- *Corresponding Author:
- C. Veeresham
University College of Pharmaceutical Sciences, Kakatiya University, Warangal-506 009, India
E-mail: ciddiveeresham@yahoo.co.in
| Date of Submission | 03 December 2016 |
| Date of Revision | 13 November 2017 |
| Date of Acceptance | 07 June 2018 |
| Indian J Pharm Sci 2018;80(4):676-685 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study was aimed at selecting suitable biocatalysts for the enantioselective conversion of racemic sotalol to R(-)-sotalol and to determine the effect of immobilization on enantiomeric purity of the product. Among the various biocatalysts used, Aspergillus niger (GUFCC5443), Cunninghamella blakesleeana (CBS133.27), Cunninghamella elegans (NCIM689), Pseudomonas putida (NCIB9494), Rhodococcus erythropolis (DSM43188), Rhizopus oryzae (NCIM1009), the enzyme lipase AP6 (triacylglycerol lipase from A. niger) demonstrated the capability for enantioselective conversion. Enantioselectivity of all the cultures was improved by immobilization, which resulted in enhanced enantiomeric purity of the product. Catalysis studies with immobilized R. erythropolis and A. niger cultures yielded good enantiomeric ratios of 55.87 and 53.34 as well as good enantiomeric excesses as 96.48 and 96.45 %, respectively. Catalysis with free lipase AP6 resulted in a product of good enantiomeric purity. This enzyme’s enantioselectivity was improved by immobilization from 11.51 to 168.49, which resulted in a product with very high enantiomeric purity of 98.82 %. Hence, the immobilized-lipase AP6 was proved to be an efficient catalyst for the enantioselective conversion of racemic sotalol to R(-)-sotalol with high enantiomeric purity followed by immobilized R. erythropolis and A. niger.
Keywords
Racemic sotalol, enantioselective conversion, lipase AP6 enzyme, immobilization, enantiomeric purity
Most pharmaceutical compounds have asymmetric nature and are optically active. Compounds with one asymmetric centre (chiral centre) would exhibit two enantiomeric forms and among them one is pharmacologically active (eutomer) while the other one contributed side effects or toxic effects or totally inert (distomer) [1]. Hence, current interest is towards development and administration of eutomer instead of its racemic mixture to improve therapeutic efficacy and reduce the unwanted effects due to the distomer [2]. Biocatalysts have excellent selectivity under mild reaction conditions [2] and hence in this study various whole-cell microorganisms like Bacillus subtilis [3], Escherichia coli [4], Pseudomonas putida [5], Sphingomonas paucimobilis [6], Rhodococcus erythropolis [7], Streptomyces halstedii [8], Aspergillus niger [9], Candida parapsilosis [10], Geotrichum candida [11], Rhizopus oryzae [12], Cunninghamella elegans [13] and Cunninghamella blakesleeana [14] were employed as biocatalysts for the stereo inversion of racemic sotalol to its active enantiomer. However, a limitation for using whole-cell microorganisms as biocatalysts is reduced stereo selectivity due to competition between multiple enzymes of the microorganism for the same substrate [15]. The use of isolated pure enzymes with suitable cofactors as biocatalysts would help overcome this limitation of using whole-cell microorganisms [16] and the reactions catalysed by pure enzymes also serves some other advantages like they are highly selective under mild conditions, more catalytic in nature and environmentally benign [2]. Lipases have great versatility in catalysing different reactions like aminolysis, esterification, transesterification and interesterification. The means followed by lipases to catalyse these reactions can be described as a ping-pong bi-bi mechanism. Accordingly, first step involves nucleophilic attack on the carbonyl group of a drug, which results in acyl-enzyme intermediate. This step is promoted by serine, histidine and aspartate residues (catalytic triad) of the enzyme lipase. The acyl-enzyme intermediate then reacts with a nucleophile like water, alcohol or amine regenerating the enzyme along with the product [17]. Hence one pure enzyme, lipase AP6 (triacylglycerol lipase from A. niger) [18] has also been employed in the present study as a biocatalyst to chiral switch the racemic sotalol.
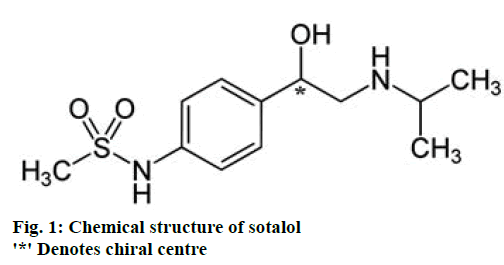
All β-blockers used in clinical practice contain an asymmetric carbon atom and the majority has an aryloxypropanolamine structure. For this type of compounds, the levorotatory l/(-)-enantiomer shows ‘S’ configuration while the dextrorotatory d/(+)-enantiomer shows ‘R’ configuration. S-enantiomers are usually more potent in blocking β-adrenergic receptors in comparison to the corresponding R-enantiomers [19]. For example, carvedilol is a non-selective, β-adrenergic receptor antagonist and α1-adrenoceptor blocker [20]. The overall cardioprotective action is attributed to its S(–)- enantiomer, which is less hepatotoxic than its R(+)- enantiomer [21]. An exception to this rule is sotalol, (RS)- N-{4-[1-hydroxy-2-(propan-2-ylamino)ethyl]phenyl} methanesulfonamide. It is a hydrophilic non-selective β-blocker, characterized more often as a class-III antiarrhythmic agent [22]. It has a phenylethanolamine structure and its asymmetric carbon atom is located in an ethanolamine type side chain (Figure 1). In this case, the priority rules of the four substituents and consequently the absolute configuration of the stereogenic centre changes so that R(–)-sotalol is a much more potent β-blocker than S(+)-sotalol (equivalent to d-sotalol) [19,23]. Because of its enantioselectivity, sotalol mechanism of action is divided between two antiarrhythmic drug classes. The R(–)-sotalol has both β-blocking (class-II antiarrhythmic agent) and potassium channel blocking activity (class-III antiarrhythmic agent), while the S(+)-sotalol has only the potassium channel blocking activity and its affinity towards β-adrenergic receptor is 30-60 times lower [22]. Hence, R(–)-enantiomer would cause decrease in heart rate and a limited reduction in the force of contraction of the heart. This reduction in cardiac work would result in reduced myocardial oxygen demand [24].
