- *Corresponding Author:
- Ping Cao
Department of Gastroenterology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi Province 030032, China
E-mail: success19830322@163.com
| Date of Received | 07 February 2022 |
| Date of Revision | 12 March 2023 |
| Date of Acceptance | 04 October 2023 |
| Indian J Pharm Sci 2023;85(6):1755-1760 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study explored Sapindus saponins role in the proliferation and metastasis of colorectal cancer cells and its possible mechanism. SW620 cells were divided into 8 groups; control group, Sapindus saponin-low group, Sapindus saponin-middle group, Sapindus saponin-high group, microRNA+negative control group, microRNA- 5590-3p group, Sapindus saponin+anti-microRNA-negative control group and Sapindus saponin+anti-microRNA-5590-3p group. Cell proliferation and metastasis were determined using colony formation assay, wound healing assay, 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay and transwell assay. MicroRNA-5590-3p expression was detected by quantitative reverse transcription-polymerase chain reaction. Matrix metalloproteinases-2 and matrix metalloproteinases-9 protein levels were examined by Western blot. SW620 cell proliferation inhibition rate and microRNA-5590-3p expression were increased, while colony numbers, invaded cell numbers, wound healing rate, matrix metalloproteinases-2 and matrix metalloproteinases-9 protein levels were decreased in Sapindus saponin-low group, Sapindus saponin-middle group, Sapindus saponin-high group in a dose-dependent manner. Besides, microRNA-5590-3p overexpression increased SW620 cell proliferation inhibition rate, while suppressed colony numbers, invaded cell numbers, wound healing rate, matrix metalloproteinases-2 and matrix metalloproteinases-9 protein levels. Compared with the Sapindus saponin+anti-microRNA-negative control group, SW620 cell proliferation inhibition rate was reduced, while colony numbers, invaded cell numbers, wound healing rate, matrix metalloproteinases-2 and matrix metalloproteinases-9 protein levels were enhanced in the Sapindus saponin+anti-microRNA-5590- 3p group. Sapindus saponin could repress colorectal cancer cell proliferation and metastasis by upregulating microRNA-5590-3p.
Keywords
Colorectal cancer, Sapindus saponin, microRNA-5590-3p, matrix metalloproteinases, chemotherapy
Colorectal Cancer (CRC) is a common clinical malignant tumor[1,2]. Chemotherapy drugs and other drugs have great toxic and side effects, and patients are prone to develop drug resistance leading to poor treatment effect[3,4]. It is of great significance to find drugs that can inhibit CRC cell proliferation and metastasis. Traditional Chinese Medicine (TCM) has anti-tumor effects and can regulate CRC cell biological behaviors[5,6]. It has been reported that Sapindus saponin inhibits proliferation in lung cancer and hepatocellular carcinoma[7,8]. However, the effect of Sapindus saponin on CRC progression is still unknown.
MicroRNA (miRNA) is widely involved in regulating human disease processes[9,10]. Studies had shown that miR-5590-3p downregulation facilitated proliferation in prostate cancer cells[11]. Besides, miR-5590-3p had been confirmed to restrain cell invasion in ovarian cancer and renal cancer[12,13]. Therefore, miR-5590-3p may serve as a tumor suppressor. However, its role in CRC progression has not been elucidated.
In this, we found that Sapindus saponin has a promotion effect on miR-5590-3p expression, but whether Sapindus saponin mediates CRC progression by increasing miR-5590-3p remains unclear. Basing on the above, we hypothesized that Sapindus saponin inhibited CRC cell functions by upregulating miR-5590-3p.
