- *Corresponding Author:
- M. Srinivas Reddy
Department of Pharmaceutical Chemistry, Vaageswari College of Pharmacy, Karimnagar-505 001
E-mail: msr.srinivas@gmail.com
| Date of Submission | 19 September 2016 |
| Date of Revision | 14 December 2016 |
| Date of Acceptance | 29 March 2017 |
| Indian J Pharm Sci 2017;79(2):321-327 |
Abstract
A new series of tetrahydroindeno[1,2-b]pyrrole-3-carboxylate derivatives(4) were synthesized by reaction of 5-amino-2-mercapto benzimidazole with 1,3-dicarbonyls and activated carbonyl compounds using ceric ammonium nitrate catalyst by a three component one-pot reaction. Structures of these compounds were established on the basis of infrared spectroscopy, proton nuclear magnetic resonance, carbon-13 nuclear magnetic resonance, mass spectrometry and elemental analyses. The title compounds 4a-h were evaluated for antiinflammatory activity using the carrageenan-induced paw edema method at a dose of 100 mg/kg in comparison to ibuprofen as a reference drug. Compounds 4a, 4b, 4c and 4d exhibited potent antiinflammatory activity comparable to that of ibuprofen.
Keywords
Tetrahydroindeno[1,2-b]pyrrole-3-carboxylates, multi-component reaction, ceric ammonium nitrate, antiinflammatory activity
Multi-component reactions (MCRs) can be defined as convergent chemical processes where three or more reagents are combined in such a way that the final product retains significant portions of all starting materials [1-5]. In MCRs, a high degree of molecular diversity can be introduced by variation of a single component at a time. Considering that, speed and diversity are key factors in modern drug discovery, MCR strategies offer significant advantages over conventional linear-type syntheses, owing to their exceptional synthetic efficiency [6]. MCRs contribute to the requirements of an ecofriendly process by reducing the number of synthetic steps, energy consumption and waste production. The pyrrole nucleus is widespread in nature, and as previously mentioned, is the key structural fragment of heme and chlorophyll, two pigments essential for life. These include some antibacterial 3-halopyrroles such as pentabromopseudodiline and pyoluteorin, both isolated from bacterial sources. Some of the recently isolated pyrrole-containing marine natural products have been found to exhibit considerable cytotoxicity and function as multidrug resistant reversal agent [7]. In addition poly substituted pyrroles are also been used as antioxidants, antibacterial, ionotropic, antitumor, antiinflammatory and antifungal agents [8-13]. It is known that dihydroxy-oxoindeno[1,2-b]pyrroles exhibit a wide range of biological activities [14-17]. Eboracin, a substituted indenopyrrole/trioxyindenopyrrole, inhibited the tonic component of convulsive seizures induced in mice by pentylenetetrazol, electroshock or auditory stimulation [18-23].
All melting points were determined on a Cintex melting point apparatus and are uncorrected. Analytical thin layer chromatography (TLC) was performed on Merck pre-coated 60 F254 silica gel plates. Visualization was done by exposing to iodine vapour. Infrared (IR) spectra (KBr pellet) were recorded on a PerkinElmer BX series Fourier transform infrared (FTIR) spectrometer. Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Varian Gemini 300 MHz spectrometer. Carbon-13 nuclear magnetic resonance (13C NMR) spectra were recorded on a Bruker 75 MHz spectrometer. Chemical shift values were given in δ ppm with tetramethylsilane as an internal standard. Mass spectral measurements were carried out by electron ionization (EI) method on a Joel JMC-300 spectrometer at 70 eV. Elemental analyses were performed on a Carlo Erba 106 and Perkin-Elmer model 240 analysers.
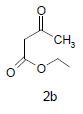
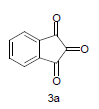
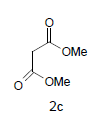
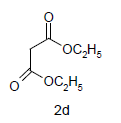
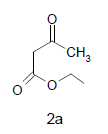
Mixture of 5-amino-2-mercapto-benzimidazole (1) (1 mmol) and 1,3-dicarbonyl (2) (1 mmol) was stirred in ethanol (20 ml) in presence of ceric ammonium nitrate (CAN) 10 mol % at 70° for 10 min. Then activated carbonyl (3) (1 mmol) was added slowly. The reaction mixture was continued stirring for 20 min. After the completion of the reaction (monitored by TLC), the reaction mixture was poured into 20 ml ice cold water. The separated solid is filtered and recrystallized from ethyl acetate to afford the title compounds. The structures of compounds are given in Table 1.