Similar to other β-blockers, sotalol inhibits renin release and subsequently reduces the blood pressure in hypertensive subjects but it will not affect the blood pressure in normotensives [24]. As the R(–)-enantiomer is more potent than its antipode, in the present study racemic sotalol has converted to its active enantiomer in pure form using various biocatalysts.
Materials and Methods
Sotalol hydrochloride in the racemic form was purchased from Neuland Laboratories Ltd., Medak, India. Enantiomers of sotalol were isolated from its racemate by Daicel Chiral Technologies Pvt. Ltd., Hyderabad, India. Sodium alginate, sodium chloride, calcium chloride and dipotassium hydrogen phosphate were purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Laboratory grade ethyl acetate purchased from Finar Limited, Ahmadabad, India was used for sample extraction. HPLC grade acetonitrile, diethylamine, formic acid and n-hexane were purchased from Sigma Aldrich Chemicals Pvt. Ltd., Thane, India.
Racemic drug and biocatalyst mixture was incubated in a refrigerated shaker incubator, Model Innova 4230, New Brunswick Scientific Co., Inc., NJ, USA. Samples were analysed on an Ultrafast Liquid Chromatograph, (Shimadzu, Kyoto, Japan) equipped with a binary pump (LC 20AD), a UV/Vis detector (LC 20A) and a Rheodyne injector port. The output signal was monitored and processed using Lab Solutions software. Lux Cellulose-4 chiral column (250×4.6 mm; 5 μ particle size) was purchased from Phenomenex, USA.
Cultures and growth media
Lyophilized cultures of B. subtilis, E. coli, P. putida, S. paucimobilis, R. erythropolis, S. halstedii, A. niger, C. parapsilosis, G. candidum, R. oryzae, C. blakesleeana, and C. elegans were purchased from microbial type culture collection (MTCC), Chandigarh, India and National collection of industrial microorganisms (NCIM), NCL, Pune, India. Suitable growth media were purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India. All the lyophilized cultures were rejuvenated and incubated in refrigerated shaker incubator under MTCC/NCIM specified growth conditions (Table 1). Sub-culturing onto solid and liquid media in regular intervals was continued for maintaining the cultures, which were stored in refrigerator at 4°. The growth media and growth conditions were specified by MTCC for selected cultures of microorganisms (by NCIM for C. elegans) and were shown in Table 1. Another biocatalyst, lipase AP6 enzyme (triacylglycerol lipase from A. niger) was also purchased from Hi Media Laboratories Pvt. Ltd., Mumbai, India.
| Name of the microorganism MTCC/NCIM Accession no. (Equivalent strain no.) | Growth media | Temperature (°) |
Incubation period (d) | Growth condition |
|---|---|---|---|---|
| E. coli MTCC448 (ATCC9637) |
Nutrient agar medium | 37 | 1 | Aerobic |
| B. subtilis MTCC 1305 |
Nutrient agar medium | 37 | 1 | Aerobic |
| P. putida MTCC 102 (NCIB9494) |
Nutrient agar medium | 25 | 2 | Aerobic |
| Sphingomonas paucimobilis MTCC 1919 (NCTC11030) |
Nutrient agar medium | 30 | 1 | Aerobic |
| C. parapsilosis MTCC 998 (13) |
Malt yeast agar medium | 28 | 2 | Aerobic |
| Geotrichum candidum MTCC 3993 (HA780) |
Malt yeast agar medium | 25 | 2 | Aerobic |
| R. erythropolis MTCC 1548 (DSM43188) |
Streptomyces medium | 28 | 4 | Aerobic |
| S. halstedii MTCC 6817 (CKM-2) |
Streptomyces medium | 30 | 2-3 | Aerobic |
| A. niger MTCC 9687 (GUFCC5443) |
Malt extract agar medium | 25 | 7 | Aerobic |
| R. oryzae MTCC 262 (NCIM1009) |
Potato dextrose agar medium | 30 | 7 | Aerobic |
| C. elegans NCIM 689 |
Potato dextrose agar medium | 28 | 4 | Aerobic |
| C. blakesleeana MTCC 3729 (CBS133.27) | Potato dextrose agar medium | 30 | 4 | Aerobic |
Table 1: Growth Conditions for Selected Microbial Cultures
Incubation protocol
For the biotransformation experiments, a two-stage fermentation protocol would enhance the metabolic activities of microorganisms and in the present study, incubation protocol reported by Venisetty et al. [25] was followed with a few modifications. Briefly, first stage culture was initiated by inoculating 25 ml of sterile liquid medium with a loop-full of freshly grown culture, under laminar flow cabinet. These cultures were incubated at 120 rpm under MTCC/NCIM specified growth conditions for particular microorganism. Second stage culture was initiated by inoculating 25 ml of fresh sterile liquid media with 5-10 % v/v inoculum from the first stage culture. Sterile liquid media used for fungal cultures was supplemented with 0.02 % Triton X-100 in order to get good dispersion of fungi in the media.
