- *Corresponding Author:
- T. Poongodi
Department of Biochemistry, Rathnavel Subramaniam College of Arts and Science, Coimbatore-641 402, India
E-mail: poongodi.btw@gmail.com
| Date of Received | 29 November 2019 |
| Date of Revision | 28 March 2020 |
| Date of Acceptance | 15 July 2020 |
| Indian J Pharm Sci 2020;82(4):692-697 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Antiinflammatory potential of Polyherbal formulation comprising of three selected plants Mimusops elengi L., Kedrostis foetidissima (Jacq.) Cogn. and Artemisia vulgaris L. MKA were studied in Lipopolysaccharide induced RAW 264.7 macrophage cell line. Initially, the MTT cell viability assay was performed to assess the safety level of MKA for anti-inflammatory studies. The MKA was studied for its anti-inflammatory property in selected four different concentrations (25µg/ml, 50 µg/ml, 75 µg/ml and 100 µg/ml). It was observed that increase in MKA concentration decreased the nitrite release from Lipopolysaccharide induced macrophage cells. Microscopic analysis also revealed that MKA restored the normal cell morphology by reducing the cell damage caused by Lipopolysaccharide. The intracellular reactive oxygen species was detected by 2’-7’-Dichlorodihydrofluorescein diacetate labelling and flow cytometry. It was found that, MKA decreased the intracellular Reactive oxygen species level in Lipopolysaccharide stimulated macrophages in a dose-dependent manner, which proved its enhanced anti-inflammatory effect.
Keywords
Polyherbal formulation, Anti-inflammatory, Macrophages, flow cytometry, microscopy
From ancient times, plants are used as medicines for all sorts of diseases[1]. The use of Polyherbal formulation is widely accepted due to its lesser side effects[2]. As multiple herbs are used in the preparation of herbal formulation, multiple compounds act against multiple targets involved in pathogenesis of diseases[3]. Synergistic action of multiple compounds is the key process underlying the mode of action of polyherbal formulation.
Free radicals are produced as by-products or endproducts of biochemical pathway in our human system. These free radicals can damage macromolecules like DNA, proteins and lipids leading to inflammation, which is the key process involved in pathogenesis of almost all diseases like heart disease, cancer, diabetes, skin diseases, Alzheimer’s disease, kidney and liver disorders, etc[4,5,6].
The inflammatory damage is due to the release of ROS from activated neutrophils and macrophages. This further stimulates the release of inflammatory cytokines like tumor necrosis factor-α, interleukin-1 and interleukin-α, which recruits additional neutrophils and macrophages thereby enhancing the rate of inflammation. Free radicals are the key mediators in inflammatory mechanism and free radical scavengers or antioxidants have neutralisation effect, which attenuates inflammation[7,8].
The secondary metabolites in the plants have antioxidant property and they can neutralize the free radicals and prevents the macromolecular damage in biological system. The Plants are screened for their antiinflammatory activity, thereby can be used as medicine for treatment of diseases.
Mimusops elengi L. leaves have potent antiinflammatory property, because of the rich source of antioxidants[9]. Due to the presence of vital secondary metabolites like alkaloids and flavanoids, the leaf infusion of Kedrostis foetidissima (Jacq.) Cogn. is used in curing asthma, skin diseases and snake bite[10]. The leaves of Artemisia vulgaris L. was found to have potent free radical scavenging activity[11]. Also, this plant showed antitumor, antibacterial, antirheumatic, antiseptic and hepatoprotective activities[12,13].
The aim of the present study was to investigate the anti-inflammatory activity of polyherbal formulation (MKA) comprising of three plants Mimusops elengi L., Kedrostis foetidissima (Jacq.) Cogn. And Artemisia vulgaris L. in LPS induced RAW 264.7 macrophage cell line. This study is the continuation of the previous research entitled “Evaluation of free radical scavenging capacity and reducing power of polyherbal formulation comprising of three selected plants”[3].
Materials and Methods
Plant collection and Authentication
Three plants, Mimusops elengi L., Kedrostis foetidissima (Jacq.) Cogn. and Artemisia vulgaris L., were collected from in and around areas of Sulur, Coimbatore, Tamilnadu, India. The plants were authenticated from Botanical Survey of India (BSI), TNAU, Coimbatore. The authentication reference number for Mimusops elengi L. is BSI/SRC/5/23/2018/ Tech/1224, Kedrostis foetidissima (Jacq.) Cogn. is BSI/ SRC/5/23/2018/Tech/1225 and Artemisia vulgaris L. is BSI/SRC/5/23/2018/Tech/1226[3].