| S. No | 5-amino-2-meracpto benzimidazole | 1,3-dicarbonyls | Activated carbonyl | Compound |
|---|---|---|---|---|
| 1 |  |
 |
 |
 4a |
| 2 | 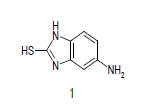 |
 |
 |
 4b |
| 3 | 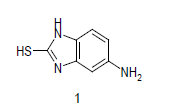 |
 |
 |
 4c |
| 4 | 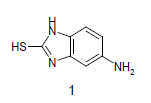 |
 |
 |
 4d |
| 5 | 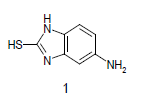 |
 |
 |
 4e |
| 6 | 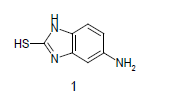 |
 |
 |
 4f |
| 7 | 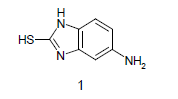 |
 |
 |
 4g |
| 8 | 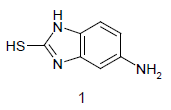 |
 |
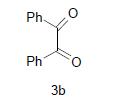 |
 4h |
Table 1: The structures of the synthesized compounds
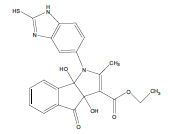
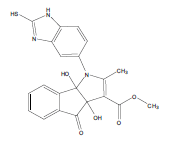
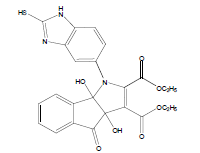
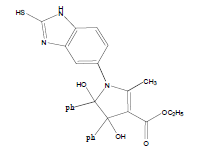
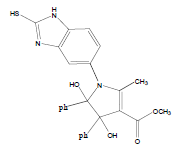
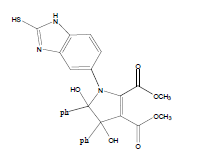
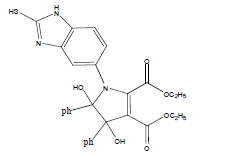
E t h y l - 3 a , 8 b - d i h y d r o x y - 2 - m ethyl-4-oxo-1- (2-sulfanyl-1H-benzo[d]imidazol-5-yl)-1,3a,4,8btetrahydroindeno[ 1,2-b]pyrrole-3-carboxylate (4a); MP 183-185°, yield 91%.1H-NMR (CDCl3, 300 MHz): δ 0.98 (t, 3H, OCH2CH3), 2.21 (pyrrole-CH3), 4.03 (s, 1H, SH), 4.26 (q, 2H, OCH2CH3), 4.53(s, 1H, OH, D2O exchangeable), 4.83 (s, 1H, OH, D2O exchangeable), 7.00-7.63 (m, 7H, ArH), 8.26 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1:3352 (OH), 3243 (OH), 3140 (NH), 1710, 1680 (CO).13C-NMR (75 MHz, CDCl3) δ ppm: 13.43, 14.15, 67.45, 81.01, 90.23, 92.01, 102.11, 114.46, 115.02, 122.23, 125.56, 125.10, 128.12, 133.78, 135.10, 136.15, 140.65, 146.23, 150.12, 168.32, 169.01, 197.23. EI-MS (70 eV) m/z:437(M+). Anal. calcd. for C22H19N3O5S: C, 60.40; H, 4.38; N, 9.61. Found: C, 60.43; H, 4.45; N, 9.53.
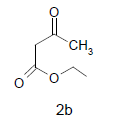
Methyl-3a,8b-dihydroxy-2-methyl-4-oxo-1-(2- sulfanyl-1H-benzo[d]imidazol-5-yl)-1,3a,4,8btetrahydroindeno[ 1,2-b]pyrrole-3-carboxylate(4b); MP 176-178°, yield 87%. 1H-NMR (CDCl3, 300 MHz): δ 2.23 (pyrrole-CH3), 3.89 (s, 3H, OCH3), 4.11 (s, 1H, SH), 4.49 (s, 1H, OH, D2O exchangeable), 4.9 (s, 1H, OH, D2O exchangeable), 7.13-7.62 (m, 7H, ArH), 8.01 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1: 3341(OH), 3253(OH), 3138(NH), 1735, 1678(CO). 13C-NMR (75MHz, CDCl3) δ ppm: 13.95, 52.13, 81.21, 90.12, 93.12, 102.56, 116.91, 117.10, 121.12, 123.10, 125.81, 128.11, 135.12, 133.10, 138.01, 140.18, 147.21, 150.12, 166.34, 169.00, 197.21. EI-MS (70 eV) m/z: 423(M+). Anal. calcd. for C21H17N3O5S: C, 59.45; H, 4.11; N, 9.76. Found: C, 59.57; H, 4.05; N, 9.92.
