastasis[1,2]. During recent years, chemotherapeutic drugs and other conventional treatments have toxic side effects and most patients are prone to develop drug resistance, thereby reducing the effectiveness of treatment[3,4]. Accordingly, finding safe and effective therapeutic drugs has become a priority in OSCC treatment. Convincing evidence has indicated that the active ingredients of traditional Chinese medicine have antioxidant, anti-inflammatory and anti-tumor effects, but the specific mechanism has not been clarified[5,6]. Of note, Phellinus igniarius (PI) is the fungus of the family Hymenochaetaceae, whose main components include polysaccharides, polyphenols and other active ingredients[7]. Further research suggested that Extracts from PI (EPI) have anti-tumor effects[8], but their influences on the biological behavior of OSCC remain unknown. Micro Ribonucleic Acids (miRNAs) are small non-coding single-stranded RNA molecules, which can participate in the occurrence and development of multiple tumors[9]. Recent reports have described that miR-655-3p, a well-known tumor-suppressor miRNA, partakes in the modulation of different human tumors[10,11], containing OSCC[12]. Yet, no report has presented whether miR-655-3p is a potential target for EPI- mediated OSCC development. Hence, this project is designed to investigate whether EPI can affect OSCC properties via regulating miR-655-3p.
Materials and Methods
Reagents:
CAL27 cells were purchased from Bioshhy (Shanghai, China) and PI was provided by Jiekangtang Pharmaceutical Industry (Bozhou, China). Dulbecco's Modified Eagle Medium (DMEM) medium, Fetal Bovine Serum (FBS), trypsin, and 3-(4,5-Dimethylthiazol-2-yl)-2,5- Diphenyl tetrazolium bromide (MTT) reagent were acquired from Beyotime (Shanghai, China). Invitrogen (Carlsbad, California, United States of America (USA)) offered LipofectamineTM 3000 transfection reagent. Solarbio (Beijing, China) supplied Trizol reagent. Tiangen (Beijing, China) provided reverse transcription and fluorescent quantitative Polymerase Chain Reaction (qPCR) reagents. Transwell chamber and Maribel were respectively supplied by Corning Costar (Corning, USA) and BD Biosciences (Franklin Lakes, New Jersey, USA). miR-Negative Control (NC), miR- 655-3p mimics, anti-miR-NC and anti-miR-655-3p were provided by Ribobio (Guangzhou, China). Rabbit anti-human Matrix Metallopeptidase (MMP) 2, MMP9, and Proliferating Cell Nuclear Antigen (PCNA) antibodies together with Horse Radish Peroxidase (HRP)-labeled goat anti-rabbit Immunoglobulin G (IgG) secondary antibodies were supplied by Cell Signaling Technology (CST) (Danvers, Massachusetts, USA).
Methods:
Preparation of EPI: EPI preparation was implemented as previously described[13]. After drying, slicing, grind, PI was filtered through a 40-mesh sieve and weighed 100 g. Following the addition of 2 l of 95 % ethanol; for reflux extraction for 1 h, the filtrate was collected and subjected to 2 l of 95 % ethanol for reflux extraction for 1 h. After being concentrated at 45° under reduced pressure, the collected filtrate was dried to give a 95 % ethanol extract, which was diluted with Dimethyl Sulfoxide (DMSO) at a concentration of 150, 300 and 600 μg/ml.
Cell treatments: 1×104 CAL27 cells at the logarithmic growth stage were routinely in DMEM medium exposed to different doses of EPI for 24 h, namely as the 150 μg/ml EPI, 300 μg/ml EPI and 600 μg/ml EPI groups. Simultaneously, normal cultured CAL27 cells were recorded as the NC group. Based on the Lipofectamine method, miR- NC or miR-655-3p were introduced into CAL27 cells, generating miR-NC or miR-655-3p groups. Meanwhile, the study performed transfection of anti-miR-NC or anti-miR-655-3p in CAL27 cells, followed by addition with 600 μg/ml EPI for 24 h, terms as 600 μg/ml EPI+anti-miR-NC or 600 μg/ ml EPI+anti-miR-655-3p group.