Whole-cell microbial cultures catalysed enantioselective resolution of sotalol
The procedure of biotransformation used was as per the reported method but with a slight modifications [26]. In brief, 25 ml of second stage suspension cultures of all selected microorganisms were added with 100 μl of racemic sotalol solution (2.5 mg/ml in methanol), which was previously sterilized by passing through syringe driven sterile PVDF hydrophilic membrane filters of pore size 0.22 μm. Immediately after adding the drug solution, the flasks were gently shaken for the even distribution of the drug avoiding the formation of foam. This study (culture+drug) was done in triplicate along with suitable drug controls and culture controls. Drug controls consisted of sterile growth medium with the same amount of the drug added whereas culture controls consisted of second stage suspension cultures of specific microorganisms with 100 μl of methanol instead of the drug solution. All these flasks were incubated for 20 d under specified growth conditions for each specific microbial culture. Samples were collected from each flask on the 3rd, 5th, 10th, 15th and 20th d. The samples were extracted and analysed using a chiral HPLC.
Immobilized-cell microbial cultures catalysed enantioselective resolution of sotalol
The microbial cultures, which gave a positive response for the enantioselective resolution were selected for this study. As the maximum extent of enantioselective conversion in whole-cell culture study was seen up to 10th d, incubation of sotalol in immobilized-cell cultures catalysed study was carried for only up to 10 d. Whole-cell microorganisms were immobilized by alginate entrapment method described by Jobanputra et al. [27] with slight modifications. Briefly, second stage suspension cultures were filtered and the cells were washed with di-potassium hydrogen phosphate (K2HPO4) buffer (pH 6.8) followed by distilled water. These cells were added to 3 % (W/V) of sodium alginate solution (1:1) with vigorous stirring to ensure even distribution. Using a syringe, the suspension was dropped into aqueous solution of calcium chloride (0.2 M) from a height of 15 cm to ensure the bead size as 2-3 mm. Resulted calcium alginate beads were allowed to stand in calcium chloride solution (1 h) for hardening and then they were washed with distilled water and gentle dried on filter paper. Then were mixed with sterile phosphate buffered solution (0.5 % K2HPO4 and 0.5 % sodium chloride) in 1:2 ratio and employed in the present study. It was performed along with suitable controls, culture controls (immobilized-cells without drug) and drug controls (drug in buffer without immobilized-cells). Except culture control all the flasks were added with 100 μl of racemic sotalol solution (2.5 mg/ml in methanol) and incubated at specific growth conditions in refrigerator shaker incubator at 120 rpm for 10 d. After the incubation period the samples were collected, extracted and analysed by chiral HPLC.
Extraction and sample preparation
Samples collected were vortex mixed with three volumes of ethyl acetate for 1 min to get the drug extracted into the ethyl acetate layer. This process was repeated three times. The separated ethyl acetate layers were combined and evaporated under reduced pressure. These samples were further dried using in a vacuum oven [26] to remove all traces of ethylacetate from the samples. The dried samples were reconstituted with 1 ml of the mobile phase, filtered through syringe driven PVDF hydrophilic membrane filters (pore size 0.22 μm) and analysed by the chiral HPLC.
Free-lipase AP6 enzyme catalysed enantioselective resolution of sotalol
The reaction mixture consisted of isopropanol and n-heptane (6:4) to which 100 μl of racemic sotalol solution (2.5 mg/ml in methanol) was added. Then 10 mg/ml of lipase AP6 was added to the reaction mixture to start the reaction [28-30]. The flask containing this reaction mixture was sealed and incubated at 25° for 36 h in a refrigerated shaker incubator at 120 rpm. Samples were collected from the reaction flask at 4 hourly intervals for 36 h. These samples were evaporated to dryness and the residues were dissolved in 1 ml of mobile phase that was used for chiral HPLC analysis of sotalol. The reconstituted samples were filtered using sterile syringe driven PVDF hydrophilic membrane filter (pore size 0.22 μm) and analysed by chiral HPLC.
Immobilized-lipase AP6 catalysed enantioselective resolution of sotalol
In the present study, the enzyme used was immobilized according to the method reported by Jobanputra et al. [27] with a few modifications. Lipase AP6 (10 g/l) was suspended in sterile phosphate buffer (pH 6.8) and was mixed with an equal volume of sodium alginate solution, in order to get the final concentration of sodium alginate as 3 % w/v. This suspension was added drop-wise to calcium chloride solution (0.2 M) with the help of syringe from a height of 15 cm to yield calcium alginate entrapped enzyme beads of size 2 mm. These beads were kept for 1 h in calcium chloride solution to for hardening the beads. Prior to using in biotransformation study, these hardened immobilizedenzyme beads were washed with distilled water three times and gentle dried on a filter paper. Then, 5 g of beads were mixed with sterile phosphate buffered solution (0.5 % K2HPO4 and 0.5 % sodium chloride) in a 1:2 ratio and employed in the present study.
This study was performed along with suitable controls, blank (reaction mixture with enzyme alginate beads and without drug) and drug control (reaction mixture with drug and without enzyme alginate beads). For the biotransformation study, the reaction mixture (isopropanol:n-heptane, 6:4) was added with 100 μl of racemic sotalol solution (2.5 mg/ml in methanol) and the enzyme alginate beads. Then these flasks were incubated at 25° with shaking at 120 rpm for 36 h in an incubator shaker and samples were collected at 4 hourly intervals for 36 h. All these samples were evaporated to dryness and the residues obtained were reconstituted in mobile phase used for chiral HPLC analysis of sotalol. Then these samples were filtered using sterile syringe driven PVDF hydrophilic membrane filters (pore size 0.22 μm) and analysed by chiral HPLC.