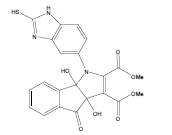
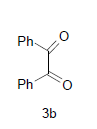
D i m e t h y l - 3 a , 8 b - d i h y d r o x y - 4 - o x o - 1 - ( 2 - sulfanyl-1H-benzo[d]imidazol-5-yl)-1,3a,4,8btetrahydroindeno[ 1,2-b]pyrrole-2,3-dicarboxylate(4c); MP 201-203°, yield 80%. 1H-NMR (CDCl3, 300 MHz): δ 3.98 (s, 6H, 2OCH3), 4.03 (s, 1H, SH), 4.56 (s, 1H, OH, D2O exchangeable), 4.73 (s, 1H, OH, D2O exchangeable), 7.08-7.51 (m, 7H, ArH), 8.26 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1:3342 (OH), 3230 (OH), 3146 (NH), 1715, 1700 (CO). 13C-NMR (75MHz, CDCl3) δ ppm: 52.30, 52.83, 84.12, 92.10, 102.34, 116.71, 117.34, 121.02, 122.21, 125.45, 127.34, 128.03, 133.23, 135.71, 138.18, 140.23, 143.54, 150.21, 163.10, 168.12, 169.32, 199.21. EI-MS (70 eV) m/z: 467(M+). Anal. calcd. for C22H17N3 O7 S: C, 56.61; H, 3.56; N, 8.78. Found: C, 56.53; H, 3.67; N, 8.99.
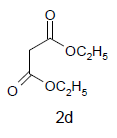
Diethyl-3a,8b-dihydroxy-4-oxo-1-(2-sulfanyl- 1 H - b e n z o [ d ] i m i d a z o l - 5 - y l ) - 1 , 3 a , 4 , 8 b - tetrahydroindeno[1,2-b]pyrrole-2,3-dicarboxylate(4d); MP 193-195°, yield 85%. 1H-NMR (CDCl3, 300 MHz): δ 0.98 (t, 6H, 2OCH2 CH3), 4.01 (s, 1H, SH), 4.26 (q, 4H, 2OCH2CH3), 4.53 (s, 1H, OH, D2O exchangeable), 4.62 (s, 1H, OH, D2O exchangeable), 7.13-7.49 (m, 7H, ArH), 7.96 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1:3338 (OH), 3263 (OH), 3125 (NH), 1705, 1670 (CO). 13C-NMR (75MHz, CDCl3) δ ppm : 14.21, 14.70, 61.34, 61.67, 63.02, 92.23, 102.43, 116.23, 117.32, 120.34, 122.12, 123.05, 127.78, 128.25, 133.45, 135.54, 138.12, 140.17, 142.08, 150.32, 165.10, 168.15, 169.41, 199.10. EI-MS (70 eV) m/z: 495 (M+). Anal. calcd. forC24H21N3O7S: C, 58.21; H, 4.31; N, 8.50. Found: C, 58.18; H, 4.27; N, 8.48.
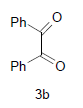
Ethyl-4,5-dihydroxy-2-methyl-4,5-diphenyl-1-(2- sulfanyl-1H-benzo[d]imidazol-5-yl)-4,5-dihydro-1H- 3-pyrrolecarboxylate (4e); MP 180-182°, yield 93%. 1H-NMR (CDCl3,300 MHz): δ 0.95 (t, 3H, OCH2CH3), 2.20 (pyrrole-CH3), 4.12 (s, 1H, SH), 4.21 (q, 2H, OCH2CH3), 4.60 (s, 1H, OH, D2O exchangeable), 4.81 (s, 1H, OH, D2O exchangeable), 7.12-7.72 (m, 13H, ArH), 8.01 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1: 3348 (OH), 3240 (OH), 3145 (NH), 1721 (CO). 13C-NMR (75MHz, CDCl3) δ ppm: 13.18, 14.42, 61.07, 99.12, 102.01, 102.21, 104.23, 116.56, 117.04, 124.11, 125.02, 125.44, 126.05, 126.31, 127.00, 127.25, 128.22, 129.09, 129.21, 129.56, 136.34, 140.22, 143.02, 146.05, 148.31, 166.09, 169.20. EI-MS (70 eV) m/z: 487(M+). Anal. calcd. for C27H25N3O4S: C, 66.56; H, 5.19; N, 8.76. Found: C, 66.51; H, 5.17; N, 8.62.