MTT assay: After being collected, 3×103 CAL27 cells were added with 20 μl MTT solution at 37° and 5 % Carbon dioxide (CO2) for 4 h. Following centrifugation at 3000 r/min for 5 min, the supernatant was discarded, 150 μl DMSO was added, and incubated for 5 min by oscillating against the light. The absorbance value (A value) was assessed by an enzyme labeling instrument at 490 nm.
Transwell assay: In migration experiment, CAL27 cells in each group were prepared into single-cell suspension (2×105 cells/ml), which was added to the upper chamber (200 μl/well). 600 μl medium containing 10 % FBS was introduced into lower counterpart, which was cultured in the incubator for 24 h and stained for 15 min. Migration number was observed under a microscope. In invasion experiment, Matrigel matrix gel diluent was added to the upper chamber (40 μl/well) and incubated. The follow-up assay procedure was the same as for cell migration assay.
Real Time-qPCR (qRT-PCR): RNA from CAL27 cells was extracted using a Trizol reagent. Reverse transcription system was as follows; deoxyribonucleic Acid (DNA) buffer 2 μl, 10×King RT buffer 2 μl, Fast King RT enzyme mix 1 μl, FQ-RT primer mix 2 μl, RNA (2 μg), RNase-Free double-distilled Water (ddH2O) supplement system to 20 μl. Reaction conditions at 42° for 15 min and 95° for 3 min. Then, complementary DNA (cDNA) template was used for qRT-PCR amplification and ABI Step OnePlus fluorescence quantitative PCR was used to detect gene relative expression.
Western blot: Total protein from CAL27 cells was prepared using 500 μl Radioimmunoprecipitation Assay (RIPA) buffer. 50 μg protein samples were taken for Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) transmembrane and blocked for 2 h. After incubation with MMP2 (1:800), MMP9 (1:800), PCNA (1:1000), and Beta (β)-actin (1:2000) at 4° for 24 h, the member was added with secondary antibody (1:300). ImageJ software analyzed gray values.
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 21.0 was employed to analyze data, which were expressed as (x͞ ±s). The t-test and one-way analysis of variance was used for comparison. Difference were deemed significant when p<0.05.