Preparation of Polyherbal Formulation
For preparation of polyherbal formulation, Mimusops elengi L., Kedrostis foetidissima (Jacq.) Cogn. and Artemisia vulgaris L. were used in ratio 1:1:1, followed by soxhlet extraction using 80% ethanol. Polyherbal extract was evaporated using rotavaporator until no solvent remained. The resultant sample was stored in refrigerator for future studies after dissolving in DMSO[3].
Cell culture
RAW 264.7 macrophage cell line was procured from NCCS, Pune and cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum, 100 units/ml penicillin and 100μg/ml streptomycin at 37°C with 5% CO2. Cultured macrophage cells were washed using DMEM and 0.25% trypsin-EDTA was used to detach the cells[14].
MTT Cell Viability Assay
The safety level of MKA for anti-inflammatory activity was assessed by MTT assay. The 50μl of MTT was added to 100μl of treated cells and incubated at 37°C for 3 hours. Then, 200μl of PBS was added and excess MTT was removed by aspiration. The formazan crystals formed were solubilised using 200μl of acid-propanol by overnight incubation in dark. The absorbance was read at 650nm in microtitre plate reader. The percentage cell viability was calculated using the formula [(control OD-sample OD)/control OD] x 100[15].
Macrophage cell treatment
The inflammatory macrophage cell model was created by Khan et al. (1995) modified method[16]. RAW 264.7 cells were seeded in 6-well plate at a concentration of 5x103 cells per well and incubated at 37°C in 5%CO2 for 24 hours. Cells were washed with DMEM and supplemented with 1600μl of growth medium. Cells were treated with 200μl of four different concentrations of MKA (25, 50, 75 and 100μg/ml) for 1-2 hours before LPS treatment. 25μM Quercetin was used as standard and cell treatment was done similar to MKA. Then, it was treated with LPS (1μg/ml) and incubated at 37°C in 5% CO2 for 24 hours. Cell control was maintained without LPS and MKA. Also, positive LPS without MKA treatment was also studied. The mixture was then centrifuged at 2000xg for 10 minutes and pelleted cells were used to study cell morphology by Inverted phase contrast microscope and Reactive Oxygen Species(ROS) by DCF-DA Flow cytometry. The Supernatant was used to study Nitrite release by Griess assay[17].
Effect of MKA on cell morphology
Morphological changes were studied in Inverted phase contrast microscopy in 200X magnification. Cell morphology was studied for all cell treatments including control and positive LPS.
Nitrite (NO2) determination
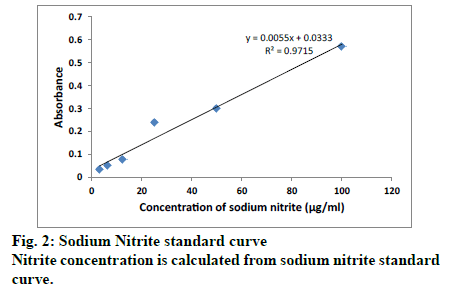
Nitrite is an oxidative end product of Nitric oxide. Nitrite concentration was analysed by Griess assay. 100μl of cell supernatant was incubated with equal volume of Griess reagent and incubated for 10 minutes at room temperature. The absorbance value was measured at 550nm using microplate reader. Sodium nitrite standard curve was used to calculate nitrite levels[14].
Assessment of Intracellular ROS levels
Oxidative damage in intact cells can be assessed using the cell permeable stain 2’, 7’-dichlorofluorescein diacetate (DCF-DA). This molecule DCF-DA can be converted to fluorescent substance 2’, 7’-dichlorofluorescein (DCF) upon oxidation. Measure of this fluorescent substance is an indicator of ROS level in the cell. RAW 264.7 cells were seeded into culture dishes with glass coverslips. Following various pre-treatments, cells were washed using PBS. This is followed by addition of 20μM DCFDA and the cells were incubated for 30 minutes at 37°C in the dark condition. After washing the cells with cold PBS, the DCF fluorescence was analysed in FACS Verse flow cytometer (BD Biosciences) at excitation wavelength of 488nm and emission wavelength of 535nm[18].