Methyl-4,5-dihydroxy-2-methyl-4,5-diphenyl-1-(2- sulfanyl-1H-benzo[d]imidazol-5-yl)-4,5-dihydro- 1H-3-pyrrolecarboxylate(4f); MP 205-207°, yield 90%. 1H-NMR (CDCl3, 300 MHz): δ 2.21 (pyrrole- CH3), 3.74 (s, 3H, OCH3), 4.13 (s, 1H, SH), 4.43 (s, 1H, OH, D2O exchangeable), 4.58 (s, 1H, OH, D2O exchangeable), 7.03-7.51 (m, 13H, ArH), 8.06 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1: 3351 (OH), 3243 (OH), 3135 (NH), 1700 (CO). 13C-NMR (75MHz,CDCl3) δ ppm: 13.23, 52.11, 99.10, 102.01, 102.31, 106.08, 114.05, 117.21, 126.01, 126.41, 127.17, 125.21, 128.02, 128.04, 127.22, 127.42, 129.23, 129.90, 129.92, 136.23, 140.21, 143,04, 143.20, 147.42, 166.08, 169.31. EI-MS (70 eV) m/z: 473 (M+). Anal. calcd. for C26H23N3O4S: C, 65.98; H, 4.88; N, 8.75. Found: C, 65.95; H, 4.90; N, 8.87.
Dimethyl-4,5-dihydroxy-4,5-diphenyl-1-(2-sulfanyl- 1H-benzo[d]imidazol-5-yl)-4,5-dihydro-1H-2,3- pyrroledicarboxylate(4g); MP 179-181°, yield 89%. 1H-NMR (CDCl3, 300 MHz): δ 3.94 (s, 6H, 2OCH3), 4.10 (s, 1H, SH), 4.51 (s, 1H, OH, D2O exchangeable), 4.64 (s, 1H, OH, D2O exchangeable), 7.01-7.50 (m, 13H, ArH), 8.00 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1: 3347 (OH), 3242 (OH), 3141 (NH), 1700 (CO). 13C-NMR (75MHz, CDCl3) δ ppm: 51.03, 52.42, 98.32, 101.21, 102.11, 116.21, 117.34, 121.10, 124.09, 124.16, 127.09, 127.18, 127.31, 127.33, 127.54, 128.20, 128.34, 129.03, 129.21, 136.32, 138.11, 143.03, 143.12, 143.14,165.03, 168.21, 169.32. EI-MS (70 eV) m/z: 517(M+). Anal. calcd. for C27H23N3O6S: C, 62.73; H, 4.55; N, 8.22. Found: C, 62.66; H, 4.48; N, 8.12.
Diethyl-4,5-dihydroxy-4,5-diphenyl-1-(2-sulfanyl- 1H-benzo[d]imidazol-5-yl)-4,5-dihydro-1H-2,3- pyrroledicarboxylate(4h); MP. 211-213°, yield 86%.1H-NMR (CDCl3, 300 MHz): δ 0.97 (t, 6H, 2OCH2CH3), 4.12 (s, 1H, SH), 4.26 (q, 4H, 2OCH2CH3), 4.51 (s, 1H, OH, D2O exchangeable), 4.76 (s, 1H, OH, D2O exchangeable), 7.10-7.62 (m, 13H, ArH), 8.23 (s, 1H, NH, D2O exchangeable). IR (KBr) cm-1:3341(OH), 3230 (OH), 3142 (NH), 1725 (CO). 13C-NMR (75 MHz, CDCl3) δ ppm:12.09, 13.21, 62.70, 61.40, 98.32, 101.21, 102.92, 114.09, 115.24, 120.31, 126.10, 126.21, 127.26, 127.43, 127.48, 128.12, 129.32, 129.39, 128.45, 128.59, 128.60, 138.12, 140.12, 143.12, 145.20, 147.23, 165.45, 169.02, 171.13. EI-MS (70 eV) m/z: 545 (M+). Anal. calcd. for C29H27N3O6S: C, 63.78; H, 4.50; N, 7.54. Found: C, 63.84; H, 4.99; N, 7.70.