Materials and Methods
Cell culture and grouping:
SW620 cells (Procell, Wuhan, China) were grown in Dulbecco's Modified Eagle Medium (DMEM) plus 10 % Fetal Bovine Saline (FBS) and 1 % dualantibiotic (Invitrogen, Carlsbad, California, United States of America (USA)). Cells were treated with Sapindus saponin (Pushida, Wuhan, China) for 24 h[8], and recorded as Sapindus saponin+low (25 μg/ml) group, Sapindus saponin+middle (50 μg/ ml) group and Sapindus saponin+high (100 μg/ml) group. Normal cultured SW620 cells were used as control group. Lipofectamine 3000 (Invitrogen) was used for cell transfection. miR-5590-3p mimic and miR-NC were transfected into SW620 cells, recording as miR-NC group and miR-5590- 3p group. Besides, SW620 cells were transfected with anti-miR-5590-3p and anti-miR-NC followed by treated with 100 μg/ml Sapindus saponin for 24 h, recording as Sapindus saponin+anti-miR-NC group and Sapindus saponin+anti-miR-5590-3p group.
3-[4,5-Dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide (MTT) assay:
SW620 cells were inoculated on 96-well plates and incubated with MTT solution (Beyotime, Shanghai, China) followed by treated with Dimethyl Sulfoxide (DMSO). Absorbance at 490 nm was detected by microplate reader to count cell proliferation inhibition rate.
Colony formation assay:
SW620 cells were cultured for 14 d in 6-well plates. After that, colonies were fixed and colony numbers were counted under a microscope.
Wound healing assay:
After reached 90 % confluences in 6-well plates, SW620 cells were scratched by a 200 μl pipette tip. Wound area was observed under a microscope to count wound healing rate.
Transwell assay:
SW620 cells were seeded into Matrigel-coated transwell upper chamber (Corning Inc., Corning, New York, USA), and complete medium were filled in lower chamber. Then, invaded cell numbers were counted under a microscope.
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
Extracted Ribonucleic Acid (RNA) was reversetranscribed into complimentary Deoxyribonucleic Acid (cDNA), and PCR amplification was performed with SYBR Green (Takara, Dalian, China). miR-5590-3p expression was analyzed by 2−ΔΔCt method.
Wesern blot:
Protein extracted by Radioimmunoprecipitation Assay (RIPA) buffer was transferred onto Polyvinylidene Difluoride (PVDF) membranes after separated by Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel. Membrane was incubated with anti-Matrix Metalloproteinase (MMP)-2, anti-MMP-9 or anti-Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) followed by treated with secondary antibody. Protein signals were displayed using Enhanced Chemiluminescence (ECL) reagents (Beyotime).
Statistical analysis:
Measurement data were expressed as x̄ ±s, and analyzed by Statistical Package for the Social Sciences (SPSS) 20.0 software. Comparison was assessed by Student’s t-test or Analysis of Variance (ANOVA). p<0.05 was considered statistically significant.
Results and Discussion
Has showed in Table 1, cell proliferation inhibition rate was increased, while colony numbers were decreased in the Sapindus saponin-low, Sapindus saponin-middle, and Sapindus saponin-high groups in a dose-dependent manner.
| Group | Inhibition rate (%) | Colony numbers |
|---|---|---|
| Control | 0.00±0.00 | 111.70±10.26 |
| Sapindus saponin+low | 24.57±2.07* | 85.38±7.95* |
| Sapindus saponin+middle | 45.13±5.02*# | 65.50±5.12# |
| Sapindus saponin+high | 71.72±5.97*#& | 48.38±4.43*#& |
| F | 513.020 | 124.501 |
| p | 0.000 | 0.000 |
Note: Compared to control group, *p<0.05; compared to Sapindus saponin+low group, #p<0.05 and compared to Sapindus saponin+middle group, &p<0.05
Table 1: Effect of Sapindus Saponin on Sw620 Cell Proliferation (X̄±S, N=9)
Moreover, MMP-2 and MMP-9 protein levels, wound healing rate and invaded cell numbers were reduced in the Sapindus saponin-low, Sapindus saponin-middle, and Sapindus saponin-high groups in a dose-dependent manner as shown in fig. 1 and Table 2. miR-5590-3p expression was elevated in the Sapindus saponin-low, Sapindus saponin-middle and Sapindus saponin-high groups in a dose-dependent manner as shown in Table 3.