Statistical analysis
All experiments were performed in triplicates and the results were expressed as Mean±Standard Deviations (SD). Data were analyzed using one way ANOVA followed by post hoc Dunnett’s multiple comparision test using SPSS software (Version 21). P<0.01 were considered as statistically significant.
Results and Discussion
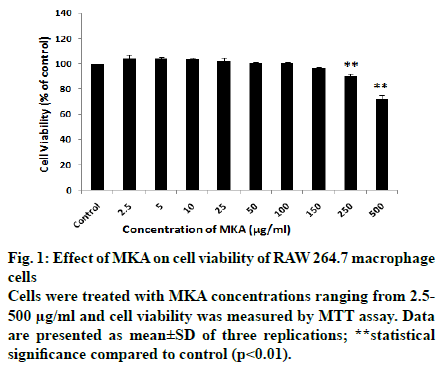
The cell viability of RAW 264.7 Macrophage cells was studied after treatment with different concentrations of Polyherbal formulation (MKA) ranging from 2.5-500 μg/ml by MTT assay. The cell viability was calculated as % of control.
From the MTT results, it was inferred that MKA does not affect the cell viability at concentrations 2.5, 5, 10, 25, 50 and 100 μg/ml (Fig. 1). The cell viability of 96.65±0.95% was seen at 150 μg/ml and 90.05±1.65% was seen in 250 μg/ml MKA treatment. At 500 μg/ml, the cell viability decreased to 71.99±3.05%. There is significant decrease in cell viability in MKA treatments 250 μg/ml and 500 μg/ml compared to control.
Concentrations 2.5, 5, 10, 25, 50, 100 μg/ml were chosen for anti-inflammatory studies as almost 100% cell viability was seen. Similar studies were performed in RAW 264.7 macrophage cells by various researchers to identify the cytotoxiciy and assess the safety level of their drug[ 19,20,21].
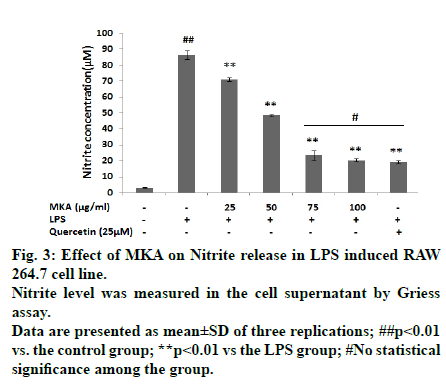
Anti-inflammatory potential of MKA was analysed in LPS stimulated macrophages by nitrite assay. Nitrite level is an indicator of extent of inflammation in the cell. Any anti-inflammatory drug has a tendency to reduce the nitrite production, signifying its pharmacopotential nature. Nitrite concentrations in treated cells were determined using standard sodium nitrite curve (Fig. 2).
LPS treatment significantly increased nitrite concentration to 86.62±2.58 μM. Increase in MKA concentration decreased the nitrite release from macrophage cells in a dose-dependent manner which is an indicator of its anti-inflammatory property (Fig. 3). In 25 μg/ml MKA treatment in LPS induced cell, nitrite production was 71.18±1.25 μM, 50 μg/ml of MKA treatment reduced nitrite level to 48.43±0.60 μM, 75 μg/ml of MKA further reduced nitrite release to 23.32±2.95 μM and lesser amount of nitrite release 20.32±1.02 μM was seen in 100 μg/ml MKA treatment. Nitrite release in standard quercetin was found to be 19.05±1.06 μM. There is no significant difference in nitrite concentration in standard quercetin, 75 μg/ml MKA and 100 μg/ml MKA treatments, which were almost found to have similar efficacy in reducing the nitrite level. From these results, it can be confirmed that MKA has potent anti-inflammatory property.
Fig. 3: Effect of MKA on Nitrite release in LPS induced RAW 264.7 cell line.
Nitrite level was measured in the cell supernatant by Griess assay.
Data are presented as mean±SD of three replications; ##p<0.01
vs. the control group; **p<0.01 vs the LPS group; #No statistical
significance among the group.