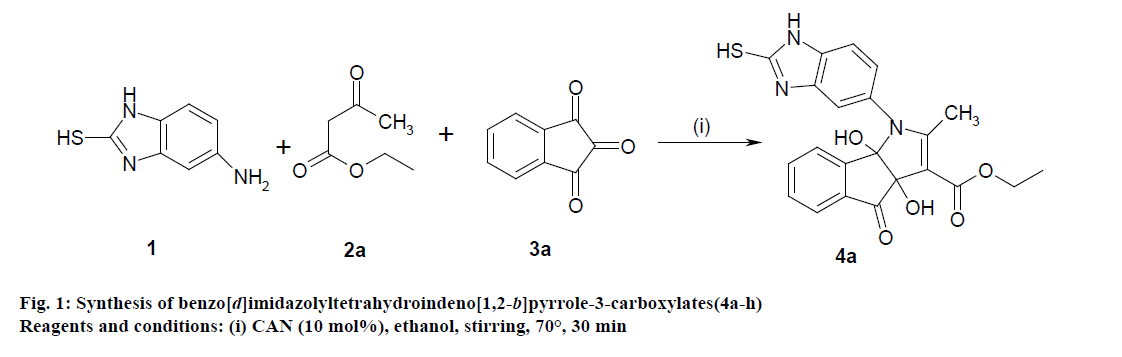
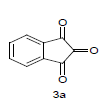
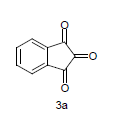
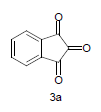
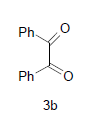
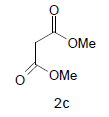
Literature survey revealed that when one biodynamic heterocyclic system was coupled with another, a molecule with enhanced biological activity was produced [24,25]. Therefore, we investigated a multi component reaction of 5-amino-2-mercaptobenzimidazole (1), ethyl acetoacetate (2a) and ninhydrine (3a) in ethanol (20 ml), which afforded benzo[d]imidazolyltetrahydroindeno[1,2-b]pyrrole- 3-carboxylate (4a) under the optimized reaction condition, a systematic study was carried out for the catalytic evaluation of CAN in this cyclization. The best overall yield (91%) was obtained with CAN (10 mol%) and is found to be more effective in terms of shorter reaction time (30-40 min) and yield (83-91%). It has been found that these three component process works well for any tested combination of 5-amino-2- meracptobenzimidazole (1), 1,3-dicarbonyls (2) and activated carbonyl compounds (3) in ethanol using 10 mol% CAN as catalyst. By adopting similar procedure, eight new derivatives (4a-h) have been synthesized by a one-pot three component reaction (Table 1, Figure 1).
The IR spectra of 4a-h showed absorption bands around 3352, 3243 for two OH functional groups, 3140 cm-1 for NH functional group and 1710, 1680 cm-1 due to C=O functional group. 1H NMR spectra of compounds 4a-h exhibited four singlets at δ 0.98, 2.21, 4.53 and 4.83 due to OCH2CH3, pyrrole-CH3, and two OH protons, respectively and a quartet at δ 4.26 due to OCH2CH3 protons confirms the formation of pyrrole dicarboxylate ring. The structure of the products was elucidated with the help of IR, 1H NMR, 13C NMR, mass spectrometry (MS) and elemental analyses.