Results and Discussion
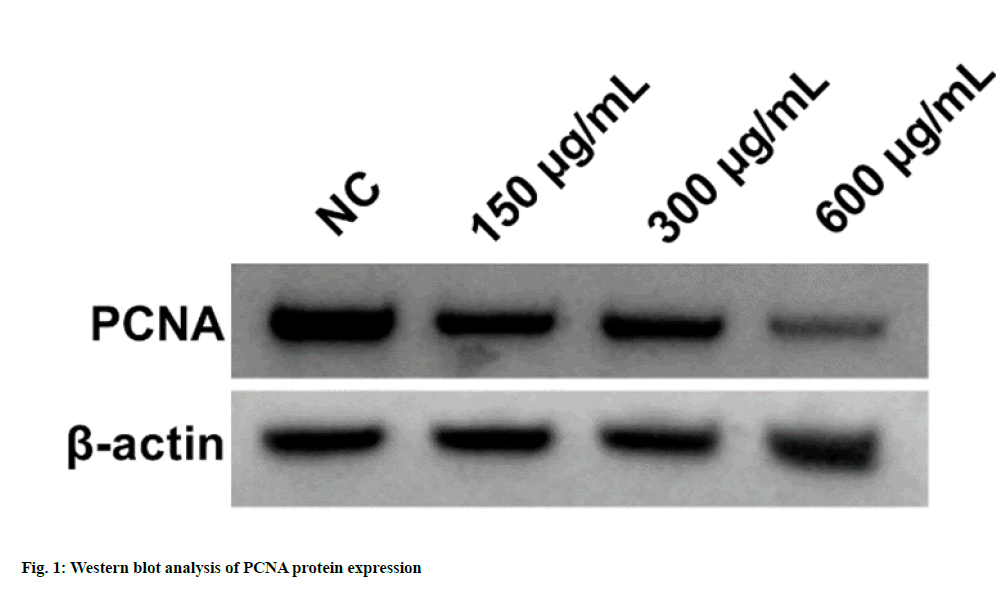
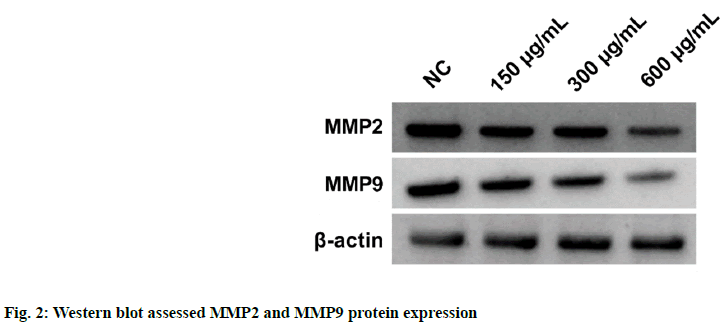
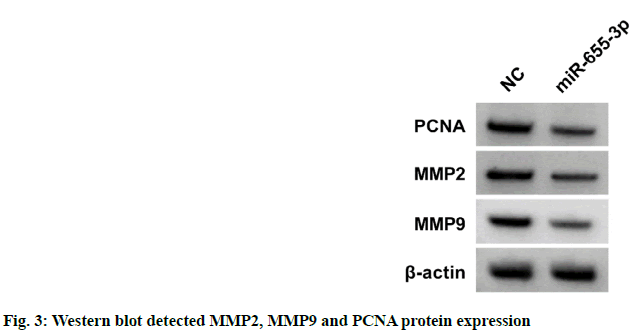
According to the data illustrated in fig. 1 and Table 1, cell proliferation and PCNA protein levels were reduced after EPI treatment in dose-dependent manners (p<0.05). As displayed in fig. 2 and Table 2, migration and invasion number, and MMP2 and MMP9 protein levels were decreased after EPI exposure in concentration-dependent ways (p<0.05). Data exhibited that miR-655-3p was upregulated in EPI-induced CAL27 cells in a dose-dependent ways (p<0.05) as shown in Table 3. As presented in fig. 3 and Table 4, cell proliferation, PCNA, MMP2 and MMP9 protein levels, and the numbers of migration and invasion were obviously declined in the miR-655-3p group vs. the miR-NC group (p<0.05).
| Group | PCNA | A value |
|---|---|---|
| NC | 0.91±0.08 | 1.128±0.10 |
| 150 μg/ml EPI | 0.75±0.07* | 0.943±0.07* |
| 300 μg/ml EPI | 0.57±0.04* | 0.725±0.07* |
| 600 μg/ml EPI | 0.42±0.04* | 0.613±0.06* |
| F | 112.779 | 80.875 |
| p | 0.000 | 0.000 |
Note: *p<0.05, relative to NC group
Table 1: Effects of Various Doses of EPI on CAL27 Cell Proliferation (x̄±s, n=9)
| Group | Migration number | Invasion number | MMP2 | MMP9 |
|---|---|---|---|---|
| NC | 218±18.16 | 172±12.58 | 0.88±0.07 | 0.75±0.07 |
| 150 μg/ml EPI | 181±17.03* | 149±12.37* | 0.72±0.07* | 0.65±0.05* |
| 300 μg/ml EPI | 152±13.67* | 102±8.71* | 0.58±0.05* | 0.48±0.04* |
| 600 μg/ml EPI | 114±8.19* | 73±6.09* | 0.42±0.03* | 0.38±0.03* |
| F | 80.051 | 170.117 | 105.091 | 100.485 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to NC group
Table 2: Effects of EPI on CAL27 Cell Migration and Invasion (x̄±s, n=9)
| Group | miR-655-3p |
|---|---|
| NC | 1.00±0.10 |
| 150 μg/ml EPI | 1.38±0.11* |
| 300 μg/ml EPI | 1.78±014* |
| 600 μg/ml EPI | 2.24±0.19* |
| F | 131.167 |
| p | 0.000 |
Note: *p<0.05, relative to NC group
Table 3: EPI Triggered Effect on miR-655-3p (x̄±s, n=9)