HPLC analysis
The samples collected from different biotransformation studies at different time points were extracted and reconstituted with the mobile phase used for chiral HPLC analysis of sotalol. These were analysed using a chiral HPLC method reported by Dossou et al. [31]. The chromatographic conditions of the method were presented in Table 2. Chiral HPLC analysis of the samples revealed the enantioselectivity of various biocatalysts used in the study for enantioselective resolution of sotalol. Enantiomeric ratio (E) is the parameter that is characteristic of both the enantioselectivity of a particular biocatalyst and the degree of conversion but it is independent of substrate concentration [32]. For a resolution process to be valuable it must have high selectivity and which can be determined by estimating the enantiomeric ratio (E) of the process. E can be calculated using the following Eqn. 1, E = 1+EEP/1– EEP, where, EEP is the enantiomeric excess of the product [29]. Generally in biocatalytic reactions, the E value is considered as worst if it is in between 1-5, poor if it is in between 5-10, moderate if in between 10-20 and good if in between 20-100. If ‘E’ value is above 100 then it is considered as excellent [32]. If the reaction to yield high enantiomeric purity (high EE), then it should exhibit excellent enantioselectivity. For example, % EEP values yielded by the reactions with E values of approximately 19, 40 and 100 are 90, 95 and 98 %, respectively. If E value is above 100 then it yields excellent enantiomeric purity (% EE >99 %) [29].
| Stationary phase | Phenomenex Lux Cellulose-4 column (250×4.6 mm; 5 µ particle size) |
| Mobile phase | Acetonitrile:n-hexane:diethylamine:formic acid (100:7.5:0.1:0.2 %) |
| Flow rate | 1 ml/min |
| Injection volume | 20 µl |
| Cell temperature | 40° |
| Detection wavelength | 254 nm |
Table 2: HPLC Chromatographic Conditions of Sotalol
The enantiomeric purity of an optically active molecule is described by % EE and the EE of the substrate and the product (% EES and % EEP) of a reaction can be calculated using the following Eqns. 2 and 3 [33], % EES = R–S/R+S×100 and EEP = R–S/R+S×100. For R>S; where S and R represent the chromatographic peak areas of the S- and R-enantiomers, respectively. The conversion yield (C) of the reaction catalysed by various biocatalysts for the enantioselective conversion of a racemate can be calculated by the following Eqn. 4 [34], C = 1–[A]+[B]/[A]˳+[B]˳, where, [A] and [B] are peak areas of R- and S-enantiomers, respectively while [A]˳ and [B]˳ are the initial peak areas of R- and S-enantiomers, respectively [35]. The conversion yield is being expressed in %.
Results and Discussion
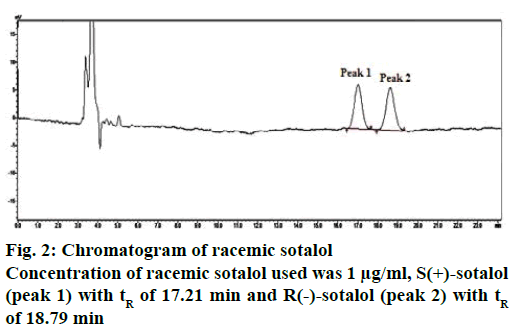
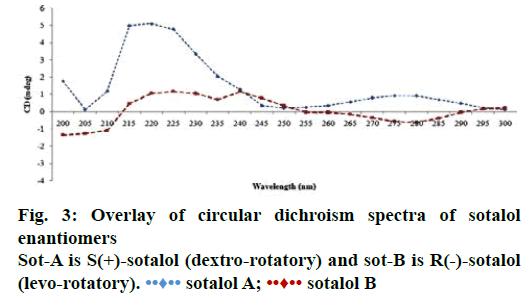
Racemic sotalol was analysed according to an earlier reported chiral HPLC method [31] (Figure 2) and the chromatographic conditions were shown in Table 2. The retention time of S(+)- and R(–)- enantiomers were found as 17.21 and 18.79 min, respectively. The enantiomers of sotalol were isolated and their molecular conformation was determined using circular dichroism (CD) spectroscopy. CD is the difference in the absorption of left-handed circularly polarized light (L-CPL) and right-handed circularly polarized light (R-CPL) and this occurs when a molecule contains one or more chiral chromophores (light absorbing groups). It can be calculated using the following Eqn. 5 [36], ΔA(λ) = A(λ)LCPL–A(λ)RCPL, where, λ is the wavelength and ΔA is difference in absorption.
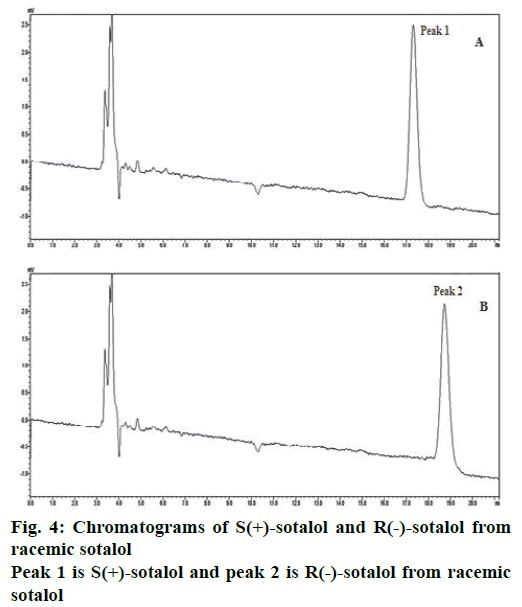
In CD spectra, if a molecule peak is at above the origin (in positive zone) then its conformation is considered as dextro(+) rotatory, while if a molecule peak is below the origin (in negative zone) then is considered as levo(–) rotatory [36]. Overlay of CD spectra of sotalol enantiomers was shown in Figure 3. From which, SOT-A the first eluted peak in chiral HPLC was conformed as dextro(+) rotatory while SOT-B, the second eluted peak in chiral HPLC) as levo(–) rotatory. The configuration of (+)-sotalol is always ‘S’ and of (–)-sotalol is R [18]. Hence SOT-A is S(+)-sotalol while SOT-B is R(–)- sotalol. S(+)- and R(–)-sotalol were analysed using the chiral HPLC method and the peaks were shown in Figure 4A and B, respectively.