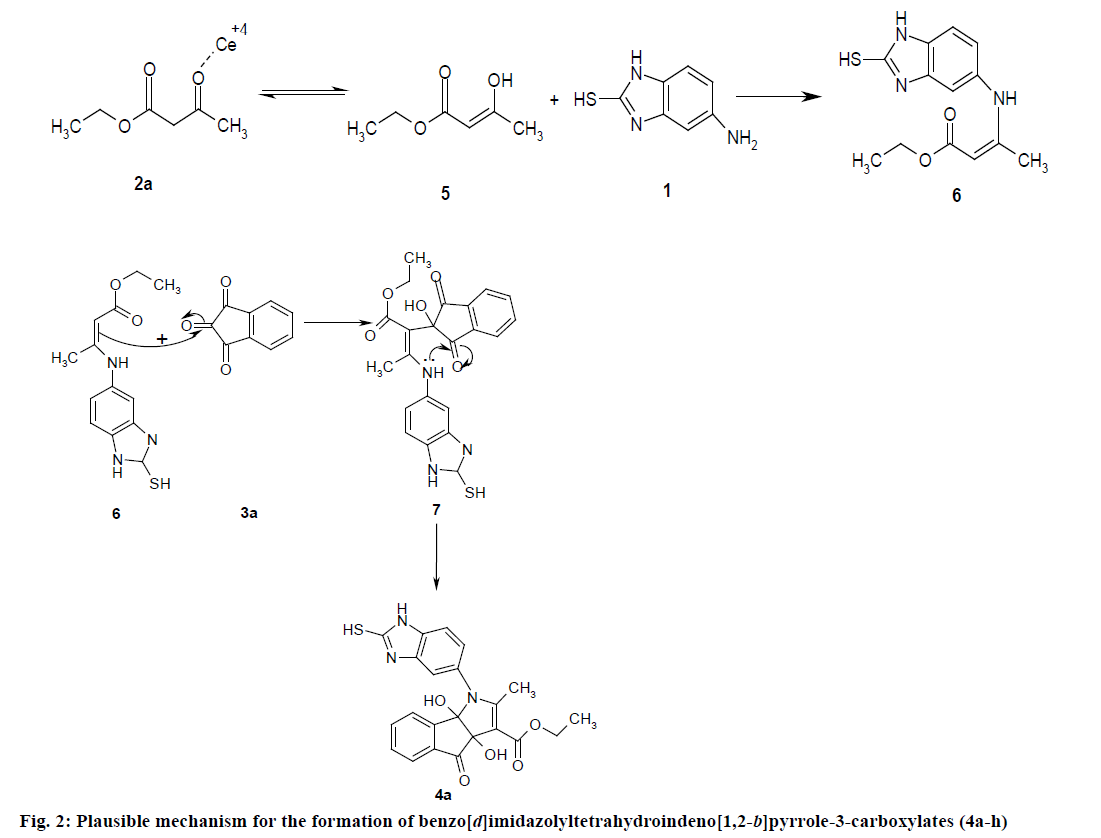
The formation of benzo[d]imidazolyl tetrahydroindeno[1,2-b]pyrrole-3-carboxylates 4a could be explained by the following plausible mechanism (Figure 2). The reaction involves the initial formation of enaminones 6 between 1,3-dicarbonyl 5 and 5-amino-2-mercaptobenzimidazole 1 activated by CAN. Enaminones 6 react with carboxyl group of ninhydrine 3a via a Michael type addition to form 7, which further undergo cyclization leads to the corresponding title compound. CAN may be activating the double bond of 1,3-dicarbonyl 2a by forming a complex, there by the reaction is facilitated and also enhances the rate of reaction.
Antiinflammatory activity was determined in the carrageenan-induced rat paw edema model [26]. Wistar rats of either sex weighing 150-200 g were divided into 6 groups (n=6) and they were fasted 18 h before the experiment with water ad libitum. Group I received 1% sodium carboxymethyl cellulose (CMC) (negative control), group II received ibuprofen at a dose of 100 mg/kg (positive control) and groups III to VI were given the compounds 4a-h (100 mg/kg). All the compounds 4a-h were given in oral route. After 30 min, 0.1 ml of 1% carrageenan suspension in normal saline was injected into the sub plantar region of the left hind paw of each rat to induce edema. The edema volumes of the injected paw measured with the help of plethysmograph at the interval of 0, 1, 2, 4 and 6 h. The difference between the paw volumes of treated animals were compared with that of the control group and the mean edema volume was calculated. Percentage inhibition was calculated as per the Eqn., percent inhibition = (Vo–Vt)/Vo)×100, where, Vo=volume of the paw control at time t, Vt=volume of the paw of drug treated at time t. Results were expressed as a mean ± SE. The antiinflammatory properties were recorded at successive intervals of 0, 1, 2, 4 and 6 h and compared with that of standard ibuprofen. The antiinflammatory activity data (Table 2) indicated that all the compounds 4a-h exhibited significant activity by decreasing the paw volume that was produced by carrageenan. Among all the compounds tested, it is interesting to note that the compounds 4a, 4b, 4c and 4d showed better antiinflammatory activity, may be due to the basic skeleton indeno[1,2-b]pyrrole-3-carboxylate.