| Group | miR-655-3p | Migration number | Invasion number | MMP2 | MMP9 | PCNA | A value |
|---|---|---|---|---|---|---|---|
| miR-NC | 1.00±0.09 | 215±17.52 | 175±14.28 | 0.86±0.07 | 0.76±0.07 | 0.92±0.09 | 1.124±0.09 |
| miR-655-3p | 2.57±0.21* | 124±10.05* | 91±8.02* | 0.41±0.04* | 0.36±0.03* | 0.46±0.04* | 0.643±0.05* |
| t | 20.615 | 13.516 | 15.386 | 16.745 | 15.757 | 14.012 | 14.016 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to miR-NC group
Table 4: Influences of miR-655-3p on Cal27 Cell Malignant Behaviors (x̄±s, n=9)
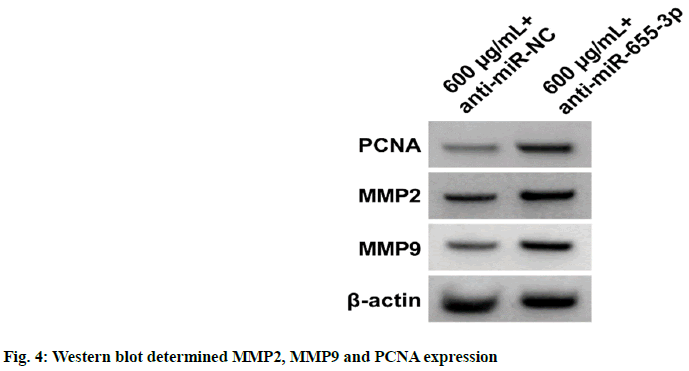
Results from fig. 4 and Table 5 exhibited that cell proliferation, PCNA, MMP2 and MMP9 protein levels, and migration and invasion numbers were reduced in the EPI+anti-miR-655-3p group relative to the EPI+anti-miR-NC group (p<0.05).
| Group | miR-655-3p | Migration number | Invasion number | MMP2 | MMP9 | PCNA | A value |
|---|---|---|---|---|---|---|---|
| 600 μg/ml EPI+anti-miR-NC | 1.00±0.10 | 111±9.73 | 75±7.05 | 0.43±0.03 | 0.37±0.02 | 0.40±0.03 | 0.617±0.06 |
| 600 μg/ml EPI+anti-miR-655-3p | 0.46±0.05* | 231±19.86* | 157±12.38* | 0.78±0.07* | 0.79±0.07* | 0.94±0.08* | 1.179±0.07* |
| t | 14.49 | 16.278 | 17.267 | 13.787 | 17.307 | 18.961 | 18.287 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: *p<0.05, relative to EPI+miR-NC group
Table 5: Anti-miR-655-3p Might Abate EPI-Evoked Effects on CAL27 Cell Proliferation, Migration and Invasion (x̄±s, n=9)
As a highly prevalent malignancy worldwide, the incidence of OSCC is expected to rise, with a projected increase of >40 % by 2040[14]. Due to the unique advantages of anti-tumor, traditional Chinese medicine extracts have attracted extensive attention in OSCC treatment[15]. Overwhelming evidence suggested that numerous miRNAs might partake in modulating the biological behaviors of OSCC cells via interaction with target genes, offering a possibility of miRNAs as a target for OSCC[16,17]. Yet, whether miRNAs may be potential targets for OSCC treatment with traditional Chinese medicine has not been elucidated.