| Group | Wound healing rate (%) | Invaded cell numbers |
|---|---|---|
| Control | 72.71±5.09 | 127.41±11.23 |
| Sapindus saponin+low | 55.81±4.69* | 93.26±7.02* |
| Sapindus saponin+middle | 42.39±3.43*# | 73.43±6.66# |
| Sapindus saponin+high | 25.61±2.05*#& | 54.85±8.25*#& |
| F | 225.313 | 120.483 |
| p | 0.000 | 0.000 |
Note: Compared to control group, *p<0.05; compared to Sapindus saponin+low group, #p<0.05 and compared to Sapindus saponin+middle group, &p<0.05
Table 2: Effect of Sapindus Saponin on Sw620 Cell Migration and Invasion (X̄±S, N=9)
| Group | miR-5590-3p |
|---|---|
| Control | 1.00±0.01 |
| Sapindus saponin+low | 1.62±0.13* |
| Sapindus saponin+middle | 2.41±0.21*# |
| Sapindus saponin+high | 3.11±0.26*#& |
| F | 236.986 |
| p | 0.000 |
Note: Compared to control group, *p<0.05; compared to Sapindus saponin+low group, #p<0.05 and compared to Sapindus saponin+middle group, &p<0.05
Table 3: Effect of Sapindus Saponin on Mir-5590-3p Expression in Sw620 Cells (X̄±S, N=9)
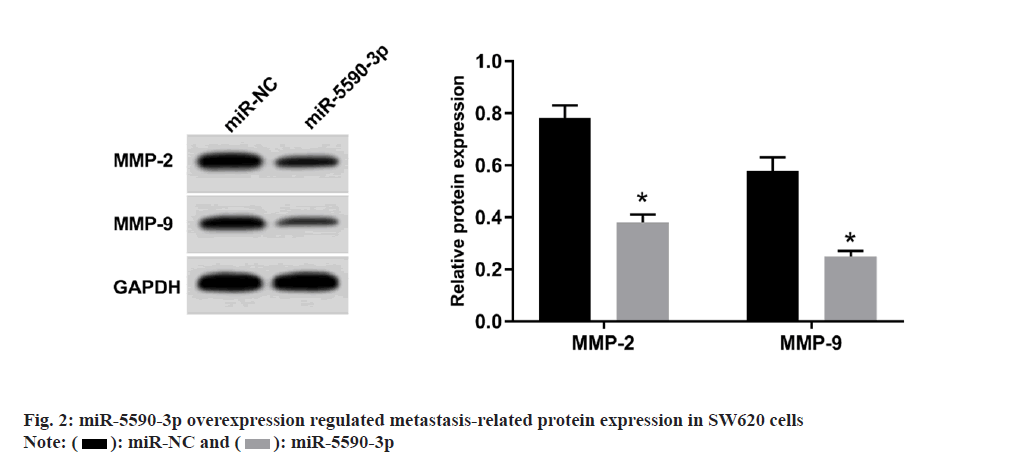
miR-5590-3p mimic was used to overexpress miR-5590-3p expression in SW620 cells. Besides, miR-5590-3p overexpression decreased colony numbers, invaded cell numbers, wound healing rate, MMP-2 and MMP-9 protein levels, while elevated cell proliferation inhibition rate as shown in fig. 2 and Table 4.