| Paw volume (ml of Hg)b | |||||
|---|---|---|---|---|---|
| Groupa | 0 h | 1 h | 2 h | 4 h | 6 h |
| 4a | 0.18 ± 0.03 | 0.55 ± 0.07 | 0.55 ± 0.05 | 0.52 ± 0.01 | 0.37 ± 0.05*** |
| 4b | 0.21 ± 0.01 | 0.42 ± 0.03 | 0.58 ± 0.04 | 0.50 ± 0.02 | 0.30 ± 0.02*** |
| 4c | 0.15 ± 0.01 | 0.40 ± 0.05 | 0.51 ± 0.01 | 0.59 ± 0.01 | 0.40 ± 0.02 |
| 4d | 0.18 ± 0.03 | 0.58 ± 0.1 | 0.50 ± 0.04 | 0.52 ± 0.01 | 0.41 ± 0.07 |
| 4e | 0.37 ± 0.02 | 0.63 ± 0.08 | 0.57 ± 0.02 | 0.53 ± 0.06 | 0.46 ± 0.01 |
| 4f | 0.41 ± 0.07 | 0.59 ± 0.02 | 0.60 ± 0.05 | 0.60 ± 0.02 | 0.31 ± 0.05 |
| 4g | 0.44 ± 0.05 | 0.52 ± 0.04 | 0.92 ± 0.04 | 0.61 ± 0.07 | 0.47 ± 0.03 |
| 4h | 0.58 ± 0.01 | 0.57 ± 0.02 | 0.81 ± 0.02 | 0.55 ± 0.01 | 0.57 ± 0.02 |
| Control | 0.36 ± 0.3 | 0.90 ± 0.05 | 1.07 ± 0.08 | 1.2 ± 0.05 | 0.96 ± 0.03 |
| Ibuprofen | 0.30 ± 0.5 | 0.66 ± 0.03 | 0.70 ± 0.05*** | 0.60 ± 0.05*** | 0.40 ± 0.05*** |
Table 2: Antiinflammatory activity of Tetrahydroindeno[1,2-B]Pyrrole-3-Carboxylates#(4a-H)
In conclusion, the synthesis of novel benzo[d] imidazolyltetrahydroindeno[1,2-b]pyrrole-3- carboxylates was achieved through a one step process from readily accessible starting materials in moderate to good yields. This synthesis benefits from a simple method of purification, which does not require chromatography. The ease of purification complimented this synthetic technology practical, easy to perform and facile. The title compounds can be considered as future drug candidates for antiinflammatory activity by simple modifications in their structure and of course required further evaluations to reveal the exact mechanism of action, so that using structure activity relationship a new potent analogue can be generated with desired efficacy. The newly synthesized novel benzo[d]imidazolyl-tetrahydroindeno[1,2-b]pyrrole-3- carboxylates were tested for antiinflammatory activity and compounds 4a, 4b, 4c and 4d exhibited significant activity, when compared to standard.
Acknowledgments
The authors thank the Head, Department of Chemistry, Kakatiya University, Warangal for providing the facilities and the Director, Indian Institute of Chemical Technology, Hyderabad for recording 1H NMR, 13C NMR and mass spectra.
Conflict of interests
Authors report no conflict of interests.
Financial support and sponsorship
Nil.
References
- Orru RVA, deGreef M. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003;10:1471-99.
- Tejedor D, González-Cruz D, Santos-Expósito A, Marrero-Tellado JJ, de Armas P, García-Tellado F. Multicomponent domino processes based on the organocatalytic generation of conjugated acetylides: Efficient synthetic manifolds for diversity-oriented molecular construction. Chemistry 2005;11:3502-10.
- Domling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 2006;1:17-89.
- Guillena G, Ramon DJ, Yus M. Organocatalyticenantioselective multicomponent reactions (OEMCRs). Cheminform 2007;18:693-700.
- Toure BB, Hall DG. Natural product synthesis using multicomponent reaction strategies. Chem Rev 2009;109:4439-86.
- FayolA, Zhu J. Three-component synthesis of polysubstituted 6-azaindolines and its tricyclic derivatives. Org Lett 2005;7:239-42.
- Tao H, Hwang I, Boger DL. Multidrug resistance reversal activity of permethylningalin B amide derivatives. Bioorg Med ChemLett 2004;14:5979-81.
- Lehuede J, Fauconneau B, Barrier L, Ourakow M , Piriou A, Vierfond J. Synthesis and antioxidant activity of new tetraarylpyrroles. Eur J Med Chem 1999;34:991-6.