Whole-cell and immobilized-cell microbial cultures catalysed enantioselective resolution of sotalol, among the different whole-cell microbial cultures employed in the present study of A. niger, C. blakesleeana, C. elegans, P. putida, R. erythropolis and R. oryzae were responded for the enantioselective resolution of racemic sotalol. Though incubation of the biocatalyst with drug was done for 20 d, maximum enantioselective conversion of racemate to its active enantiomer i.e. maximum enantiomeric excess was seen in 10th d samples. This was observed in case of almost all whole-cell biocatalysts used in this study. Depletion of the concentration of the drug with no further enantioselective conversion was observed in samples collected after 10 d of incubation. Hence in immobilization study, the cultures previously responded for the enantioselective conversion were immobilized and incubated with the racemic drug for only up to 10 d. Cultures were immobilized to improve their enantioselectivity [37] and subsequently to obtain the product with enhanced enantiomeric purity [29]. The 10th d samples collected from the whole-cell and immobilize-cell culture catalysed biotransformation studies were assessed for the enantiomeric purity of sotalol by calculating % EE, E and conversion yield (% C). Enantiomeric purity of sotalol after incubation with the whole-cell as well as immobilized-cell cultures for 10 d was depicted in Table 3. Enantiomeric purity of the drug obtained by the reaction catalysed by whole-cell R. erythropolis culture was found to be high, followed by whole-cell P. putida, C. blakesleeana, R. oryzae, A. niger and C. elegans cultures. E-value of the reaction catalysed by whole-cell R. erythropolis culture was moderate while those of the reactions catalysed by remaining whole-cell microbial cultures were found to be poor. This indicates that the enantioselectivity of the whole-cell microbial cultures was very less and hence it was improved by immobilization [37]. Sample collection and extraction was simplified by immobilization, which has resulted in the efficient extraction of the product. From Table 3, it was clear that immobilization has improved the enantioselectivities (E values) of all the cultures. The E value of the reaction catalysed by immobilized-cell R. oryzae culture was improved from poor to moderate while those of reactions catalysed by remaining all the immobilized-cell cultures were found to be enhanced from poor to good. Hence, the enantiomeric purity of the drug (% EE) also enhanced by the reactions catalysed by all these microbial cultures after immobilization. The enantiomeric purity of the product obtained by the reaction catalysed by immobilized-cell R. erythropolis culture was found to be high, followed by immobilizedcell A. niger, C. blakesleeana, P. putida, C. elegans and R. oryzae cultures. These results were in line with those reported by Tamalampudi et al. [38] and according to them (RS)-1-phenylethanol was enantioselectively transesterified to (R)-1-phenylethyl acetate using immobilized-recombinant A. oryzae expressing lipase-encoding gene from Candida antarctica, which resulted in a product with maximum yield of 88.1 % with a % EE of >99 %. The enantiomeric purity of (R)-1-phenylethyl acetate was found to be high when compared to that of the reaction catalysed by wholecell cultures. The results obtained in the present study were also in line with Wang et al. [39]. According to them racemic (R,S)-2,2-dimethylcyclopropane carboxamide was converted to (S)-2,2-dimethylcyclopropane carboxamide by alginate immobilized-cells of Delftia tsuruhatensis CCTCC M 205114 harboring R-amidase with >49 % yield and a % EE of 97.7.
| Microorganism | Whole-cell culture incubation | Immobilized-cell culture incubation | ||||
|---|---|---|---|---|---|---|
| EE (%) |
E* | C (%) | EE (%) |
E* | C (%) | |
| R. erythropolis | 82.37 | 10.34 | 59.30 | 96.48 | 55.87 | 55.75 |
| P. putida | 80.22 | 9.11 | 74.74 | 93.82 | 31.36 | 72.53 |
| C. blakesleeana | 78.26 | 8.20 | 57.05 | 95.13 | 40.07 | 60.41 |
| R. oryzae | 77.49 | 7.89 | 78.05 | 86.28 | 13.58 | 48.64 |
| A. niger | 75.16 | 7.05 | 59.13 | 96.45 | 53.34 | 53.24 |
| C. elegans | 70.43 | 5.76 | 48.35 | 90.91 | 21.00 | 55.71 |
Table 3: Enantiomeric Purity of Sotalol Incubated with Whole-cell And Immobilized-cell cultures
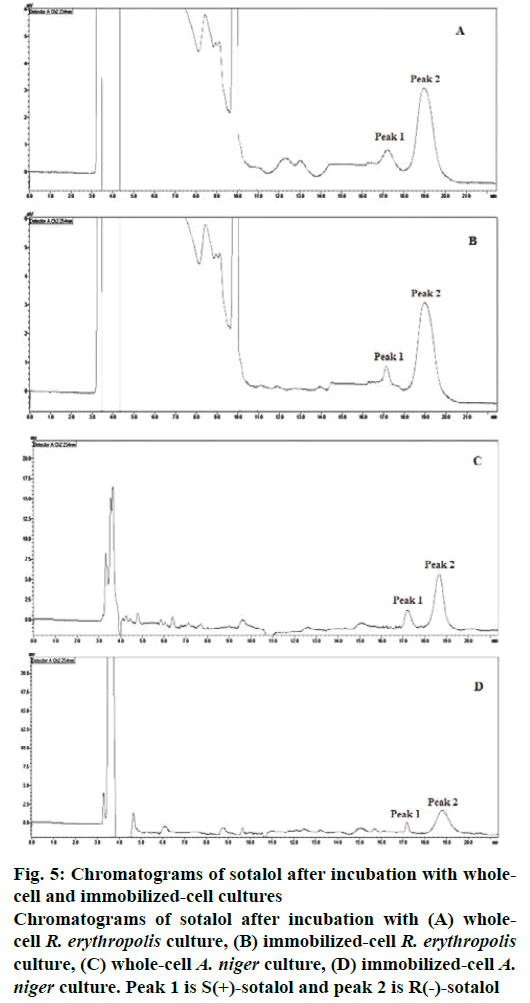
On the whole, all the whole-cell microbial cultures were resulted in good enantiomeric purity but among them R. erythropolis and P. putida cultures showed good % EE. The enantioselectivity of all these wholecell cultures were improved from poor to good by immobilization and hence the enantiomeric purity of the product also enhanced. The reactions catalysed by immobilized-cell R. erythropolis and A. niger cultures have yielded good enantiomeric purity. The chromatograms of sotalol after incubation with wholecell and immobilized-cell R. erythropolis and A. niger cultures were shown in Figure 5A-D, respectively.