| Group | miR-5590-3p | Inhibition rate (%) | Colony numbers | Wound healing rate (%) | Invaded cell numbers |
|---|---|---|---|---|---|
| miR-NC | 1.00±0.00 | 7.38±0.57 | 113.07±12.13 | 73.15±6.35 | 129.16±10.13 |
| miR-5590-3p | 3.02±0.27* | 25.34±2.08* | 53.76±4.62* | 32.37±3.45* | 60.68±4.96* |
| t | 22.444 | 24.983 | 13.708 | 16.929 | 18.214 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared to miR-NC group, *p<0.05
Table 4: miR-5590-3p Overexpression Regulated Sw620 Cell Proliferation and Metastasis (x̄±s, n=9)
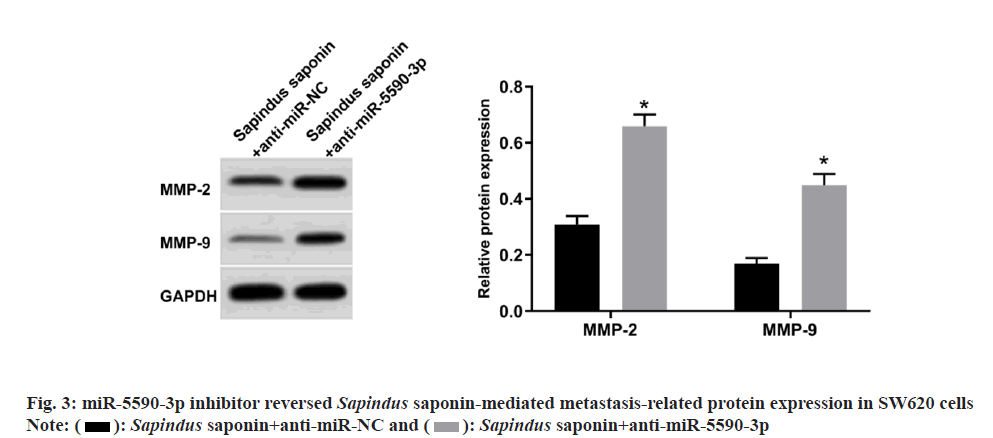
Meanwhile, anti-miR-5590-3p was used to reduce miR-5590-3p expression in Sapindus saponintreated SW620 cells. Functional experiments showed that colony numbers, invaded cell numbers, wound healing rate, MMP-2 and MMP-9 protein levels were enhanced, while cell proliferation inhibition rate was reduced in Sapindus saponin+anti-miR-5590-3p group as shown in fig. 3 and Table 5.
| Group | miR-5590-3p | Inhibition rate (%) | Colony numbers | Wound healing rate (%) | Invaded cell numbers |
|---|---|---|---|---|---|
| Sapindus saponin+anti-miR-NC | 1.00±0.00 | 72.18±5.62 | 46.52±4.51 | 23.53±2.11 | 53.82±4.69 |
| Sapindus saponin+anti-miR-5590-3p | 0.43±0.04* | 27.17±2.69* | 89.28±6.58* | 60.74±4.84* | 106.86±11.85* |
| t | 42.75 | 21.672 | 16.081 | 21.142 | 12.486 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared to Sapindus saponin+anti-miR-NC group, *p<0.05
Table 5: miR-5590-3p Inhibitor Reversed Sapindus Saponin-Mediated Sw620 Cell Proliferation and Metastasis (x̄±s, n=9)
TCM may exert anti-CRC effects by regulating multiple signaling pathways or gene expression[14,15]. miRNAs are aberrantly expressed in CRC tissues and can affect CRC cell biological behaviors[16,17]. However, whether miRNAs can be a potential target for CRC treatment with TCM need to be further explored.
Sapindus saponin plays an important role in many cancers. Sapindus saponin could facilitate apoptosis in lung cancer cells[7]. Also, Sapindus saponin was found to inhibit proliferation in hepatocellular carcinoma cells[8]. However, Sapindus saponins role in CRC has been reported relatively little. Our data showed that SW620 cell proliferation was markedly reduced after treatment with Sapindus saponin, suggesting that Sapindus saponin could suppress CRC cell proliferation. MMP-2 and MMP-9 belong to matrix metalloproteinases, whose expression are up-regulated in CRC and can facilitate cell metastasis[18,19]. Here, Sapindus saponin was found to reduce wound healing rate, invaded cell numbers, MMP-2 and MMP-9 levels in SW620 cells, indicating that Sapindus saponin inhibited CRC cell metastasis.
miR-5590-3p is aberrantly expressed in many cancers[11,20]. miR-5590-3p overexpression suppressed proliferation in breast cancer[21]. miR- 5590-3p upregulation inhibited renal carcinoma cell metastasis[22]. In this study, miR-5590-3p was upregulated in Sapindus saponin-treated SW620 cells. Meanwhile, miR-5590-3p overexpression reduced SW620 cell proliferation and metastasis, whereas its inhibition also reversed the effect of Sapindus saponin on SW620 cell functions, indicating that Sapindus saponin repressed CRC cell progression by promoting miR-5590-3p expression.