The nitrite assay results of MKA corroborate with the findings of Oh YC et al., who conducted research using polyherbal formulation Hwangryunhaedoktang (HR)[19]. Similar research was conducted using a simplified 2-herb formula NF-3 used in traditional Chinese medicine for treating inflammation in diabetic foot ulcer. Nitrite release was studied in LPS induced RAW 264.7 macrophage upon treatment with NF-3 in different concentrations and a decrease in nitrite concentration was seen with increased concentration of NF-3[22].
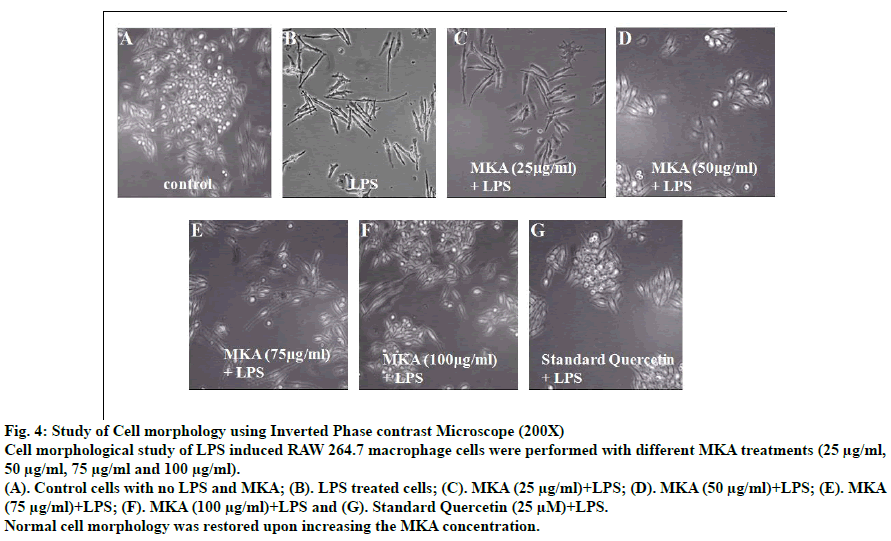
The cell morphological changes of RAW 264.7 macrophage cells were studied using Inverted Phase contrast microscope (200X) after treatment with different concentrations of MKA and LPS stimulation (Fig. 4). The macrophage cells on LPS stimulation were prone to cell damage and showed drastic change in cell morphology. Varied levels of cell repair were seen with different concentrations of MKA treatments on LPS induced macrophage cells. Microscopic picture revealed that the cell damage caused by LPS is reduced in the presence of MKA. Also repair mechanism increased and normal cell morphology was restored in higher concentration of MKA (100 μg/ml).
Fig. 4: Study of Cell morphology using Inverted Phase contrast Microscope (200X)
Cell morphological study of LPS induced RAW 264.7 macrophage cells were performed with different MKA treatments (25 μg/ml,
50 μg/ml, 75 μg/ml and 100 μg/ml).
(A). Control cells with no LPS and MKA; (B). LPS treated cells; (C). MKA (25 μg/ml)+LPS; (D). MKA (50 μg/ml)+LPS; (E). MKA
(75 μg/ml)+LPS; (F). MKA (100 μg/ml)+LPS and (G). Standard Quercetin (25 μM)+LPS.
Normal cell morphology was restored upon increasing the MKA concentration.