EPI can promote apoptosis in gastric cancer cells and also has anti-tumor effects[18,19]. Here, EPI could reduce OSCC cell viability in a concentration- dependent manner. A recent report indicated that increased PCNA could boost OSCC cell proliferation and migration[20]. In this research, with the increase in the doses of EPI, the level of PCNA protein decreased, implying that EPI can hinder OSCC cell proliferation. Beyond that, MMP2 and MMP9 belong to matrix metalloproteinase, and their increased expression levels can expedite OSCC cell metastasis[21]. Herein, EPI could reduce the migration and invasion ability of OSCC cells, and constrain MMP2 and MMP9 protein expression, suggesting that EPI could retard OSCC cell migration and invasion.
Enhanced miR-655-3p might inhibit retinoblastoma cell metastasis[22]. Meanwhile, miR-655-3p is down-regulated in hepatocellular carcinoma, and it’s up-regulation can inhibit the progression of the tumor[11]. Furthermore, some literature exhibited that miR-655 might impede OSCC proliferation and invasion through different pathways[12,23]. In this paper, the expression of miR-655-3p was increased in OSCC cells after treatment with EPI. Elevated miR-655-3p could diminish OSCC cell proliferation, migration and invasion, while its inhibition could antagonize the repression of EPI on OSCC cell proliferation, migration and invasion. These findings disclosed that EPI could exert anti-OSCC effects by accelerating miR-655- 3p expression.
In summary, EPI could dwindle the development of OSCC cells via boosting miR-655-3p expression. Apart from that, miR-655-3p might be a potential target of EPI for the treatment of OSCC and might provide a new direction for the development of therapeutic drugs for OSCC. However, it needs to be further investigated whether EPI could play an anti-OSCC role via regulating other genes or signaling pathways.
Author’s contributions:
Deying Li and Tingting Xu have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ling Z, Cheng B, Tao X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int J Cancer 2021;148(7):1548-61.
[Crossref] [Google Scholar] [PubMed]
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73(1):17-48.
[Crossref] [Google Scholar] [PubMed]
- Meng X, Lou QY, Yang WY, Wang YR, Chen R, Wang L, et al. The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential. Cancer Commun 202;41(10):981-1006.
[Crossref] [Google Scholar] [PubMed]
- Choi HS, Kim YK, Yun PY. Assessing gene expression related to cisplatin resistance in human oral squamous cell carcinoma cell lines. Pharmaceuticals 2022;15(6):704.
[Crossref] [Google Scholar] [PubMed]
- Hou F, Liu Y, Cheng Y, Zhang N, Yan W, Zhang F. Exploring the mechanism of Scutellaria baicalensis georgi efficacy against oral squamous cell carcinoma based on network pharmacology and molecular docking analysis. Evid Based Complement Altern Med 2021;2021:5597586.
[Crossref] [Google Scholar] [PubMed]
- Wei D, Yang H, Zhang Y, Zhang X, Wang J, Wu X, et al. Nano-traditional Chinese medicine: A promising strategy and its recent advances. J Mater Chem B 2022;10(16):2973-94.
- Zhou LW, Vlasák J, Qin WM, Dai YC. Global diversity and phylogeny of the Phellinus igniarius complex (Hymenochaetales, Basidiomycota) with the description of five new species. Mycologia 2016;108(1):192-204.
[Crossref] [Google Scholar] [PubMed]
- He P, Zhang Y, Li N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: A review. Food Function 2021;12(5):1856-81.
[Crossref] [Google Scholar] [PubMed]
- Hill M, Tran N. miRNA interplay: Mechanisms and consequences in cancer. Dis Mod Mech 2021;14(4):047662.