In conclusion, Sapindus saponin suppressed CRC cell proliferation and metastasis by upregulating miR-5590-3p. However, whether Sapindus saponin can exert anti-cancer effects by regulating the other genes expression or signaling pathways needs to be further investigated.
Conflict of interests:
The authors declared no conflict of interests.
References
- Mahmoud NN. Colorectal cancer: Preoperative evaluation and staging. Surg Oncol Clin 2022;31(2):127-41.
[Crossref] [Google Scholar] [PubMed]
- Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021;325(7):669-85.
[Crossref] [Google Scholar] [PubMed]
- Riesco-Martinez MC, Modrego A, Espinosa-Olarte P, La Salvia A, Garcia-Carbonero R. Perioperative chemotherapy for liver metastasis of colorectal cancer: Lessons learned and future perspectives. Curr Treat Options Oncol 2022;23(9):1320-37.
[Crossref] [Google Scholar] [PubMed]
- Wen F, Li Q. Treatment dilemmas of cetuximab combined with chemotherapy for metastatic colorectal cancer. World J Gastroenterol 2016;22(23):5332.
[Crossref] [Google Scholar] [PubMed]
- Zhao H, He M, Zhang M, Sun Q, Zeng S, Chen L, et al. Colorectal cancer, gut microbiota and traditional Chinese medicine: A systematic review. Am J Chin Med 2021;49(4):805-28.
[Crossref] [Google Scholar] [PubMed]
- Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu Y, et al. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother 2021;133:111044.
[Crossref] [Google Scholar] [PubMed]
- Wang ML, Xh H. Experimental study on apoptosis induced by Sapindus saponin in human pulmonary carcinoma cell A549 and its molecular mechanisms. Guiding J Tradit Chin Med Pharm 2015;21(4):35.
- Liu WX, Zhou ZH, Chen SZ. Proliferation and apoptosis effects of Sapindus-saponin on human hepatocellular carcinoma Huh7 cells. J Fujian Normal Univ 2019;35(2):90-3.
- Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet 2022;38(6):613-26.
[Crossref] [Google Scholar] [PubMed]
- Ho PT, Clark IM, Le LT. microRNA-based diagnosis and therapy. Int J Mol Sci 2022;23(13):7167.
[Crossref] [Google Scholar] [PubMed]
- Luo ZF, Peng Y, Liu FH, Ma JS, Hu G, Lai SL, et al. Long noncoding RNA SNHG14 promotes malignancy of prostate cancer by regulating with miR-5590-3p/YY1 axis. Eur Rev Med Pharmacol Sci 2020;24(9):4697-4709.
[Crossref] [Google Scholar] [PubMed]
- Wu X, Zhong Y, Zhang H, Li M. miR-5590-3p inhibits the proliferation and invasion of ovarian cancer cells through mediating the Wnt/β-catenin signaling pathway by targeting TNIK. Histol Histopathol 2023:18636.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Zhu A, Gao W, Gui F, Zou Y, Zhou X, et al. miR-5590-3p inhibits the proliferation and metastasis of renal cancer cells by targeting ROCK2 to inhibit proliferation, migration and invasion. Oncol Lett 2022;24(4):1-2.
[Crossref] [Google Scholar] [PubMed]
- Sui H, Zhao J, Zhou L, Wen H, Deng W, Li C, et al. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett 2017;403:86-97.
[Crossref] [Google Scholar] [PubMed]
- Wang R, Liu H, Shao Y, Wang K, Yin S, Qiu Y, et al. Sophoridine inhibits human colorectal cancer progression via targeting MAPKAPK2. Mol Cancer Res 2019;17(12):2469-79.