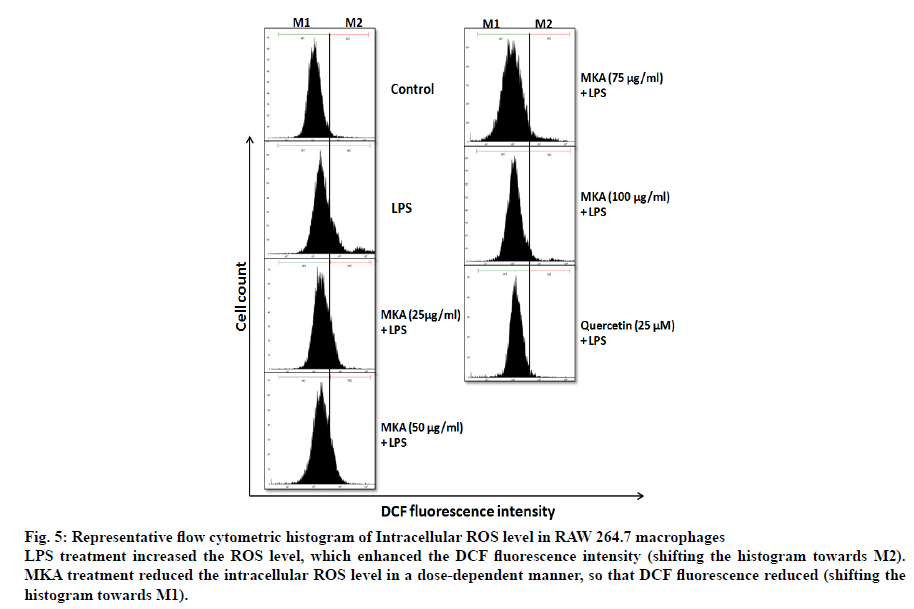
Intracellular ROS is a measure of extent of inflammation in the cells. LPS treatment induced inflammation in macrophages which resulted in the increase of ROS level. Prior treatment of macrophage cells with MKA reduced the production of ROS (Fig. 5). DCF fluorescence increased in LPS treated cells with increased ROS production. In Fig. 5, M1 represents the percentage of normal cells and M2 represents the percentage of cells that increase in ROS production. It was evident that MKA treatment shifted the DCF fluorescence histogram from M2 towards M1. This shift is an indicator of reduction in ROS production by MKA.
Fig. 5: Representative flow cytometric histogram of Intracellular ROS level in RAW 264.7 macrophages
LPS treatment increased the ROS level, which enhanced the DCF fluorescence intensity (shifting the histogram towards M2). MKA treatment reduced the intracellular ROS level in a dose-dependent manner, so that DCF fluorescence reduced (shifting the
histogram towards M1).
The Polyherbal formulations like APR (Angelica gigas Nakai, Panax ginseng and Rhus verniciflua) and OCB (Oryza sativa, Camellia sinensis and Bacillus subtilis) were also reported by researchers to reduce ROS release in LPS induced macrophages[18,23].
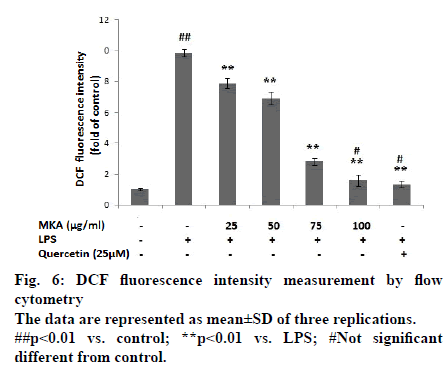
In Fig. 6, ROS levels were illustrated in terms of DCF fluorescence intensity. It is inferred that, LPS treatment significantly increased DCF fluorescence intensity compared to control.
MKA treatments (25 μg/ml, 50 μg/ml, 75 μg/ml and 100 μg/ml) significantly (p<0.01) reduced DCF fluorescence intensity compared to LPS, which implies the reduction of ROS level in a dose-dependent manner. Also it is found that, there is no significant difference in DCF fluorescence intensity between MKA (100 μg/ml) and standard Quercetin .
The present experiment demonstrated for the first time that MKA comprising of Mimusops elengi L., Kedrostis foetidissima (Jacq.) Cogn. And Artemisia vulgaris L. were proven to have anti-inflammatory property in LPS induced RAW 264.7 macrophages, by inhibiting the production of intracellular Reactive oxygen species (ROS). The polyherbal formulation (MKA) was also proven to restore the normal cell morphology by protecting the cells from LPS induced cell membrane damage. Nitrite, the key inflammatory mediator, was also significantly reduced by MKA in LPS induced macrophage cells. It is evident from the current research that MKA has potent anti-inflammatory effect in LPS induced RAW 264.7 macrophages.
Acknowledgement
The authors would like to thank the faculty of Department of Biochemistry, Biotechnology and Bioinformatics, Avinashilingam University for providing the instrumental facilities to complete the present research study.
References
- Petrovska BB. Historical review of medicinal plants' usage. Pharmacogn Rev 2012;6:1-5.
- Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of ayurveda. Pharmacogn Rev 2014;8:73-80.