Figure 5: Chromatograms of sotalol after incubation with wholecell and immobilized-cell cultures
Chromatograms of sotalol after incubation with (A) wholecell R. erythropolis culture, (B) immobilized-cell R. erythropolis culture, (C) whole-cell A. niger culture, (D) immobilized-cell A. niger culture. Peak 1 is S(+)-sotalol and peak 2 is R(-)-sotalol
Free and immobilized-lipase AP6 enzyme catalysed enantioselective resolution of sotalol, though the samples from the reaction catalysed by free-enzyme was collected up to 36th h, maximum enantiomeric purity of the drug was found only in 24th h and incubation of drug for further longer periods haven’t shown any improvement in % EE. Hence in the immobilized-enzyme catalysed study, the samples were collected only up to 24th h. Comparative enantiomeric purity of the drug after incubating with free as well as immobilized-lipase AP6 at specific time intervals was shown in Table 4. From this it was clear that the enantiomeric excess was gradually increased up to 20th h and there was a slight increase of % EE in 24th h sample also. When compared to the reaction catalysed by free-enzyme, the immobilized-enzyme catalysed study has yielded good enantiomeric purity at each time point. This was because immobilization will improve the stability, enantioselectivity and catalytic activity of the enzyme [37,40,41].
| Time interval (h) | Enantiomeric excess (% EE) | |
|---|---|---|
| Free-lipase AP6 enzyme | Immobilized-lipase AP6 enzyme | |
| 4 | 3.75 | 5.45 |
| 8 | 10.12 | 12.23 |
| 12 | 25.61 | 38.92 |
| 16 | 51.27 | 66.87 |
| 20 | 83.06 | 97.12 |
| 24 | 84.01 | 98.82 |
Table 4: Enantiomeric Purity of Sotalol after Incubation with free and Immobilized-lipase AP6
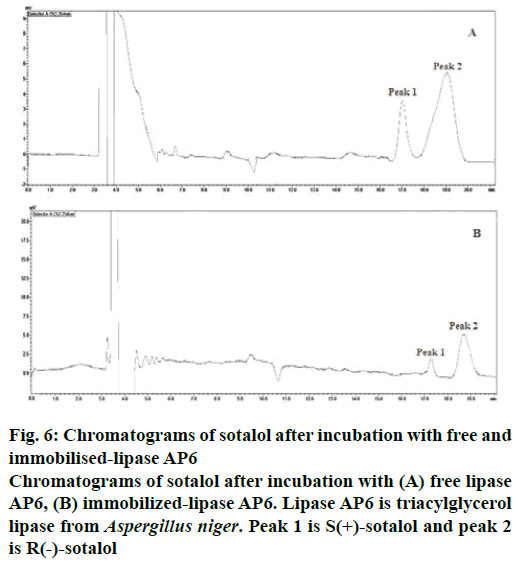
The maximum enantiomeric purity was found at 24th h in both free as well as immobilized-lipase AP6 enzyme catalysed reactions and hence the enantioselective conversion of sotalol was estimated in 24th h samples by calculating % EE, E and % C (Table 5). E is the prime parameter for describing the enzyme’s enantioselectivity. The E value of the reaction catalysed by free lipase AP6 enzyme was found to be moderate at 24th h but it was excellent (E>100) after immobilization and hence, immobilized lipase AP6 catalysed study has yielded very good enantiomeric purity of the drug. Percent C of the drug also found to be high in immobilized-lipase AP6 catalysed study. The results obtained were in line with Peña-Montes et al. [42]. According to them, the recombinant NStcI esterase of A. nidulans has retained its pharmacological activity and enantioselectivity after immobilization for longer periods, when compared to that of the free-enzyme. This resulted in high yield (42 %) and enantiomeric purity (71.7 %) of (R)-enantiomer of racemic (R,S)- 1-phenylethanol. The results were also in line with the results of Huang et al. [41], who reported that the activity and enantioselectivity of lipase from Tsukamurella tyrosinosolvents E105 is enhanced by immobilization and is resulted in improved yield of (S)-2-(2- oxopyrrolidin-1-yl)butyric acid after enantioselective resolution of racemic ethyl 2-(2-oxopyrrolidin-1-yl) butyrate. Chromatograms of sotalol after incubation with free and immobilized-lipase AP6 were shown in Figure 6A and B, respectively.