[Crossref] [Google Scholar] [PubMed]
- Li SS, Zhu HJ, Li JY, Tian LM, Lv DM. miRNA-875-3p alleviates the progression of colorectal cancer via negatively regulating PLK1 level. Eur Rev Med Pharmacol Sci 2020;24(3):1126-33.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Shao Y, Hu W, Zhang J, Shi Y, Kong X, et al. A novel long noncoding RNA SP100-AS1 induces radioresistance of colorectal cancer via sponging miR-622 and stabilizing ATG3. Cell Death Differ 2023;30(1):111-24.
[Crossref] [Google Scholar] [PubMed]
- Jahangiri B, Khalaj-Kondori M, Asadollahi E, Dizaj LP, Sadeghizadeh M. MSC-derived exosomes suppress colorectal cancer cell proliferation and metastasis via miR-100/mTOR/miR-143 pathway. Int J Pharm 2022;627:122214.
[Crossref] [Google Scholar] [PubMed]
- Lu Y, Yu M, Ye J, Liang Y, Gao J, Ji Z, et al. Sauchinone inhibits the proliferation and immune invasion capacity of colorectal cancer cells through the suppression of PD-L1 and MMP2/MM9. Anticancer Agents Med Chem 2023;23(12):1406-14.
[Crossref] [Google Scholar] [PubMed]
- Liang F, Fu X, Wang L. miR-5590-3p-YY1 feedback loop promotes the proliferation and migration of triple-negative breast cancer cells. J Cell Biochem 2019;120(10):18415-24.
[Crossref] [Google Scholar] [PubMed]
- Chen FY, Zhou ZY, Zhang KJ, Pang J, Wang SM. Long non-coding RNA MIR100HG promotes the migration, invasion and proliferation of triple-negative breast cancer cells by targeting the miR-5590-3p/OTX1 axis. Cancer Cell Int 2020;20(1):508.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Dong MH, Hu HM, Min QH, Xiao L. lncRNA FGD5-AS1/miR-5590-3p axis facilitates the proliferation and metastasis of renal cell carcinoma through ERK/AKT signalling. Eur Rev Med Pharmacol Sci 2020;24(17):8756-66.
[Crossref] [Google Scholar] [PubMed]
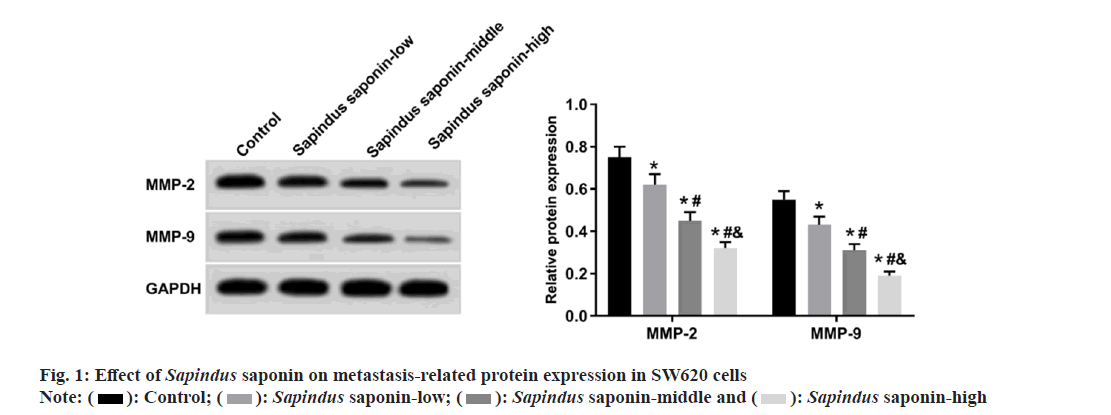
 ): Control; (
): Control; ( ): Sapindus saponin-low; (
): Sapindus saponin-low; ( ): Sapindus saponin-middle and (
): Sapindus saponin-middle and ( ): Sapindus saponin-high
): Sapindus saponin-high