- Bürli RW, Jones P, McMinn D, Le Q, Duan JX, Kaizerman JA, et al. DNA binding ligands targeting drug-resistant Gram-positive bacteria. Part 2: C-terminal benzimidazoles and derivatives. Bioorg Med ChemLett 2004;14:1259-63.
- Jonas R, KlockowM, Lues I, Prucher H, Schliep HJ, Wurziger H. Synthesis and biological activities of meribendan and related heterocyclic benzimidazolo-pyridazinones. Eur J Med Chem 1993;2:129-40.
- Denny WA, Rewcastle GW, Baguley BC. Potential antitumor agents. 59. Structure-activity relationships for 2-phenylbenzimidazole-4-carboxamides, a new class of "minimal" DNA-intercalating agents, which may not act via topoisomerase II. J Med Chem 1990;33:814-9.
- DemopoulosVJ, Rekka E. Isomeric benzoylpyrroleaceticacids:some structural aspects for aldose reductase inhibitory and antiinflammatory activities. J Pharm Sci 1995;84:79-82.
- Del Poeta M, Schell WA, Dykstra CC, Jones S, Tidwell RR, Czarny A, et al. Structure in vitro activity relationships of pentamidine analogues and dication-substituted bis-benzimidazoles as new antifungal agents. Antimicrob Agents Chemother 1998;42:2495-502.
- Wang L, Price HL,Juusola J, Kline M, Phanstiel O. Influence of polyamine architecture on the transport and topoisomerase II inhibitory properties of polyamine DNA-intercalator conjugates. J Med Chem 2001;44:3682-691.
- Tsuchiya T, Takagi Y, Yamada H. Preparation of 5-(2,6-dideoxy-2-fluoro-alpha-L-talopyranosyloxy)-6-hydroxynaphtho[2,3-f]quinoline-7,12-dione (FT-Alz), a new-type, potentially antitumor substance with various biological activities. Bioorg Med ChemLett 2000;10:203-7.
- Brana MF, Ramos A. Naphthalimides as anticancer agents: synthesis and biological activity. Curr Med Chem Anticancer Agents 2001;1:237-55.
- Mereyala HB, Joe M. Cytotoxic activity of styryl lactones and their derivatives. Curr Med Chem Anticancer Agents 2001;1:293-300.
- Alexander GJ, Chatterjie N, Kopeloff LM. Effect of eboracin on convulsive seizures in mice: enhancement of myoclonus and inhibition of the tonic phase. Res CommunChemPatholPharmacol 1982;36:153-6.
- Ravindernath A, Srinivas Reddy M. Synthesis, antimicrobial, antiinflammatory and antioxidant activity of novel Spiro (imidazo[4′,5′:4,5′]benzo[1,2-e][1,4]thiazepine)-9,3′-indolines. J SulfChem 2012;3:363-72.
- Ravindernath A, Srinivas Reddy M.A mild and efficient three component one-pot synthesis of novel 9-aryl-2-sulfanyl-5,9-dihydro-1H-Imidazo[4,5-h][4,1]benzothiazepin-6[7h]-ones. Het Lett 2012;2:291-6.
- Ravindernath A, Srinivas Reddy M, Sunil V. Synthesis and biological evaluation of benzo[d] imidazolylchromeno[2,3-d]pyrimidinones. Med Chem Res 2014;23:759-64.
- Rajanarendar E, Ramakrishna S, Ramamurthy K. Synthesis of novel isoxazolylbis-thiazolo[3,2-a] pyrimidines. Chin ChemLett 2012;23:899-02.
- Rajanarendar E, Ramakrishna S, Govardhan Reddy K, Nagaraju D, Reddy YN. A facile synthesis, antiinflammatory and analgesic activity of isoxazolyl-2,3-dihydrospiro [benzo[f]isoindole-1,3′-indoline]-2′,4,9-triones. Bioorg Med ChemLett 2013;23:3954-58.
- Boschi D, Cena C, Di Stilo A, Fruttero R, Gasco A. Nicorandil analogues containing NO-donor furoxans and related furazans. Bioorg Med Chem 2012;8:1727-32.
- Moloney GP, Martin GR, Mathews N, Milne A, Hobbs H, Dodsworth S, et al. Synthesis and serotonergic activity of 2-oxa diazolyl-5-substituted-N,N-dimethyltryptamines: novel antagonists for the vascular 5-HT1B-like receptor. J Med Chem 1999;42:2504-26.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paws of the rat as an assay for antiiflammatory drugs. ProcSocExpBiol Med 1962;111:544-7.