| State of biocatalyst | Enantiomeric excess (% EE) |
Enantiomeric ratio (E) |
Conversion yield (% C) |
|---|---|---|---|
| Free-lipase AP6 | 84.01 | 11.51 | 49.37 |
| Immobilized-lipase AP6 | 98.82 | 168.49 | 71.80 |
Table 5: Enantiomeric Purity of Sotalol after Incubation with free and Immobilized-lipase AP6 for 24 H
On the whole, enantioselectivity of the microbial cultures and the enzyme used in this study was enhanced by immobilization, which resulted in good enantiomeric purity of sotalol. In conclusion, the enantioselectivity of the biocatalyst was improved by immobilization, which resulted in very good enantiomeric purity of the drug. Among all the microbial cultures used, immobilized- R. erythropolis and A. niger cultures have shown good enantioselectivity and resulted in a product with good enantiomeric purity. These results agreed with the conclusions drawn by Suzuki et al. [43] and Wang et al. [39]. Lipase from A. niger also shown excellent enantioselectivity after immobilization and resulted in excellent enantiomeric purity of the drug. From the results of the present study along with the supporting results reported previously, it could be concluded that the immobilized-lipase AP6 was found to be a more suitable biocatalyst for the enantioselective resolution of racemic sotalol with excellent enantiomeric purity followed by immobilized-cell cultures of R. erythropolis and A. niger.
Acknowledgements
Authors gratefully acknowledge the financial assistance (SR/SO/HS/0087/2010) by the Science and Engineering Research Board, DST, New Delhi, India. Authors also thank the Neuland Laboratories Ltd., Medak, India for the generous gift of racemic sotalol hydrochloride. Authors wish to thank the officer incharge, Central Instrumentation Laboratory, University of Hyderabad, India, for assisting in the determination of molecular conformation of sotalol enantiomers by CD spectroscopy.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ali I, Gupta VK, Aboul-Enein HY, Singh P, Sharma B. Role of racemization in optically active drugs development. Chirality 2007;19:453-63.
- Rene C. Chiral switches: Problems, Strategies, Opportunities and Experiences. In: Ramesh NP, editor. Biocatalysis in the pharmaceutical and biotechnology industries. New York: CRC Press 2007. p. 699-716.
- Adam W, Heckel F, Heckel F, Moller CRS, Schreier P. Biocatalytic synthesis of optically active oxyfunctionalized building blocks with enzymes, chemoenzymes and microorganisms. J Organomet Chem 2002;661:17-29.
- Kato DI, Miyamoto K, Ohta H. Preparation of optically active 4-chlorophenylalanine from its racemate by deracemization technique using transformant Escherichia coli cells. Biocatal Biotransformation 2005;23:375-79.
- Huang HR, Xu JH. Preparation of (S)-mandelic acid from racemate using growing cells of Pseudomonas putida ECU1009 with (R)-mandelate degradation activity. Biochem Eng J 2006;30:11-5.
- Suar M, Hauser A, Poiger T, Buser HR, Müller MD, Dogra C, et al. Enantioselective transformation of α-hexachlorocyclohexane by the dehydrochlorinases LinA1 and LinA2 from the soil bacterium Sphingomonas paucimobilis B90A. Appl Environ Microbiol 2005;71:8514-8.
- Layh N, Knackmuss HJ, Stolz A. Enantioselective hydrolysis of ketoprofen amide by Rhodococcus sp. C3II and Rhodococcus erythropolis MP 50. Biotechnol Lett 1995;17:187-92.
- Levadoux W, Trani M, Lortie R, Kerr D, Groleau D. Microbial resolution of baclofen by a new isolate of Streptomyces halstedii. J Biosci Bioeng 2002;93:557-62.
- Huo KJ, Zhong CH, Yu GZ. Enantioselective hydrolysis of epichlorohydrin using whole Aspergillus niger ZJB-09173 cells in organic solvents. J Biosci 2012;37:695-702.
- Hu Q, Xu Y, Nie Y. Enhancement of Candida parapsilosis catalyzing deracemization of (R,S)-1-phenyl-1,2-ethanediol to its (S)-enantiomer by a highly productive "two-in-one" resin-based in situ product removal strategy. Bioresour Technol 2010;101:8461-3.
- Nakamura K, Inoue Y, Matsuda T, Ohno A. Microbial deracemization of 1-arylethanol. Tetrahedron Lett 1995;36:6263-66.
- Rahime S, Binnaz L, Emine B, Ülkü M, Ayhan SD. Enantioselective production of benzoin from benzoin acetate via kinetic resolution and deracemization using Rhizopus oryzae. Artif Cells Blood Substit Immobil Biotechnol 2011;39:162-68.
- Barth T, Conti R, Pupo MT, Okano LT, Bonato PS. Chiral HPLC analysis of donepezil, 5-O-desmethyl donepezil and 6-O-desmethyl donepezil in culture medium: application to fungal biotransformation studies. Anal Bioanal Chem 2012;404:257-66.
- Hu SH, Tian XF, Han GD. Novel microbial hydroxylation of 13-ethyl-17β-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one. Steroids 1998;63:88-92.
- Ramos AS, Ribeiro JB, Lopes RO, Leite SGF, deSouza ROMA. Highly enantioselective bioreduction of ethyl 3-oxohexanoate. Tetrahedron Lett 2011;52:6127-29.
- Sanchez S, Demain AL. Enzymes and bioconversions of industrial, pharmaceutical, and biotechnological significance. Org Process Res Dev 2011;15:224-30.
- Enríquez-Núñez CA, Camacho-Dávila AA, Ramos-Sánchez VH, Zaragoza-Galán G, Ballinas-Casarrubias L, Chávez-Flores D. Chemoenzymatic Kinetic resolution of (R)-malathion in aqueous media. Chem Cent J 2015;9:46.