[Crossref] [Google Scholar] [PubMed]
- Liu ZL, Wang SK, Pang L, Meng XW. circXRCC5 foster gastric cancer growth and metastasis by the HNRNPC/circXRCC5/miR-655-3p/RREB1/UBA2 positive feedback loop. Cancer Gene Ther 2022;29(11):1648-61.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Liu J, Cui J, Zhong R, Sun G. Role of lncRNA LINC01194 in hepatocellular carcinoma via the miR-655-3p/SMAD family member 5 axis. Bioengineered 2022;13(1):1115-25.
[Crossref] [Google Scholar] [PubMed]
- Yu L, Huo L, Shao X, Zhao J. lncRNA SNHG5 promotes cell proliferation, migration and invasion in oral squamous cell carcinoma by sponging miR-655-3p/FZD4 axis. Oncol Lett 2020;20(6):310.
[Crossref] [Google Scholar] [PubMed]
- Wang JH, Wang JJ, Ju TY, Huang YX, Yuan LX, Luo YH, et al. Analysis of Phellinus igniarius effects on gastric cancer cells by atomic force microscopy. Micron 2023;164:103376.
[Crossref] [Google Scholar] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-49.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Duan X, Zhang Y, Meng Z, Wang J. Traditional Chinese medicine for oral squamous cell carcinoma: A Bayesian network meta-analysis protocol. Medicine 2020;99(43).
[Crossref] [Google Scholar] [PubMed]
- Manzano-Moreno FJ, Costela-Ruiz VJ, García-Recio E, Olmedo-Gaya MV, Ruiz C, Reyes-Botella C. Role of salivary MicroRNA and cytokines in the diagnosis and prognosis of oral squamous cell carcinoma. Int J Mol Sci 2021;22(22):12215.
[Crossref] [Google Scholar] [PubMed]
- Jadhav KB, Nagraj SK, Arora S. miRNA for the assessment of lymph node metastasis in patients with oral squamous cell carcinoma: Systematic review and metanalysis. J Oral Pathol Med 2021;50(4):345-52.
[Crossref] [Google Scholar] [PubMed]
- Wang FF, Shi C, Yang Y, Fang Y, Sheng L, Li N. Medicinal mushroom Phellinus igniarius induced cell apoptosis in gastric cancer SGC-7901 through a mitochondria-dependent pathway. Biomed Pharmacother 2018;102:18-25.
[Crossref] [Google Scholar] [PubMed]
- Dong Y, Qiu P, Zhu R, Zhao L, Zhang P, Wang Y, et al. A combined phytochemistry and network pharmacology approach to reveal the potential antitumor effective substances and mechanism of Phellinus igniarius. Front Pharmacol 2019;10:266.
[Crossref] [Google Scholar] [PubMed]
- Kong Y, Feng Y, Xiao YY, Liu SC, Li XG, Yang QL, et al. lncRNA LUCAT1 promotes growth, migration and invasion of oral squamous cell carcinoma by upregulating PCNA. Eur Rev Med Pharmacol Sci 2020;24(14):7569.
[Crossref] [Google Scholar] [PubMed]
- Gao L, Dong J, Zhang N, Le Z, Ren W, Li S, et al. Cyclosporine A suppresses the malignant progression of oral squamous cell carcinoma in vitro. Anticancer Agents Med Chem 2019;19(2):248-55.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Wang Z, Gao M, Wang X, Feng H, Cui Y, et al. lncRNA MALAT1 regulated ATAD2 to facilitate retinoblastoma progression via miR-655-3p. Open Med 2021;16(1):931-43.
[Crossref] [Google Scholar] [PubMed]
- Wang Q, Lv L, Li Y, Ji H. microRNA-655 suppresses cell proliferation and invasion in oral squamous cell carcinoma by directly targeting metadherin and regulating the PTEN/AKT pathway. Mol Med Rep 2018;18(3):3106-14.
[Crossref] [Google Scholar] [PubMed]