- Poongodi T, Nazeema.TH. Evaluation of free radical scavenging capacity and reducing power of polyherbal formulation comprising of three selected plants. Int Res J Pharm 2019;10:143-9.
- Aruoma OI. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol 1994;32:671-83.
- Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest 1982;47:412-26.
- Bagchi K, Puri S. Free radicals and antioxidants in health and disease. East Mediterranean Health Jr 1998;4:350-60.
- Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov 2009;3:73-80.
- Delaporte RH, Sanchez GM, Cuellar AC, Giuliani A, de Mello JC. Anti-inflammatory activity and lipid peroxidation inhibition of iridoid lamiide isolated from Bouchea fluminensis(Vell.) Mold. (Verbenaceae). J Ethnopharmacol 2002;82:127-30.
- Kar B, Kumar RBS, Karmakar I, Dola N, Bala A, Mazumder UK et al. Antioxidant and in-vitro antiinflammatory activities of Mimusops elengi L. leaves. Asian Pac J Trop Biomed 2012;2:S976-80.
- Vasantha K, Priyavardhini S, Tresina P, Mohan VR. Phytochemical analysis and antibacterial activity of Kedrostis foetidissima (Jacq.) Cogn. Bioscience Discovery 2012;3:06-16.
- Sharmila K, Padma PR. In vitro free radical scavenging activities of Artemisia vulgaris leaf extract. Indo Am J Pharm Res 2013;3:1716-21.
- Lee SJ, Chung HY, Lee IK, Oh SU, Yoo ID. Phenolics with inhibitory activity on mouse brain monoamine oxidase (MAO) from whole parts of Artemisia vulgaris L (Mugwort). Food Sci Biotechnol 2000;9:179-82.
- Terra DA, Amorim LD, Catanho MT, Fonseca AD, Santos-Filho SD, Brandão-Neto J, et al. Effect of an extract of Artemisia vulgaris L.(Mugwort) on the in vitro labeling of red blood cells and plasma proteins with technetium-99m. Braz Archi Biol Technol 2007;50:123-8.
- Lee JY, Park W. Anti-Inflammatory effect of Myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules 2011;16:7132-42.
- Igarashi M, Miyazawa T. The growth inhibitory effect of conjugated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facilitation of lipid peroxidation in the cells. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids 2001;1530:162-71.
- Khan T, Wagener J, Bost T, Martinez J, Accurso F, Riches D. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995;151:1075-82.
- Dewi K, Widyarto B, Erawijantari PP, Widowati W. In vitro study of Myristica fragrans seed (Nutmeg) ethanolic extract and quercetin compound as anti-inflammatory agent. Int J Res Med Sci 2015;3:2303-10.
- Choi HS, Seo HS, Kim SR, Choi YK, Shin YC, Ko SG. Anti-inflammatory and anti-proliferative effect of herbal medicines (APR) in RAW264. 7 cells. Mol Med Rep 2014;9:1569-74.
- Oh YC, Jeong YH, Cho WK, Lee KJ, Kim T, Ma JY. Lactobacilli-fermented Hwangryunhaedoktang has enhanced anti-inflammatory effects mediated by the suppression of MAPK signaling pathway in LPS-stimulated RAW 264.7 cells. Pharmacogn Mag 2014;10:S645-54.
- Kang SR, Han DY, Park KI, Park HS, Cho YB, Lee HJ, et al. Suppressive effect on lipopolysaccharide-induced proinflammatory mediators by Citrus aurantium L. in macrophage RAW 264.7 cells via NF-B signal pathway. Evid Based Complement Alternat Med 2011;2011:248592.
- Kim J, Jeong SH, Lee W, Min H. In vitro anti-inflammatory activity of Pothos scandens extract in RAW 264.7 cells. Food Sci Biotechnol 2017;26:791-9.
- Tam JCW, Lau KM, Liu CL, To MH, Kwok HF, Lai KK, et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J Ethnopharmacol 2011;134:831-8.
- Rhee Y, Rhee CH, Chung P, Ahn J. Anti-oxidant and anti-inflammatory effects of rice bran and green tea fermentation mixture on lipopolysaccharide-induced RAW 264.7 macrophages. Trop J Pharm Res 2017;16:2943-51.