- Gunasekaran V, Das D. Lipase fermentation: Progress and prospects. Indian J Biotechnol 2005;4:437-45.
- Kurt S, Gerald J, Wolfgang L. Racemic beta-blockers fixed combinations of different drugs. J Clin Basic Cardiol 1998;1:15-9.
- Gagyi L, Gyeresi A, Kilar F. Role of chemical structure in stereoselective recognition of β-blockers by cyclodextrins in capillary zone electrophoresis. J Biochem Biophys Methods 2008;70:1268-75.
- Hao J, Kim CH. Comparing the effects of Carvedilol enantiomers on regression of established cardiac hypertrophy induced by pressure overload. Lab Anim Res 2010;26:75-82.
- Hancu G, Sămărghiţan C, Rusu A, Mircia E. Sotalol chiral separation by capillary electrophoresis. J Chil Chem Soc 2014;59:2559-63.
- Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics of β-adrenergic blockers in humans. J Pharm Pharm Sci 2001;4:185-200.
- http://www.medsafe.govt.nz/profs/datasheet/s/Sotaloltab.pdf.
- Venisetty RK, Keshetty S, Ciddi V. Biotransformation of silibinin (silybin) using fungal organisms. Indian J Pharm Edu Res 2011;45:384-91.
- Srisailam K, Veeresham C. Biotransformation of celecoxib by microbial cultures. Appl Biochem Biotechnol 2010;160:2075-89.
- Jobanputra AH, Karode BA, Chincholkar SB. Calcium alginate as supporting material for the immobilization of rifamycin oxidase from Chryseobacterium species. Biotechnol Bioinf Bioeng 2011;1:529-35.
- Carvalho PO, Contesini FJ, Ikegaki M. Enzymatic resolution of (r,s)-ibuprofen and (r,s)-ketoprofen by microbial lipases from native and commercial sources. Braz J Microbiol 2006;37:329-37.
- Faber K. Biotransformations in organic chemistry. 6th ed. London: Springer-Verlag Berlin Heidelberg; 2011.
- Liu N, Wang L, Wang Z, Jiang L, Wu Z, Yue H, et al. Microwave-assisted resolution of α-Lipoic acid catalyzed by an ionic liquid co lyophilized lipase. Molecules 2015;20:9949-60.
- Dossou KSS, Chiap P, Chankvetadze B, Servais AC, Fillet M, Crommen J. Optimization of the LC enantioseparation of chiral pharmaceuticals using cellulose tris(4-chloro-3-methylphenylcarbamate) as chiral selector and polar non-aqueous mobile phases. J Sep Sci 2010;33:1699-707.
- Liu R. Enzymes as catalysts in synthesis of enantiomerically pure building blocks- secondary alcohols bearing two vicinal stereocenters [dissertation]. Stockholm, Sweden: School of Chemical Science and Engineering (CHE), KTH Royal Institute of Technology; 2005.
- Smith MB, March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms and Structure. 6th ed. Hoboken, New Jersey: John Wiley & Sons; 2007. p. 180.
- Bhandarkar S, Neau S. Lipase catalyzed enantioselective esterification of flurbiprofen with n-butanol. Electron J Biotechnol 2000;3:195-201.
- Sontakke JB, Yadav GD. Optimization and kinetic modeling of lipase mediated enantioselective kinetic resolution of (±)-2-octanol. Nat Sci 2013;5:1025-33.
- Chougala SS, Niranjan MS, Raju KC, Prashanth E, Kumar KLN. Circular-Dichroism in the study of protein structure. J Pharm Chem 2015;9:36-41.
- Nicole E, Schoning KU. Immobilized biocatalysts in industrial research and production. Top Curr Chem 2004;242:273-317.
- Tamalampudi S, Hama S, Tanino T, Talukder MR, Kondo A, Fukuda H. Immobilized recombinant Aspergillus oryzae expressing heterologous lipase: An efficient whole-cell biocatalyst for Enantioselective transesterification in non-aqueous medium. J Mol Catal B Enzyme 2007;48:337.
- Wang YS, Zheng RC, Xu JM, Liu ZQ, Cheng F, Feng ZH, et al. Enantioselective hydrolysis of (R)-2,2-dimethylcyclopropane carboxamide by immobilized cells of an R-amidase-producing bacterium, Delftia tsuruhatensis CCTCC M 205114, on an alginate capsule carrier. J Ind Microbiol Biotechnol 2010;37:503-10.
- Singh RK, Tiwari MK, Singh R, Lee JK. From Protein Engineering to Immobilization: Promising Strategies for the Upgrade of Industrial Enzymes. Int J Mol Sci 2013;14:1232-77.
- Huang J, Yan R, He JY, Wang P. Purification and immobilization of a novel enantioselective lipase from Tsukamurella tyrosinosolvents for efficient resolution of ethyl 2-(2-oxopyrrolidin-1-yl) butyrate. Appl Biochem Biotechnol 2016;179:1-14.
- Peña-Montes C, Mondragón-Tintor ME, Castro-Rodríguez JA, Bustos-Jaimes I, Navarro-Ocaña A, Farrés A. Immobilization and biochemical properties of the enantioselective recombinant NStcI esterase of Aspergillus nidulans. Enzyme Res 2013:2013;1-11.
- Suzuki T, Idogaki H, Nakagawa A, Kasai N, inventor; Daiso Co., Ltd., assignee. Process for preparing (s)-3-halogeno-1,2-propanediol by Pseudomonas. European patent EP1103620. 2001.
 sotalol A;
sotalol A;  sotalol B
sotalol B